Abstract
One of the main limitations of vertebroplasty is the excessive pressure required to inject a sufficient amount of cement into a vertebral body. Based on previous work that shows that approximately 95% of the injection pressure is required to deliver the cement through the cannula, we proposed a new cannula design with a larger internal diameter in the proximal section. The objective of this study is to determine whether the new cannula geometry significantly reduces the delivery pressure and eases cement injection during vertebroplasty. Two different methods were employed to examine the delivery pressure in a conventional and two redesigned cannulae: (1) analytical model: Hagen-Poisseuille’s flow through a tube was used to predict the pressure drop in the cannulae; (2) experiment: first a Newtonian silicone oil and then an acrylic bone cement was injected through the cannulae at a constant rate of 4 cc/min, and the delivery pressure was recorded. Both the experimental and analytical findings confirmed that the redesigned cannula reduces the delivery pressure significantly. Specifically, when the internal diameter of the proximal section was increased by a factor of two, which is clinically feasible, the delivery pressure dropped by about 63%. The redesigned cannula appears to have the potential to improve vertebroplasty. The key benefits are that (1) it eases cement injection, (2) it can be easily integrated into the existing procedure, and (3) it is cost-effective.
Keywords: Cannula, Cement delivery, Injection pressure, Osteoporosis, Vertebroplasty
Introduction
Vertebroplasty is an emerging procedure to treat primarily spinal fragility fractures, but also other pathological fractures [19, 20–24]. In this procedure, bone cement is injected under pressure through a thin cannula into a vertebra. The in situ cement polymerization augments the weakened bone [1, 2].
One of the limitations of vertebroplasty is that the pressure required to inject the cement is often high [1–3, 16, 25, 28]. The injection pressure during vertebroplasties was reported to exceed 1,500 kPa, which approaches the limit of what can be applied manually [4, 5, 7, 11, 28, 29]. As a consequence of the excessive pressure, premature termination of the procedure may become necessary resulting in insufficient filling of the vertebra.
There are two methods employed to overcome the problem of high pressure: (1) to use a pressure applicator device, which increases the pressure that is applied to the cement [4, 5, 7, 11, 18, 29], and (2) to increase the recommended liquid to powder ratio of cement, which lowers cement viscosity [12, 17, 18, 23]. However, these methods introduce additional risks. With the pressure applicator device, there is the possibility that the liquid may separate from the suspended powder under excessive pressure [3, 7, 13, 28]. Moreover, the tactile feedback of the cement flow into the bone is reduced with the pressure device. Altering the recommended liquid to powder ratio may cause complications such as toxicity and reduced cement strength [12, 27].
To arrive at a targeted solution, a different approach was needed. We began by developing an analytical model to identify the underlying biomechanisms in the pressurized environment of the cement injection procedure as well as locate any pressure-generating bottleneck in the system [4, 7]. In the model, the total pressure required for cement injection has two main components:
Extravertebral or delivery pressure, which is the external delivery pressure required to overcome the friction in the cannula; and
Intravertebral pressure, which is the pressure required for the cement to infiltrate the trabecular bone inside the vertebra and displace the bone marrow through the vertebral shell.
The details of the analytical model, which calculates the pressures by means of combining the established rheological laws of both Hagen-Poiseuille’s law and Darcy’s law, have been presented in previous studies [7, 14]. To understand the injection process, the crucial individual parameters, such as bone porosity, bone permeability, cement viscosity, hydraulic resistance of a vertebral body, and cannula geometry, had to be identified and determined individually [4–11, 13, 14]. After the underlying mechanisms of the parameters had been understood and measurements obtained, they were combined into an analytical model of the injection pressure.
The most significant result obtained from the analytical model was that the delivery pressure is much larger than the intravertebral pressure. Specifically, over 95% of the total injection pressure is required to overcome the friction in the cannula, and less than 5% of the pressure is required to force the cement to infiltrate the trabecular bone and displace the bone marrow [4, 11]. Once the theoretical findings are combined with the data obtained from experimental studies that confirm that intravertebral pressure is very small, the solution seems rather obvious [4, 11, 14, 15, 26]. Since the pressure bottleneck during the injection occurs in the cannula, its geometry must be changed.
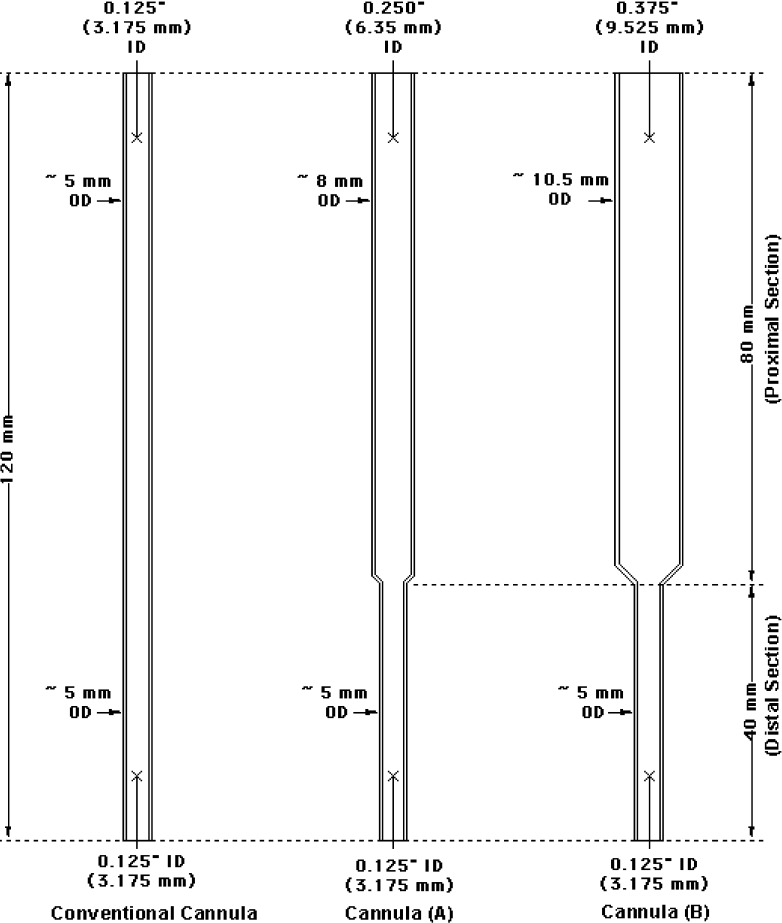
A new cannula design to ease cement delivery is therefore proposed. Instead of a straight tube with a constant internal diameter (ID), the redesigned cannula features two distinct sections with two different IDs. The diameter of the one third of the cannula that enters the pedicles (distal section) is determined by the anatomical limits posed by the pedicles; however, the diameter of the remaining two thirds (proximal section), which in part passes through the soft tissue, is increased to reduce the delivery friction and to ease the injection (Fig. 1).
Fig. 1.
Schematic drawings of a conventional cannula and two redesigned cannulae tested in this study. ID Internal diameter, OD outside diameter.
It is hypothesized that the new cannula geometry significantly reduces the delivery pressure. The objective of this paper is to employ an analytical and an experimental model to test this hypothesis.
Materials and methods
Cannula design
Three cannulae made of stainless steel were tested in this study—a conventional cannula with geometry similar to that of a typical 8-gauge cannula, and two redesigned cannulae (Fig. 1). The length of all of the cannulae was 120 mm. The length of the proximal section of the redesigned cannulae was 80 mm, and the length of the distal section was 40 mm. The IDs of the cannulae were as follows:
Conventional cannula: ID of entire cannula = 3.175 mm.
Redesigned cannula A: ID of proximal section = 6.35 mm (i.e., two times larger than the ID of the distal section and of a conventional cannula), and ID of distal section = 3.175 mm.
Redesigned cannula B: ID of proximal section = 9.525 mm (i.e., three times larger than the ID of the distal section and of a conventional cannula), and ID of distal section = 3.175 mm.
Analytical model
Since the geometry of the cannulae is known, the extravertebral delivery pressure, ΔPdel, can be estimated using Hagen-Poisseuille’s law [14]:
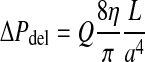 |
1 |
where a is the radius of the cannula, L is the length of the cannula, η is cement viscosity, and Q is the volume flow rate of the cement. According to this equation, the delivery pressure decreases in an overproportional fashion (a4) with an increase in the radius of the cannula and increases in a linear fashion with respect to the length of the cannula.
For the new cannulae, the delivery pressure is divided into the delivery pressure for the proximal section and the delivery pressure for the distal section. This relationship can be expressed as:
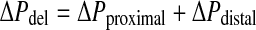 |
2 |
The benefit of this separation is that it isolates the effect that each section of the redesigned cannula has on the delivery pressure.
The separation also makes evident that since the pressure drop in the distal section, ΔPdistal, is the same in all three cannulae, it cannot be pertinent for the reduction in delivery pressure. Conversely, since it is the geometry of the proximal section that varies in the three cannulae, the pressure drop in the proximal section, ΔPproximal is the only relevant component for the delivery pressure reduction.
However, ΔPdistal is important for determining the absolute limit to which the delivery pressure, ΔPdel, can be reduced. Theoretically, the delivery pressure can be reduced by a maximum of 66.6% since the unchanged distal section represents one third of the cannula length.
Experimental model
In the experiment, two types of fluids—silicone oil and bone cement—were injected at a constant flow rate through the three cannulae described above. The delivery pressure for the six independent experiments was measured.
The three cannulae were first injected with silicone oil with a viscosity of 95 Pa s (viscosity standard 100,000; Brookfield Engineering, Middleboro, MA, USA), which is similar to the viscosity of bone cement. The main advantage of silicone oil is that it allows the effect of the cannula geometry to be isolated from the effect of the material. This isolation is possible because the viscosity of silicone oil is Newtonian, which provides greater control over and predictability of the experiment. Another benefit is that because the viscosity of silicone oil is less sensitive to changes in the ambient conditions, the results are more accurate and reproducible.
In the subsequent tests, the silicone oil was replaced by DP-Pour acrylic cement (DenPlus, Montreal, QC, Canada).1 To prepare the cement for injection, the liquid to powder ratio recommended by the manufacturer was followed. Accordingly, 18.0 ml of liquid and 30.8 g of powder were measured with a graduated cylinder and an analytical balance, respectively. The liquid was then added to the powder in a plastic beaker, and a spatula was used to mix the cement at approximately 60 beats/min for approximately 50 s until the powder had visually dissolved in the liquid. To match clinical conditions, the injections with DP-Pour were started once the cement exhibited a dough-like consistency, which was approximately 11 min after the liquid was added to the powder [10].
In the final phase of each test, a 20-cc syringe, filled with either silicone oil or DP-Pour was connected to a cannula. The cannula and syringe were then attached to a servohydraulic testing machine (Mini Bionix 856; MTS, Eden Prairie, MN, USA). The testing machine depressed the plunger of the syringe at an injection rate of approximately 4 cc/min, which is representative of a clinical situation, and recorded the force required to deliver the fluid through a cannula.
For each experimental condition (three cannulae and two fluids), three trials were conducted. This resulted in a total of 18 experiments. The testing reproducibility, which was determined by the coefficient of the variation, was on average 2.7% for the nine experiments with silicone oil and 13.4% for the nine experiments conducted using the bone cement.
Results
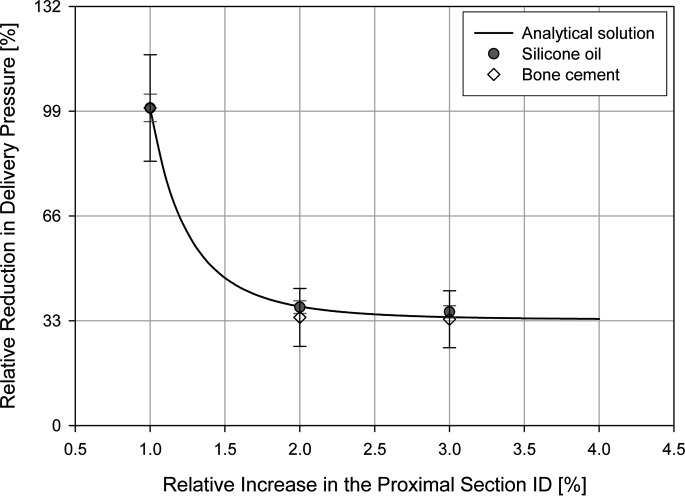
The analytical model predicted that the redesigned cannula A reduces the delivery pressure by approximately 63% and the redesigned cannula B reduces the delivery pressure by approximately 66%. Increasing the ID by any more than twice its initial value would therefore contribute only minimally toward reducing the delivery pressure.
The experimental data obtained from both test series—one using silicone oil, the other bone cement—confirmed the predictions of the analytical model (Fig. 2). The results of the analytical and experimental models are therefore consistent with one another.
Fig. 2.
A comparison of the analytical and experimental results. The pressure reduction was normalized with the readings in the conventional cannula. The error bars indicate a 95% confidence interval from the mean. The radius of the proximal section was normalized with that of the distal section. There is a good fit between the analytical and experimental findings.
These findings confirm our hypothesis that the changes in the cannula design reduce the delivery pressure significantly.
Discussion
Since previous studies had already shown the delivery portion of the vertebroplasty procedure as the bottleneck in the system, a new cannula design with a larger ID in the proximal section was proposed to significantly reduce the delivery pressure and to ease cement injection. Both the analytical and experimental results confirmed that increasing the ID of the proximal section of the cannula by a factor of two reduces the delivery pressure by about 63%. Seen in the specific context of our test data, an injection pressure value of 1,500 kPa obtained with the old cannula decreased to almost 550 kPa when the traditional cannula was exchanged for the redesigned version. This value lies well within the range of strength required from a human to perform everyday tasks: using a 5-cc syringe for cement injection, 550 kPa corresponds to a force of 60 N or 6 kg.
The primary benefit of the cannula lies in the dramatic ease with which sufficient amounts of cement can be injected. A secondary, but arguably equally important, advantage of the new cannula over the old one is that clinicians no longer have to be concerned about a possible malfunction in the cement delivery but can turn their whole attention to the overall procedure.
Another notable benefit of the new cannula is the straightforwardness of its design, which facilitates the modification of existing setups and requires neither the acquisition of new equipment nor the retraining of clinicians or technicians.
In summary, the simplicity and cost-effectiveness of acquiring, installing, operating, and maintaining the proposed cannula when added to the obvious clinical benefits arising from a significantly easier and safer cement injection process will potentially render vertebroplasty a considerably improved and more widely applicable therapeutic procedure.
Finally, one may hypothesize that the fact that the new cannula significantly decreases the delivery pressure may improve the feasibility of the injection of more viscous cements. Animal studies [15], ex vivo laboratory studies [7, 14], and anecdotal evidence reported in clinical studies [2, 20, 21] suggest that high-viscosity cements not only increase the uniformity of the cement spread pattern in the vertebral body, but they also decrease the risk of leakage. These claims and our hypotheses will, however, require further study.
Acknowledgements
This work has been supported by the Canadian Institute of Health Research (CIHR) grant number MOP 57835. The technical support of L. Beckman for designing and machining the tested tool is greatly appreciated. He was also instrumental in the inception of the two-phase cannula.
Footnotes
DP-Pour is an acrylic polymer that is used mainly in dental and research laboratories. The composition and rheological behavior of DP-Pour is similar to that of other bone cements, such as Simplex, Vertebroplastic, and Placos LV-40. DP-Pour was favored because it is less expensive.
References
- 1.Al-Assir I, Perez-Higueras A, Florensa J, Munoz A, Cuesta E. Percutaneous vertebroplasty: a special syringe for cement injection. AJNR Am J Neuroradiol. 2000;21:159–161. [PMC free article] [PubMed] [Google Scholar]
- 2.Amar AP, Larson DW, Teitelbaum GP. Use of a screw-syringe injector for cement delivery during kyphoplasty. Neurosurgery. 2003;53:380–383. doi: 10.1227/01.NEU.0000073423.09308.40. [DOI] [PubMed] [Google Scholar]
- 3.Arramon YP, McIntyre S (2002) Cannula system for hard tissue implant delivery. US Patent Application
- 4.Baroud G, Beckman L, Heini P, Ferguson S, Steffen T (2003) Clinical and laboratory analysis of the pressure at injection in a vertebroplasty. In: Annual meeting of International Society of the Study of the Lumber Spine, Vancouver (Abstract 279)
- 5.Baroud G, Heini P, Bohner M, Ferguson S, Steffen T (2003) Drop in pressure at injection and infiltration in vertebroplasty. In: 13th interdisciplinary research conference on biomaterials (GRIBOI 2003), Baltimore
- 6.Baroud G, Wu J, Bohner M, Sponagel S, Steffen T. How to determine the permeability for cement infiltration into osteoporotic cancellous bone. Med Eng Phys. 2003;25:283–288. doi: 10.1016/S1350-4533(02)00223-0. [DOI] [PubMed] [Google Scholar]
- 7.Baroud G, Bohner M, Heini P, Steffen T. Injection biomechanics of bone cements used in vertebroplasty. Biomed Mater Eng. 2004;14(4):487–504. [PubMed] [Google Scholar]
- 8.Baroud G, Falk R, Crookshank M, Sponagel S, Steffen T. Experimental and theoretical investigation of the directional permeability of cancellous bone for cement infiltration. J Biomech. 2004;37:189–196. doi: 10.1016/S0021-9290(03)00246-X. [DOI] [PubMed] [Google Scholar]
- 9.Baroud G, Matsushita C, Samara M, Steffen T. Influence of oscillatory mixing on the injectability of three acrylic and two calcium-phosphate bone cements for vertebroplasty. J Biomed Mater Res. 2004;68:105–111. doi: 10.1002/jbm.b.20010. [DOI] [PubMed] [Google Scholar]
- 10.Baroud G, Samara M, Steffen T. Influence of mixing method on the cement temperature-mixing time history and doughing time of three acrylic cements for vertebroplasty. J Biomed Mater Res. 2004;68:112–116. doi: 10.1002/jbm.b.20009. [DOI] [PubMed] [Google Scholar]
- 11.BaroudSpine 2005306815626984 [Google Scholar]
- 12.Belkoff SM, Sanders JC, Jasper LE. The effect of the monomer-to-powder ratio on the material properties of acrylic bone cement. J Biomed Mater Res. 2002;3:396–399. doi: 10.1002/jbm.10258. [DOI] [PubMed] [Google Scholar]
- 13.Bohner M, Baroud G (2003) Experimental results to assess the relevance of a theoretical model describing the injectability of powder—liquid mixtures. In: 13th interdisciplinary research conference on biomaterials (GRIBOI 2003), Baltimore
- 14.Bohner M, Gasser B, Baroud G, Heini P. Theoretical and experimental model to describe the injection of a polymethylmethacrylate cement into a porous structure. Biomaterials. 2003;24:2721–2730. doi: 10.1016/S0142-9612(03)00086-3. [DOI] [PubMed] [Google Scholar]
- 15.Breusch S, Heisel C, Mueller J, Borchers T, Mau H. Influence of cement viscosity on cement interdigitation and venous fat content under in vivo conditions. Acta Orthop Scand. 2002;73:409–415. doi: 10.1080/00016470216320. [DOI] [PubMed] [Google Scholar]
- 16.Chavali R, Resijek R, Knight SK, Choi IS. Extending polymerization time of polymethylmethacrylate cement in percutaneous vertebroplasty with ice bath cooling. AJNR Am J Neuroradiol. 2003;24:545–546. [PMC free article] [PubMed] [Google Scholar]
- 17.Cotten A, Boutry N, Cortet B, Assaker R, Demondion X, Leblond D, Chastanet P, Duquesnoy B, Deramond H. Percutaneous vertebroplasty: state of the art. Radiographics. 1998;18:311–323. doi: 10.1148/radiographics.18.2.9536480. [DOI] [PubMed] [Google Scholar]
- 18.Deramond H, Depriester C, Toussaint P, Galibert P. Percutaneous vertebroplasty. Semin Musculoskelet Radiol. 1997;1:285–295. doi: 10.1055/s-2008-1080150. [DOI] [PubMed] [Google Scholar]
- 19.Deramond H, Depriester C, Galibert P, Gars D. Percutaneous vertebroplasty with polymethylmethacrylate. Radiol Clin North Am. 1998;36:533–546. doi: 10.1016/s0033-8389(05)70042-7. [DOI] [PubMed] [Google Scholar]
- 20.Fourney DR, Schomer DF, Nader R, Chlan-Fourney J, Suki D, Ahrer R, Rhines LD, Gokaslan ZL. Percutaneous vertebroplasty and kyphoplasty for painful vertebral body in cancer patients. J Neurosurg. 2003;98(Spine 1):21–30. doi: 10.3171/spi.2003.98.1.0021. [DOI] [PubMed] [Google Scholar]
- 21.Heini PF, Walchli B, Berlemann U. Percutaneous transpedicular vertebroplasty with PMMA: operative technique and early results. A prospective study for the treatment of osteoporotic compression fractures. Eur Spine J. 2000;9:445–450. doi: 10.1007/s005860000182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jensen ME, Kallmes DF. Percutaneous vertebroplasty in the treatment of malignant spine disease. Cancer J. 2002;8:194–206. doi: 10.1097/00130404-200203000-00013. [DOI] [PubMed] [Google Scholar]
- 23.Jensen ME, Evans AJ, Mathis JM, Kallmes DF, Cloft HJ, Dion JE. Percutaneous polymethylmethacrylate vertebroplasty in the treatment of osteoporotic vertebral body compression fractures: technical aspects. Am J Neuroradiol. 1997;18:1897–1904. [PMC free article] [PubMed] [Google Scholar]
- 24.Mathis JM, Barr JD, Belkoff SM, Barr MS, Jensen ME, Deramond H. Percutaneous vertebroplasty: a developing standard of care for vertebral compression fractures. Am J Neuroradiol. 2001;22:373–381. [PMC free article] [PubMed] [Google Scholar]
- 25.Preissman HE (2002) High pressure applicator. US Patent 6,383,190 B1
- 26.Reidy D, Ahn H, Mousavi P, Finkelsein J, Whyne CM. A biomechanical analysis of intravertebral pressures during vertebroplasty of cadaveric spines with and without simulated metastases. Spine. 2003;28:1534–1539. doi: 10.1097/00007632-200307150-00011. [DOI] [PubMed] [Google Scholar]
- 27.San Millan Ruiz D, Burkhardt K, Jean B, Muster M, Martin JB, Bouvier J, Fasel JH, Rufenacht DA, Kurt AM. Pathology findings with acrylic implants. Bone. 1999;25:85S–90S. doi: 10.1016/S8756-3282(99)00140-4. [DOI] [PubMed] [Google Scholar]
- 28.Schallen EH, Gilula LA. Vertebroplasty: reusable flange converter with hub lock for injection of polymethylmethacrylate with screw-plunger syringe. Radio. 2002;222:851–855. doi: 10.1148/radiol.2223001722. [DOI] [PubMed] [Google Scholar]
- 29.Tomita S, Molloy S, Abe M, Belkoff SM (2003) Ex vivo measurement of intravertebral pressure during vertebroplasty. In: 13th interdisciplinary research conference on biomaterials (GRIBOI 2003), Baltimore [DOI] [PubMed]