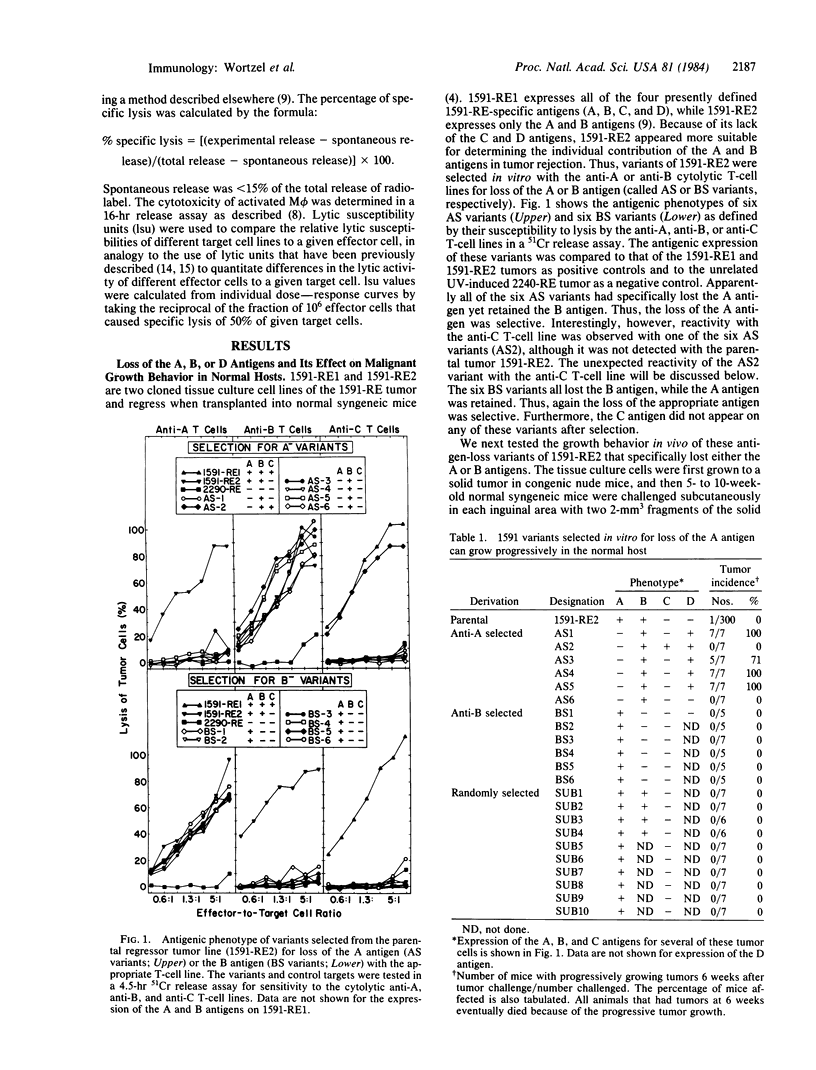
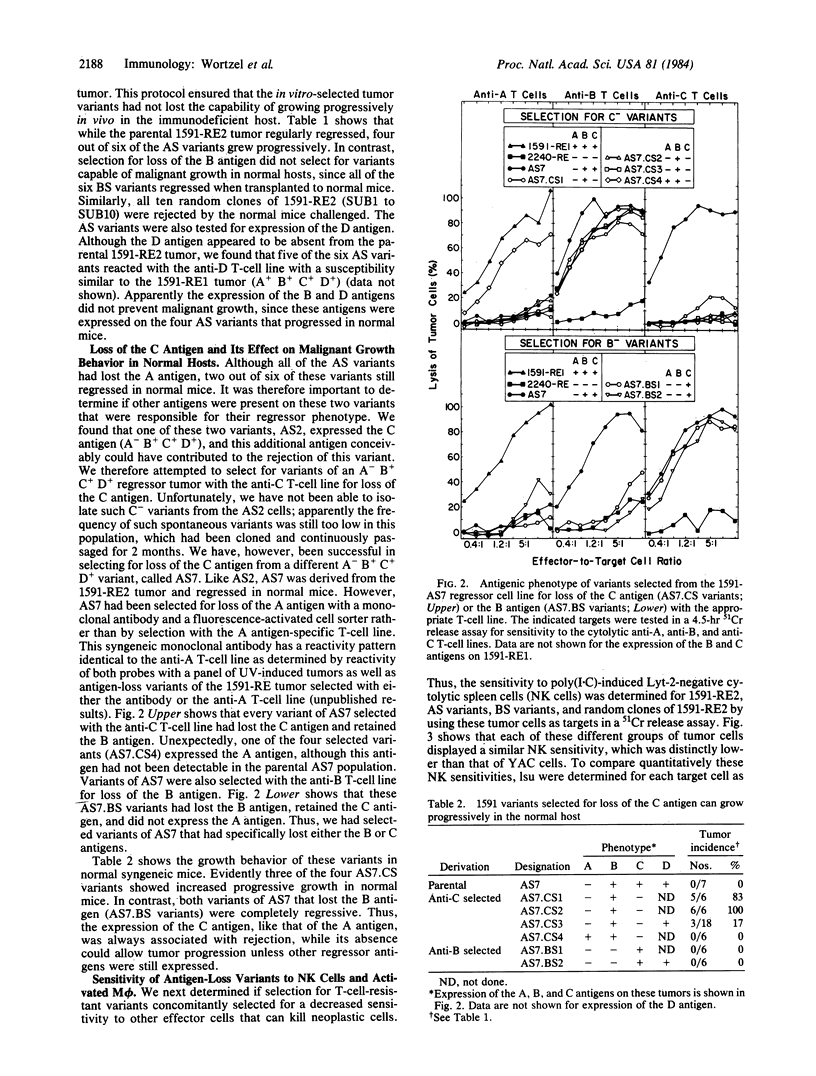
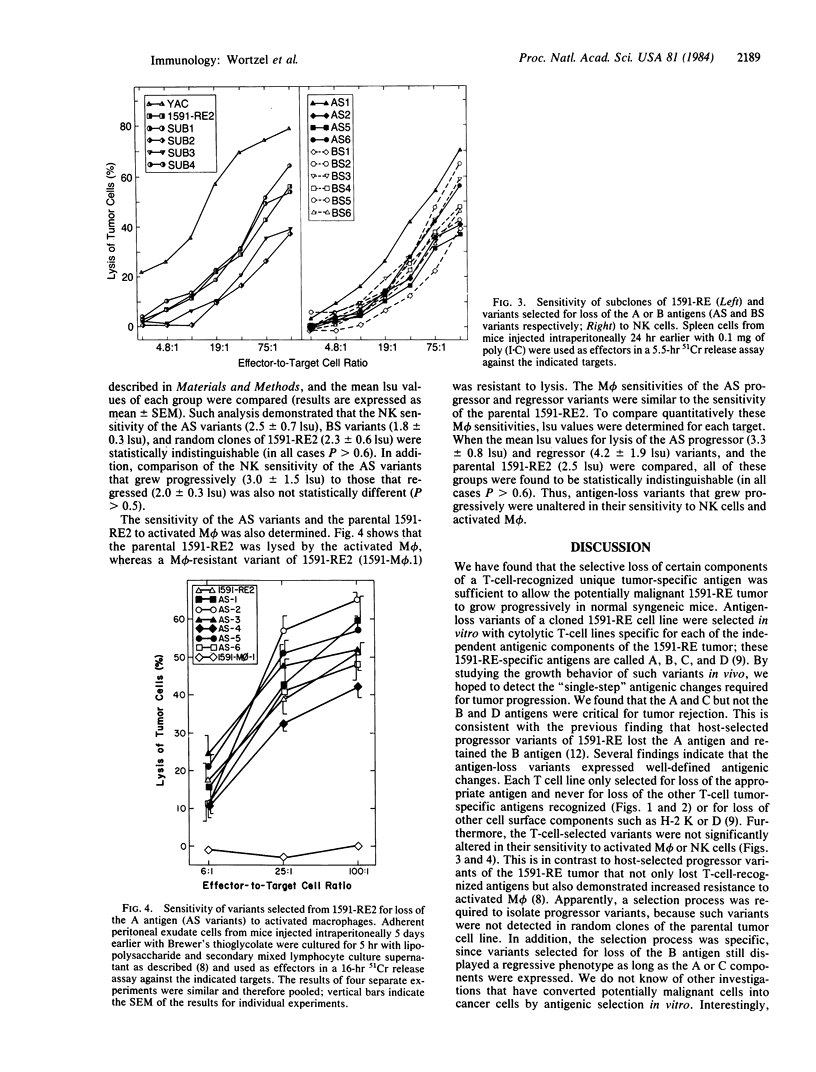
Abstract
Using variant selection in vitro, we have explored the changes necessary to convert potentially malignant cells into progressively growing cancer cells. As a model, we studied the murine ultraviolet light (UV)-induced tumor 1591-RE, which routinely regresses in the normal syngeneic host. The antigenicity of this tumor is specified by multiple independent tumor-specific antigens, named A, B, C, and D. Cells of this tumor were exposed in vitro to cytolytic T-cell lines specific for these separate antigens. Tumor variants were isolated that displayed selective antigenic losses. By challenging normal syngeneic mice with the variants, we could determine the significance of each antigen in tumor rejection--i.e., a switch from a regressor to a progressor phenotype upon selective loss of certain antigens. We found that variants which lost the A and C antigens grew progressively in normal mice, whereas variants which had lost the B antigen were still rejected like the parental tumor cells. Furthermore, the B and D antigens could be expressed on progressively growing variants. Thus, loss of the A and C antigens, but not the B and D antigens, was required for allowing malignant growth. Selection for loss of one T-cell-recognized antigen never selected simultaneously for loss of any of the other T-cell-recognized antigens, nor did such selections affect sensitivity to activated macrophages or natural killer cells. Therefore, our results strongly suggest that loss of defined components of a complex T-cell-recognized antigen are sufficient to allow escape from immunosurveillance.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhan A. K., Perry L. L., Cantor H., McCluskey R. T., Benacerraf B., Greene M. I. The role of T cell sets in the rejection of a methylcholanthrene-induced sarcoma (S1509a) in syngeneic mice. Am J Pathol. 1981 Jan;102(1):20–27. [PMC free article] [PubMed] [Google Scholar]
- Bosslet K., Schirrmacher V. Escape of metastasizing clonal tumor cell variants from tumor-specific cytolytic T lymphocytes. J Exp Med. 1981 Aug 1;154(2):557–562. doi: 10.1084/jem.154.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton R. C., Winn H. J. Studies on natural killer (NK) cells. I. NK cell specific antibodies in CE anti-CBA serum. J Immunol. 1981 May;126(5):1985–1989. [PubMed] [Google Scholar]
- Cerottini J. C., Engers H. D., Macdonald H. R., Brunner T. Generation of cytotoxic T lymphocytes in vitro. I. Response of normal and immune mouse spleen cells in mixed leukocyte cultures. J Exp Med. 1974 Sep 1;140(3):703–717. doi: 10.1084/jem.140.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins J. L., Patek P. Q., Cohn M. Cancer: a problem in somatic cell evolution. Contemp Top Immunobiol. 1980;11:1–79. doi: 10.1007/978-1-4684-3701-0_1. [DOI] [PubMed] [Google Scholar]
- Djeu J. Y., Heinbaugh J. A., Holden H. T., Herberman R. B. Augmentation of mouse natural killer cell activity by interferon and interferon inducers. J Immunol. 1979 Jan;122(1):175–181. [PubMed] [Google Scholar]
- FOULDS L. The experimental study of tumor progression: a review. Cancer Res. 1954 Jun;14(5):327–339. [PubMed] [Google Scholar]
- Hardie I. R., Strong R. W., Hartley L. C., Woodruff P. W., Clunie G. J. Skin cancer in Caucasian renal allograft recipients living in a subtropical climate. Surgery. 1980 Feb;87(2):177–183. [PubMed] [Google Scholar]
- Kripke M. L. Latency, histology, and antigenicity of tumors induced by ultraviolet light in three inbred mouse strains. Cancer Res. 1977 May;37(5):1395–1400. [PubMed] [Google Scholar]
- Loveland B. E., Hogarth P. M., Ceredig R., McKenzie I. F. Cells mediating graft rejection in the mouse. I. Lyt-1 cells mediate skin graft rejection. J Exp Med. 1981 May 1;153(5):1044–1057. doi: 10.1084/jem.153.5.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz C. T., Fitch F. W. Accessory cell requirements for the generation of cytolytic T lymphocytes. J Immunol. 1979 Jun;122(6):2598–2604. [PubMed] [Google Scholar]
- Maryanski J. L., Boon T. Immunogenic variants obtained by mutagenesis of mouse mastocytoma P815. IV. Analysis of variant-specific antigens by selection of antigen-loss variants with cytolytic T cell clones. Eur J Immunol. 1982 May;12(5):406–412. doi: 10.1002/eji.1830120509. [DOI] [PubMed] [Google Scholar]
- Mills C. D., North R. J. Expression of passively transferred immunity against an established tumor depends on generation of cytolytic T cells in recipient. Inhibition by suppressor T cells. J Exp Med. 1983 May 1;157(5):1448–1460. doi: 10.1084/jem.157.5.1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowell P. C. The clonal evolution of tumor cell populations. Science. 1976 Oct 1;194(4260):23–28. doi: 10.1126/science.959840. [DOI] [PubMed] [Google Scholar]
- Urban J. L., Burton R. C., Holland J. M., Kripke M. L., Schreiber H. Mechanisms of syngeneic tumor rejection. Susceptibility of host-selected progressor variants to various immunological effector cells. J Exp Med. 1982 Feb 1;155(2):557–573. doi: 10.1084/jem.155.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban J. L., Holland J. M., Kripke M. L., Schreiber H. Immunoselection of tumor cell variants by mice suppressed with ultraviolet radiation. J Exp Med. 1982 Oct 1;156(4):1025–1041. doi: 10.1084/jem.156.4.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban J. L., Schreiber H. Selection of macrophage-resistant progressor tumor variants by the normal host. Requirement for concomitant T cell-mediated immunity. J Exp Med. 1983 Feb 1;157(2):642–656. doi: 10.1084/jem.157.2.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uyttenhove C., Maryanski J., Boon T. Escape of mouse mastocytoma P815 after nearly complete rejection is due to antigen-loss variants rather than immunosuppression. J Exp Med. 1983 Mar 1;157(3):1040–1052. doi: 10.1084/jem.157.3.1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinhold K. J., Miller D. A., Wheelock E. F. The tumor dormant state. Comparison of L5178Y cells used to establish dormancy with those that emerge after its termination. J Exp Med. 1979 Mar 1;149(3):745–757. doi: 10.1084/jem.149.3.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wortzel R. D., Philipps C., Schreiber H. Multiple tumour-specific antigens expressed on a single tumour cell. Nature. 1983 Jul 14;304(5922):165–167. doi: 10.1038/304165a0. [DOI] [PubMed] [Google Scholar]
- Wortzel R. D., Urban J. L., Philipps C., Fitch F. W., Schreiber H. Independent immunodominant and immunorecessive tumor-specific antigens on a malignant tumor: antigenic dissection with cytolytic T cell clones. J Immunol. 1983 May;130(5):2461–2466. [PubMed] [Google Scholar]



