Abstract
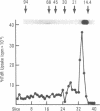
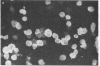
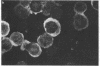
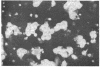
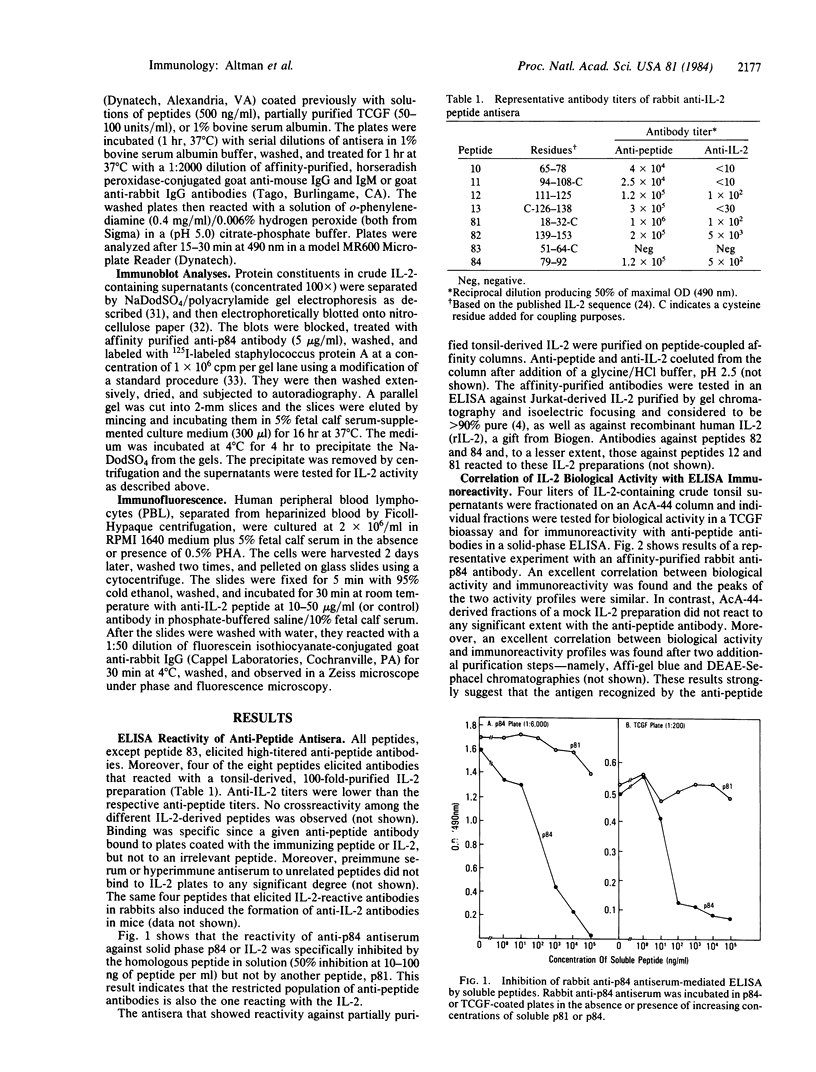
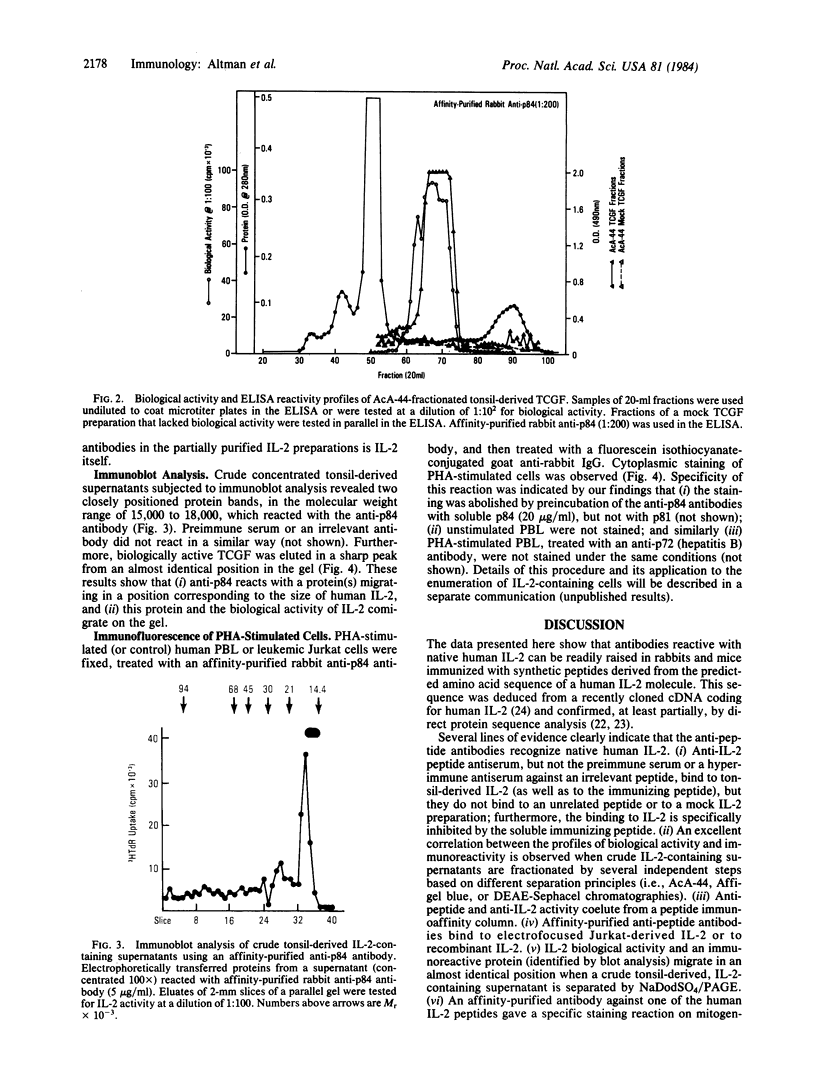
This communication describes the preparation of antibodies to human interleukin 2 (IL-2), using as immunogens synthetic peptides derived from the predicted amino acid sequence of IL-2. Rabbits and mice were immunized with protein carrier conjugates of eight chemically synthesized IL-2-derived peptides, each consisting of 13-15 amino acids. The immune antisera were screened in a solid-phase ELISA for reactivity to a native human IL-2. Antibodies to four of the eight peptides were found by a variety of biological and immuno-chemical criteria to react against human IL-2. Furthermore, an affinity-purified antibody to one of the IL-2 peptides (peptide 84) specifically stained the cytoplasm of phytohemagglutinin-stimulated human peripheral blood lymphocytes or T-leukemic cells (Jurkat). Antibodies to synthetic IL-2 peptides should serve as useful probes for studying this lymphokine and for developing quantitative assays for measuring its levels in biological fluids and its association with disease.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alcocer-Varela J., Alarcón-Segovia D. Decreased production of and response to interleukin-2 by cultured lymphocytes from patients with systemic lupus erythematosus. J Clin Invest. 1982 Jun;69(6):1388–1392. doi: 10.1172/JCI110579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander H., Johnson D. A., Rosen J., Jerabek L., Green N., Weissman I. L., Lerner R. A. Mimicking the alloantigenicity of proteins with chemically synthesized peptides differing in single amino acids. Nature. 1983 Dec 15;306(5944):697–699. doi: 10.1038/306697a0. [DOI] [PubMed] [Google Scholar]
- Altman A., Theofilopoulos A. N., Weiner R., Katz D. H., Dixon F. J. Analysis of T cell function in autoimmune murine strains. Defects in production and responsiveness to interleukin 2. J Exp Med. 1981 Sep 1;154(3):791–808. doi: 10.1084/jem.154.3.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittle J. L., Houghten R. A., Alexander H., Shinnick T. M., Sutcliffe J. G., Lerner R. A., Rowlands D. J., Brown F. Protection against foot-and-mouth disease by immunization with a chemically synthesized peptide predicted from the viral nucleotide sequence. Nature. 1982 Jul 1;298(5869):30–33. doi: 10.1038/298030a0. [DOI] [PubMed] [Google Scholar]
- Cheever M. A., Greenberg P. D., Fefer A., Gillis S. Augmentation of the anti-tumor therapeutic efficacy of long-term cultured T lymphocytes by in vivo administration of purified interleukin 2. J Exp Med. 1982 Apr 1;155(4):968–980. doi: 10.1084/jem.155.4.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flomenberg N., Welte K., Mertelsmann R., Kernan N., Ciobanu N., Venuta S., Feldman S., Kruger G., Kirkpatrick D., Dupont B. Immunologic effects of interleukin 2 in primary immunodeficiency diseases. J Immunol. 1983 Jun;130(6):2644–2650. [PubMed] [Google Scholar]
- Gentry L. E., Rohrschneider L. R., Casnellie J. E., Krebs E. G. Antibodies to a defined region of pp60src neutralize the tyrosine-specific kinase activity. J Biol Chem. 1983 Sep 25;258(18):11219–11228. [PubMed] [Google Scholar]
- Gerin J. L., Alexander H., Shih J. W., Purcell R. H., Dapolito G., Engle R., Green N., Sutcliffe J. G., Shinnick T. M., Lerner R. A. Chemically synthesized peptides of hepatitis B surface antigen duplicate the d/y specificities and induce subtype-specific antibodies in chimpanzees. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2365–2369. doi: 10.1073/pnas.80.8.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis S., Ferm M. M., Ou W., Smith K. A. T cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol. 1978 Jun;120(6):2027–2032. [PubMed] [Google Scholar]
- Gillis S., Kozak R., Durante M., Weksler M. E. Immunological studies of aging. Decreased production of and response to T cell growth factor by lymphocytes from aged humans. J Clin Invest. 1981 Apr;67(4):937–942. doi: 10.1172/JCI110143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm E. A., Mazumder A., Zhang H. Z., Rosenberg S. A. Lymphokine-activated killer cell phenomenon. Lysis of natural killer-resistant fresh solid tumor cells by interleukin 2-activated autologous human peripheral blood lymphocytes. J Exp Med. 1982 Jun 1;155(6):1823–1841. doi: 10.1084/jem.155.6.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henney C. S., Kuribayashi K., Kern D. E., Gillis S. Interleukin-2 augments natural killer cell activity. Nature. 1981 May 28;291(5813):335–338. doi: 10.1038/291335a0. [DOI] [PubMed] [Google Scholar]
- Houghten R. A., Chang W. C., Li C. H. Human beta-endorphin: synthesis and characterization of analogs iodinated and tritiated at tyrosine residues 1 and 27. Int J Pept Protein Res. 1980 Oct;16(4):311–320. doi: 10.1111/j.1399-3011.1980.tb02592.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Linker-Israeli M., Bakke A. C., Kitridou R. C., Gendler S., Gillis S., Horwitz D. A. Defective production of interleukin 1 and interleukin 2 in patients with systemic lupus erythematosus (SLE). J Immunol. 1983 Jun;130(6):2651–2655. [PubMed] [Google Scholar]
- Liu F. T., Zinnecker M., Hamaoka T., Katz D. H. New procedures for preparation and isolation of conjugates of proteins and a synthetic copolymer of D-amino acids and immunochemical characterization of such conjugates. Biochemistry. 1979 Feb 20;18(4):690–693. doi: 10.1021/bi00571a022. [DOI] [PubMed] [Google Scholar]
- McMillan S., Seiden M. V., Houghten R. A., Clevinger B., Davie J. M., Lerner R. A. Synthetic idiotypes: the third hypervariable region of murine anti-dextran antibodies. Cell. 1983 Dec;35(3 Pt 2):859–863. doi: 10.1016/0092-8674(83)90118-6. [DOI] [PubMed] [Google Scholar]
- Mills G. B., Paetkau V. Generation of cytotoxic lymphocytes to syngeneic tumor by using co-stimulator (Interleukin 2). J Immunol. 1980 Nov;125(5):1897–1903. [PubMed] [Google Scholar]
- Morgan D. A., Ruscetti F. W., Gallo R. Selective in vitro growth of T lymphocytes from normal human bone marrows. Science. 1976 Sep 10;193(4257):1007–1008. doi: 10.1126/science.181845. [DOI] [PubMed] [Google Scholar]
- Reiner N. E., Finke J. H. Interleukin 2 deficiency in murine Leishmaniasis donovani and its relationship to depressed spleen cell responses to phytohemagglutinin. J Immunol. 1983 Sep;131(3):1487–1491. [PubMed] [Google Scholar]
- Robb R. J., Kutny R. M., Chowdhry V. Purification and partial sequence analysis of human T-cell growth factor. Proc Natl Acad Sci U S A. 1983 Oct;80(19):5990–5994. doi: 10.1073/pnas.80.19.5990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb R. J., Munck A., Smith K. A. T cell growth factor receptors. Quantitation, specificity, and biological relevance. J Exp Med. 1981 Nov 1;154(5):1455–1474. doi: 10.1084/jem.154.5.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruscetti F. W., Morgan D. A., Gallo R. C. Functional and morphologic characterization of human T cells continuously grown in vitro. J Immunol. 1977 Jul;119(1):131–138. [PubMed] [Google Scholar]
- Smith K. A., Favata M. F., Oroszlan S. Production and characterization of monoclonal antibodies to human interleukin 2: strategy and tactics. J Immunol. 1983 Oct;131(4):1808–1815. [PubMed] [Google Scholar]
- Smith K. A. T-cell growth factor. Immunol Rev. 1980;51:337–357. doi: 10.1111/j.1600-065x.1980.tb00327.x. [DOI] [PubMed] [Google Scholar]
- Stadler B. M., Berenstein E. H., Siraganian R. P., Oppenheim J. J. Monoclonal antibody against human interleukin 2 (IL 2). I. Purification of IL 2 for the production of monoclonal antibodies. J Immunol. 1982 Apr;128(4):1620–1624. [PubMed] [Google Scholar]
- Steinmann G., Conlon P., Hefeneider S., Gillis S. Serological visualization of interleukin 2. Science. 1983 Jun 10;220(4602):1188–1190. doi: 10.1126/science.6344215. [DOI] [PubMed] [Google Scholar]
- Sutcliffe J. G., Shinnick T. M., Green N., Lerner R. A. Antibodies that react with predetermined sites on proteins. Science. 1983 Feb 11;219(4585):660–666. doi: 10.1126/science.6186024. [DOI] [PubMed] [Google Scholar]
- Taniguchi T., Matsui H., Fujita T., Takaoka C., Kashima N., Yoshimoto R., Hamuro J. Structure and expression of a cloned cDNA for human interleukin-2. Nature. 1983 Mar 24;302(5906):305–310. doi: 10.1038/302305a0. [DOI] [PubMed] [Google Scholar]
- Thoman M. L., Weigle W. O. Cell-mediated immunity in aged mice: an underlying lesion in IL 2 synthesis. J Immunol. 1982 May;128(5):2358–2361. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner H., Hardt C., Heeg K., Pfizenmaier K., Solbach W., Bartlett R., Stockinger H., Röllinghoff M. T-T cell interactions during cytotoxic T lymphocyte (CTL) responses: T cell derived helper factor (Interleukin 2) as a probe to analyze CTL responsiveness and thymic maturation of CTL progenitors. Immunol Rev. 1980;51:215–255. doi: 10.1111/j.1600-065x.1980.tb00323.x. [DOI] [PubMed] [Google Scholar]
- Wagner H., Hardt C., Heeg K., Röllinghoff M., Pfizenmaier K. T-cell-derived helper factor allows in vivo induction of cytotoxic T cells in nu/nu mice. Nature. 1980 Mar 20;284(5753):278–278. doi: 10.1038/284278a0. [DOI] [PubMed] [Google Scholar]
- Warren H. S., Atkinson K., Pembrey R. G., Biggs J. C. Human bone marrow allograft recipients: production of, and responsiveness to, interleukin 2. J Immunol. 1983 Oct;131(4):1771–1775. [PubMed] [Google Scholar]
- Wofsy D., Murphy E. D., Roths J. B., Dauphinée M. J., Kipper S. B., Talal N. Deficient interleukin 2 activity in MRL/Mp and C57BL/6J mice bearing the lpr gene. J Exp Med. 1981 Nov 1;154(5):1671–1680. doi: 10.1084/jem.154.5.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarling J. M., Bach F. H. Continuous culture of T cells cytotoxic for autologous human leukaemia cells. Nature. 1979 Aug 23;280(5724):685–688. doi: 10.1038/280685a0. [DOI] [PubMed] [Google Scholar]