Abstract.
Overall, vertebroplasty has a low complication rate. Nevertheless, severe complications can occur. The majority of these are related to cement extrusion. The rate of cement leakage is often obtained by X-ray, with only a single leak registration per vertebra. Detection rate of leaks in comparison with CT and inter-observer reliability for X-ray is, in large parts, unknown. We conducted this study to determine the value of fluoroscopy and X-ray used to detect cement leakage as compared to CT scans. Intraoperative findings in lateral fluoroscopy by the surgeon, and postoperative findings in X-rays by two orthopaedic surgeons, were compared with CT scans for the same study group. Multiple cement leakage was considered, and agreement rate was determined. The detection rate for leaks was 34% for lateral X-ray and 48% for lateral and AP view. Additional AP views only enhanced the detection of leaks in the segmental veins. The agreement rate between fluoroscopy/X-ray and CT scans ranged between 66% and 74%, while inter-observer reliability showed only fair agreement. The rate of cement leaks in vertebroplasty is high if multiple leaks are considered in CT scans. Detection rates using X-rays are low and complicated by only fair inter-observer agreement. Leaks in the basivertebral veins are frequently misinterpreted and can lead to severe complications. Therefore, CT scans should be obtained to calculate the exact leakage rate and to assess persistent or new pain occurring postoperatively.
Keywords: Vertebroplasty, Cement leakage, Detection rate, Interobserver reliability
Introduction
Percutaneous vertebroplasty (PVP) was introduced for the treatment of vertebral angiomas in 1984 by Galibert and Deramond [9]. Indications were extended to osteolytic neoplasms [2, 3, 8] and osteoporotic fractures [3, 6, 7, 14, 15, 19, 20, 31] in the following years. Reported pain relief is achieved in approximately 70%–100% [10, 27] of osteoporotic fractures. For this reason PVP is one of the most efficient procedures in spine surgery. Cement leaks are often found after PVP, with reported rates between 11% and 73% [10, 26, 27]. Most of the time the cement extrusion is clinically asymptomatic and, therefore, mentioned without exact localization of the pattern or recognition of multiple leaks. However, the few reported severe complications are mainly due to cement leakage [12, 13, 16, 22, 24, 26]. We conducted this study to gain an exact and detailed description of cement leaks during vertebroplasty and to determine the most efficient and reliable way to detect cement leaks. We compared fluoroscopy, standard X-rays and CT scans, taking into account the inter-observer reliability for X-rays. Possible resulting conclusions for the technical performance of vertebroplasties were drawn.
Materials and methods
Below are the details of the vertebroplasties we performed in our institution between January 2003 and 2004 . The study group consisted of 21 patients, 15 female and six male, with 29 treated vertebrae. The average age of the participants was 73.1 years. The women ranged between the ages of 62 and 84 (average age 74.8) years, while the men were aged between 51 and 77 (average age 69.0) years. Indication for vertebroplasty was, in all cases, an osteoporotic vertebral fracture with refractory pain under conservative treatment. Conservative treatment consisted of analgesics, rest and gentle physical therapy. The average pain duration before vertebroplasty was close to 6.5 weeks, with a range from 2–24 weeks. In one patient, additionally, a spinal canal stenosis was treated by the undercutting of L3/4 and L4/5. In two other patients a biopsy was performed in the same procedure before the vertebroplasty. Preoperative X-ray and CT were carried out in all patients. MRI was done if multiple fractures of different ages were evident, to determine the pain-causing level and, thereby, minimize the number of levels to be treated. Three vertebrae (Th12, L1, L4) in different patients showed that the posterior wall was affected in the CT scans (Fig. 1). According to Magerl et al. [18], they were all classified as incomplete cranial burst fractures, type A 3.1.1. Vertebroplasty was performed in the OR under general anaesthesia. 15 times a short median approach and six times a bilateral approach according to Wiltse et al. [29] was chosen. The pedicle entrance point was located under fluoroscopy. After insertion of the needle (11G), vertebral venography was carried out. If a fast leak occurred, the needle was adjusted until lower drainage appeared. Cement (Vertebroplastic, DePuy Acromed) was mixed to a paste-like consistency, and application was started with an injector (V-Max, DePuy Acromed) under lateral fluoroscopic control. Direct postoperative X-ray in two planes (all patients) and CT scan (18 patients) was carried out. CT (Siemens Somatom Plus4) was performed with a slice thickness of 3 mm, pitch 4.5 mm and reconstruction increment 1.5. Postoperative mobilization was without restrictions. One orthopaedic surgeon and a radiologist, experienced in spine radiographs and CT scans, examined the postoperative CT scans for cement leaks. Additionally, two orthopaedic surgeons, unaware of the patients’ histories, rated the postoperative lateral X-ray first and then the AP view. The leaks detected by the surgeon using lateral fluoroscopy during surgery were collected.
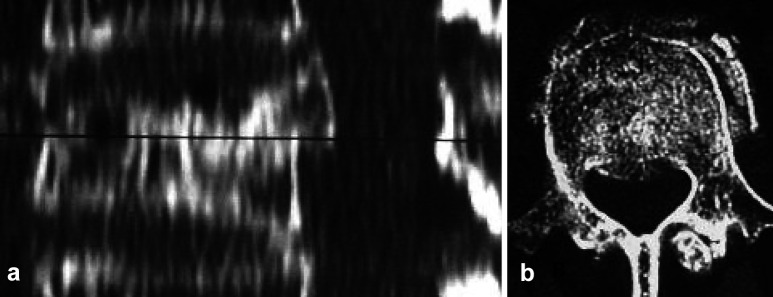
Fig. 1.
a Sagittal CT reconstruction showing posterior wall fragment. Black line level corresponding to transverse CT scan in b
First, the rate of detected leaks by the two readers (orthopaedic surgeons) using X-ray and the surgeon using lateral fluoroscopy was determined in comparison with the CT scans. Second, the agreement between CT and X-ray/fluoroscopy was measured, which included all the vertebrae with postoperative CT scans (18 patients/26 vertebrae). Third, the inter-observer reliability for the two readers was determined by Cohen’s kappa (SPSS V 9.0. for Windows), separated for lateral and lateral and AP X-ray (21 patients/29 vertebrae). Leaks were classified according to Yeom et al. [31]: type S leak via the segmental vein (Fig. 2), type B via the basivertebral vein (Fig. 3) and type C through a cortical defect (Fig. 4).
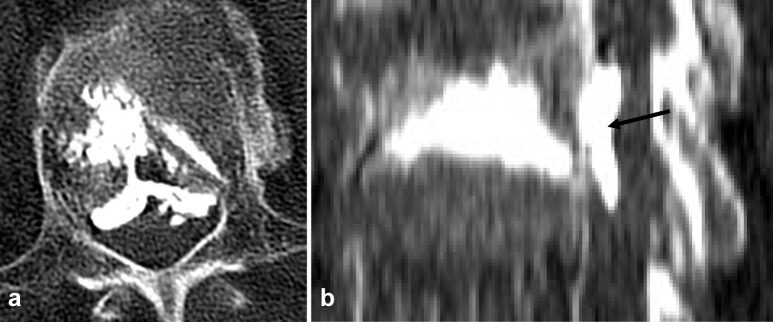
Fig. 2.
Type S leak via the segmental vein (white arrow)
Fig. 3.
a Type B leak Although cement does not reach the posterior vertebral wall, leakage in the vein has occurred. b Sagittal CT reconstruction showing cement distribution in the spinal canal (arrow)
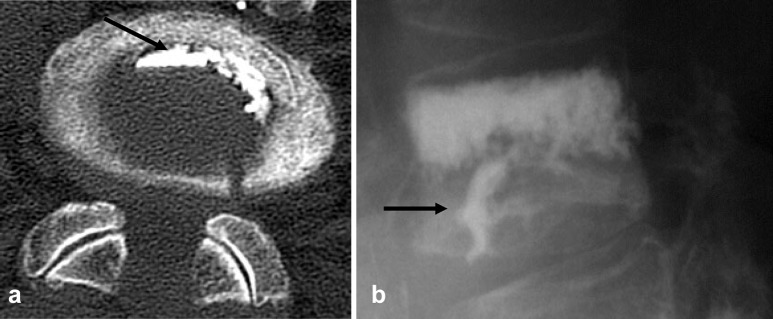
Fig. 4.
a CT and b X-ray with type C leak arrow indicates cement in the intervertebral disc
Results
Nineteen lumbar (6×L1, 4×L2, 4×L3, 3×L4, 2×L5) and ten thoracic (1×Th5, 2×Th6, 1×Th7, 2×Th8, 1×Th11, 3×Th12) vertebrae were treated. In five cases, multiple vertebroplasties were performed on a maximum of three levels. The average procedure time was 45 min. When the patients with multi-level vertebroplasties, as well as the patient with undercutting and the two patients with decompression in the same procedure, were excluded, the average time was 36.7 min for the single level vertebroplasty. The mean cement volume injected was 3.5±2.0 ml for the thoracic vertebrae and 4.9±1.6 ml for the lumbar vertebrae.
Two complications that required re-operation occured, both in the lumbar spine and both caused by type B leaks (Fig. 3). These were recognized by the surgeon intraoperatively and led to either a laminotomy or a laminectomy during the same procedure. The patient given a primary laminectomy had a postoperative CT scan that revealed spinal canal and foraminal narrowing, with clinically incomplete paraparesis, despite laminectomy. Therefore, in addition to the laminectomy, surgical removal of the cement was performed the same day. Symptoms were regressive and the patient is now mobile with a walker, and bladder function has returned to normal. Nevertheless, weakness, especially of the right leg, persisted (grade D1, according to the modified Frankel grading system [4]). The patient that underwent laminotomy complained postoperatively of radiating pain, and a further neurological examination revealed bilateral radiculopathy L3, which corresponded to the extruded cement (Fig. 3). Despite administration of analgesics and physical therapy, pain persisted, so surgical decompression was performed 6 days after the vertebroplasty, and the patient recovered. No further complications occurred.
Besides the two symptomatic type B leaks, 27 asymptomatic leaks were detected in postoperative CT scans. Eight (30%) type S, six (22%) type B and 13 (48%) type C. None of the patients with a posterior wall fragment suffered cement extrusion through the fracture gap, in the postoperative CT scans. The rate of detected leaks was 21% (six of 29 leaks) for the surgeon in lateral fluoroscopy, the analogue values of the two readers are presented in Table 1.
Table 1.
Detection of leaks by X-ray in comparison with CT scan
| Lateral X-ray/fluoroscopy | Leaks detected by CT | Type S 8 | Type B 8 | Type C 13 | Average per reader | Lateral and AP X-ray | Type S 8 | Type B 8 | Type C 13 | Average per reader |
|---|---|---|---|---|---|---|---|---|---|---|
| Reader 1 | Misinterpretation as no leak | 7 (88%) | 4 (50%) | 7 (54%) | 18 (62%) | Reader 1 | 3 (37%) | 3 (37%) | 7 (54%) | 13 (45%) |
| Correct interpretation | 1 (12%) | 4 (50%) | 6 (46%) | 11 (38%) | 5 (63%) | 5 (63%) | 6 (46%) | 16 (55%) | ||
| Reader 2 | Misinterpretation as no leak | 7 (88%) | 5 (63%) | 8 (62%) | 20 (69%) | Reader 2 | 4 (50%) | 5 (63%) | 8 (62%) | 17 (59%) |
| Correct interpretation | 1 (12%) | 3 (37%) | 5 (38%) | 9 (31%) | 4 (50%) | 3 (37%) | 5 (38%) | 12 (41%) | ||
| Surgeon | Misinterpretation as no leak | 7 (88%) | 5 (63%) | 11 (85%) | 23 (79%) | |||||
| Correct interpretation | 1 (12%) | 3 (37%) | 2 (15%) | 6 (21%) | ||||||
| Correct in average for both readers | 13% | 44% | 42% | 34% | 56% | 50% | 42% | 48% |
In order to calculate the agreement between CT and X-ray/fluoroscopy, we compared the results of the readers and surgeon with CT, including the negative (no leak) findings. Values are shown in Tables 2 and 3.
Table 2.
Comparison of CT scans versus surgeon’s findings with lateral fluoroscopy
| Surgeon | CT scan | ||||
|---|---|---|---|---|---|
| No leak | Type S | Type B | Type C | Agreement | |
| No leak | 46 (59%) | 7 (9%) | 5 (6%) | 11 (14%) | |
| Type S | 1 (1%) | 1 (1%) | – | – | |
| Type B | – | – | 3 (4%) | – | |
| Type C | 2 (3%) | – | – | 2 (3%) | 67% |
Table 3.
Readers’ findings with X-ray in comparison with CT scans
| Parameter | CT scan | ||||
|---|---|---|---|---|---|
| No leak | Type S | Type B | Type C | Agreement | |
| X-ray lateral | |||||
| Reader 1 | |||||
| No leak | 43 (55%) | 7 (9%) | 4 (5%) | 7 (9%) | 69% |
| Type S | 3 (4%) | 1 (1%) | – | – | |
| Type B | 3 (4%) | – | 4 (5%) | – | |
| Type C | – | – | – | 6 (8%) | |
| Reader 2 | |||||
| No leak | 43 (55%) | 7 (9%) | 5 (6%) | 8 (10%) | 66% |
| Type S | 3 (4%) | 1 (1%) | – | – | |
| Type B | 1 (1%) | – | 3 (4%) | – | |
| Type C | 2 (3%) | – | – | 5 (6%) | |
| X-ray lateral + ap | |||||
| Reader 1 | |||||
| No leak | 42 (54%) | 3 (4%) | 3 (4%) | 7 (9%) | 74% |
| Type S | 5 (6%) | 5 (6%) | – | – | |
| Type B | 2 (3%) | – | 5 (6%) | – | |
| Type C | – | – | – | 6 (8%) | |
| Reader 2 | |||||
| No leak | 42 (54%) | 4 (5%) | 5 (6%) | 8 (10%) | 69% |
| Type S | 4 (5%) | 4 (5%) | – | – | |
| Type B | 1 (1%) | – | 3 (4%) | – | |
| Type C | 2 (3%) | – | – | 5 (6%) | |
Although every vertebra was examined for three leak types on the same X-ray, we pooled the values and calculated Cohen’s kappa. The inter-observer comparison was made for all 21 patients, as everyone had undergone a postoperative X-ray. Kappa statistics were 0.30 for lateral X-ray and 0.37 for lateral and AP view. Kappa statistics are often interpreted as: ≤0.2, poor agreement; 0.21–0.40, fair agreement; 0.41–0.60, moderate agreement; 0.61–0.8, good agreement; greater than 0.8, very good agreement [1]. The statistics between surgeon and readers was not calculated, as different pictures (postoperative X-ray and intraoperative lateral fluoroscopy) were rated.
Discussion
The rate of reported complications for vertebroplasty are low, with values up to 10% [10, 27, 30]. Although complications are frequent, due to cement leakage, leaks are often only mentioned by a dichotomous registration (leak yes/no) per vertebra. In our study, when we applied this method to the results of our CT scans, we found a leakage rate of 81%, which corresponded to 63% by Yeom et al. [31]. In contrast, if multiple leakage were allowed, we found 29 leaks in 26 vertebrae (1.1 leaks per vertebra). Yeom et al. [31] had 74 leaks in 48 vertebrae (1.5 leaks per vertebra). As every leak can lead to severe neurological or pulmonary complications [12, 13, 16, 22, 24, 26, 28], independent of whether it drains into segmental or basivertebral veins or through a cortical cleft, it is necessary for multiple extrusions to be recorded. Moreover, a realistic leakage rate can only be determined if multiple leaks per vertebra are included.
The radiological detection of leaks is usually done by intraoperative fluoroscopy or postoperative X-ray. In comparison to the observed leaks in our CT scans, only 34% of these leaks were detected by lateral X-ray and 48% by lateral and AP X-ray (Table 1). This is in accordance with results from other studies of 31% and 50% [31]. In this study, the surgon’s intraoperative values were even lower, with 21% detected leaks (Table 1). This can be explained by the minor picture resolution of fluoroscopy in comparison to standard X-ray. Additionally, the reading time for the surgeon intraoperatively is much shorter than for the readers in evaluating the postoperative X-rays. Nevertheless, fluoroscopy is the only possibility for continuous intraoperative monitoring. The low detection rate by fluoroscopy or X-ray confirms the need for CT scans, to determine the precise rate of cement leakage in vertebroplasty.
Besides the necessity for intraoperative identification of cement leaks by the surgeon, postoperative identification is important for clinical studies. Here, the inter-observer reliability plays an essential part. The inter-observer comparison in this study showed only fair agreement for lateral and biplanar X-ray. In all probability, this will be the same, or even worse, for the intraoperatively used fluoroscopy, which can lead to different leakage rates for several readers. The inter-observer differences can eliminate the comparison of studies, e.g. leakage rate of vertebroplasty compared to kyphoplasty by different observers. In our opinion, this also leads to the need for postoperative CT scans for study purposes. Comparison studies are also facilitated if identical or similar classifications are used. The classification by Yeom et al. [31], used in this study, covers leakage with potential pulmonary sequelae (type S) and neurological sequelae (type B), as well as unpredictable leaks due to the fracture (type C). In further studies, the use of classifications could lead to new perceptions about cement leakage and possible ways to anticipate it.
The detection of type S leaks could be substantially enhanced by the AP view, which is logical, due to their course at the lateral border of the vertebra. It is questionable whether this brings the need for biplanar fluoroscopy. Type S leaks do not usually lead to neurological complications, although they can lead to foraminal narrowing if cement reflow in the vein towards the neural foramen occurs. From that standpoint, it does not seem essential for the additional AP view to be used. However, cement in the segmental vessels could drain and lead to pulmonary embolism [13, 22], which is a rare reported complication. The disadvantage of bilateral fluoroscopy is that the surgeon must observe simultaneously two fluoroscopy screens. With the current available studies a conclusive decision as to whether intraoperative biplanar fluoroscopy is needed cannot be drawn from our point of view.
Also, when additional AP views were used, type B lesions were frequently (50%–60%, Table 1) not detected. The leaks are unrecognized by overprojection of the posterior wall, or they are misinterpreted as cement reflow in the direction of the pedicles, if no crossing of the pedicle border occurs (Fig. 5). It therefore seems preferable for the needle to be inserted to the trocar after injection, to avoid reflow of cement. As the pedicle is usually not involved in the fracture, it is possible that it does not alter the clinical result. Caution must be given to the fact that insertion of the needle to the trocar leads to further cement filling. Additionally, by leaving the needle and trocar in place, cement extrusion through iatrogenic pedicle erosions (Fig. 6) can be avoided. Injection should also be stopped if cement reaches the posterior part of the vertebra. Thereby, misinterpretation of the cement localization in the posterior vertebral part or already in the spinal canal can be avoided. This also lowers the risk of a cement leak in case of posterior wall fracture (Fig. 6). None of the three patients with incomplete burst fractures in our study had cement leakage in the spinal canal. It is questionable whether or not leaks in the basivertebral vein can be prevented with this technique. The basivertebral vein drains the whole vertebral body, and leakage can occur before cement reaches the posterior wall (Fig. 3).
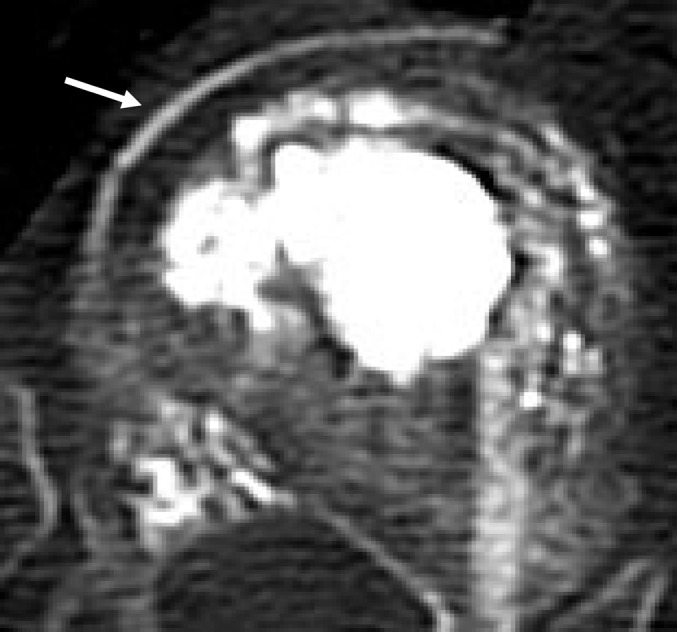
Fig. 5.
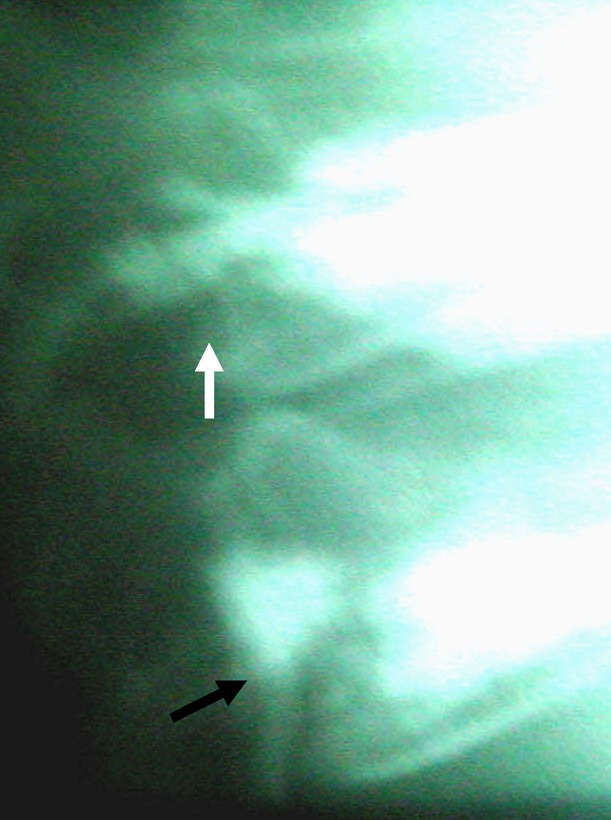
Type B leak (black arrow) with crossing of pedicle border and reflow of cement towards pedicle with no leak (white arrow)
Fig. 6.
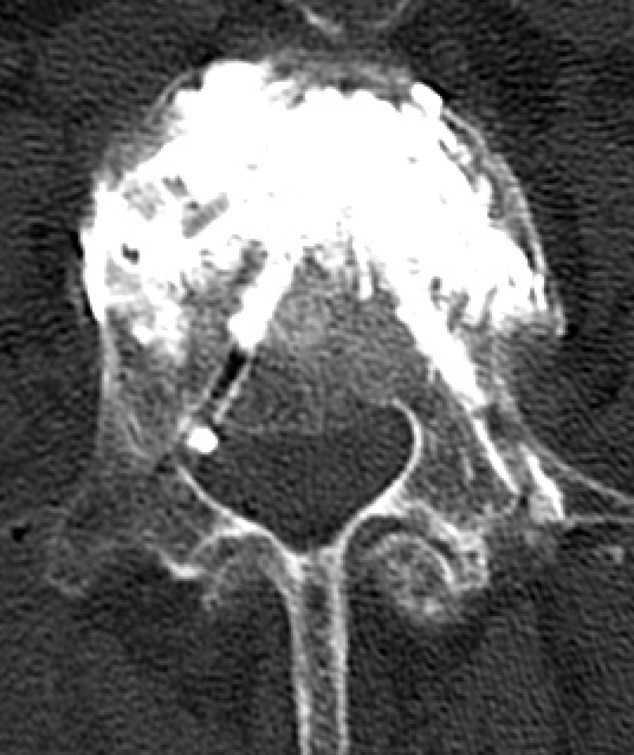
CT corresponding to Fig. 1 after PVP, showing small leak by iatrogenic pedicle wall erosion. Filling only anterior and middle part of the vertebra, with no leak through posterior wall fracture
If cement crosses the pedicle border, cranially or caudally, a leak into the spinal canal can be assumed (Fig. 5). In our study, both of the complications that required further surgery were due to type B leaks. This is in discordance with Yeom et al. [31], who state that type B leaks probably do not lead to neurological complications, as distribution resembles normal venous engorgement and cement is contained in the veins. Besides our study, Wenger et al. [28] and Lee et al. [16] also had neurological complications from type B leaks. In our patient with paraparesis, cement was contained in the vein during removal; however, the spinal canal was narrowed by up to 50%. Thus, surgeons should be on the look out for type B patterns, as they can lead to symptomatic complications as well as type C leaks, which can occur in any place around the vertebra.
In clinical studies, pain reduction by vertebroplasty was observed with various amounts of cement, mainly ranging from 2–15 ml [2, 3, 5, 6, 14, 27]. Objective parameters for a satisfying filling are missing. The determination of parameters with regard to the amount of cement needed for vertebroplasty would be a great step forward in decreasing cement leakage rates, as the leakage is dose dependent [25], and the amount of cement should be reduced as far as possible without affecting the clinical outcome. Nevertheless, the view that leaks are used to indicate satisfactory filling [19] is not shared by us. Eventually, this problem could be partially obviated by kyphoplasty, which may lower the risk of cement extrusion [8, 17, 23].
The results of this study are limited by the small number of patients. Nevertheless, it is quite obvious that the detection of leaks by X-ray or fluoroscopy has its limitations. After completing this study and the subsequent study of the literature, we believe that cement leakage is an underestimated problem when compared to the reported number of leaks. Although most leaks are clinically asymptomatic, they all bear the risk for sequelae of either transient or permanent nature. In most reported complications, surgeons found no obvious technical mistakes. It appears that even with a perfect technique, cement leakage is, up to a certain percentage, an inevitable and unpredictable complication [11, 21]. On the basis of this assumption, the occurrence of symptomatic cement leakage is something of which surgeons should be aware while performing vertebroplasty.
Conclusions
The rate of cement leakage during vertebroplasty is quite high if precise determination by CT scan, as in this study, is performed. Detection of leaks by fluoroscopy or X-ray is difficult and further complicated by only fair inter-observer agreement. Therefore, for the accurately determination of the rate of leakage CT, with consideration of multiple leaks, is the method of choice. Although the rate of clinically symptomatic leaks is low, the potential for severe complications exists. CT scans are also the best tools to use when it is necessary for one to determine whether postoperative clinical symptoms, such as new or persistent pain, are related to cement extrusion.
References
- 1.Altman DG. Practical statistics for medical research. London: Chapman and Hall; 1991. [Google Scholar]
- 2.Alvarez L, Pérez-Higueras A, Quinones D, Calvo E, Rossi RE. Vertebroplasty in the treatment of vertebral tumors: postprocedural outcome and quality of life. Eur Spine J. 2003;12:356–360. doi: 10.1007/s00586-003-0525-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barr JD, Barr MS, Lemley TJ, McCann RM. Percutaneous vertebroplasty for pain relief and spinal stabilization. Spine. 2000;25:923–928. doi: 10.1097/00007632-200004150-00005. [DOI] [PubMed] [Google Scholar]
- 4.Bradford DS, McBride GG. Surgical management of thoracolumbar spine fractures with incomplete neurologic deficits. Clin Orthop. 1987;218:201–216. [PubMed] [Google Scholar]
- 5.Cotten A, Dewatre F, Cortet B, Assaker R, Leblond D, Duquesnoy B, Chastanet P, Clarisse J. Percutaneous vertebroplasty for osteolytic metastases and myeloma: effects of the percentage of lesion filling and the leakage of methyl methacrylate at clinical follow-up. Radiology. 1996;200:525–530. doi: 10.1148/radiology.200.2.8685351. [DOI] [PubMed] [Google Scholar]
- 6.Deramond H, Depriester C, Galibert P, Le Gars D. Percutaneous vertebroplasty with polymethylmethacrylate. Technique, indications, and results. Radiol Clin North Am. 1998;36:533–546. doi: 10.1016/s0033-8389(05)70042-7. [DOI] [PubMed] [Google Scholar]
- 7.Diamond TH, Champion B, Clark WA. Management of acute osteoporotic vertebral fractures: a nonrandomized trial comparing percutaneous vertebroplasty with conservative therapy. Am J Med. 2003;114:257–265. doi: 10.1016/S0002-9343(02)01524-3. [DOI] [PubMed] [Google Scholar]
- 8.Fourney DR, Schomer DF, Nader R, Chlan Fourney J, Suki D, Ahrar K, Rhines LD, Gokaslan ZL. Percutaneous vertebroplasty and kyphoplasty for painful vertebral body fractures in cancer patients. J Neurosurg. 2003;98:21–30. doi: 10.3171/spi.2003.98.1.0021. [DOI] [PubMed] [Google Scholar]
- 9.Galibert P, Deramond H, Rosat P, Le Gars D. Note preliminaire sur le traitement des angiomes vertebraux par vertebroplastie acrylique percutanee. Neurochirurgie. 1987;33:166–168. [PubMed] [Google Scholar]
- 10.Garfin SR, Yuan HA, Reiley MA. New technologies in spine: kyphoplasty and vertebroplasty for the treatment of painful osteoporotic compression fractures. Spine. 2001;26:1511–1515. doi: 10.1097/00007632-200107150-00002. [DOI] [PubMed] [Google Scholar]
- 11.Gaughen JR, Jensen ME, Schweickert PA, Kaufmann TJ, Marx WF, Kallmes DF. Relevance of antecedent venography in percutaneous vertebroplasty for the treatment of osteoporotic compression fractures. AJNR Am J Neuroradiol. 2002;23:594–600. [PMC free article] [PubMed] [Google Scholar]
- 12.Harrington KD. Major neurological complications following percutaneous vertebroplasty with polymethylmethacrylate: a case report. J Bone Joint Surg Am. 2001;83A:1070–1073. doi: 10.2106/00004623-200107000-00014. [DOI] [PubMed] [Google Scholar]
- 13.Jang JS, Lee SH, Jung SK. Pulmonary embolism of polymethylmethacrylate after percutaneous vertebroplasty: a report of three cases. Spine. 2002;27:E416–E418. doi: 10.1097/00007632-200210010-00021. [DOI] [PubMed] [Google Scholar]
- 14.Jensen ME, Evans AJ, Mathis JM, Kallmes DF, Cloft HJ, Dion JE. Percutaneous polymethylmethacrylate vertebroplasty in the treatment of osteoporotic vertebral body compression fractures: technical aspects. AJNR Am J Neuroradiol. 1997;18:1897–1904. [PMC free article] [PubMed] [Google Scholar]
- 15.Lane JI, Maus TP, Wald JT, Thielen KR, Bobra S, Luetmer PH. Intravertebral clefts opacified during vertebroplasty: pathogenesis, technical implications, and prognostic significance. AJNR Am J Neuroradiol. 2002;23:1642–1646. [PMC free article] [PubMed] [Google Scholar]
- 16.Lee BJ, Lee SR, Yoo TY. Paraplegia as a complication of percutaneous vertebroplasty with polymethylmethacrylate: a case report. Spine. 2002;27:E419–E422. doi: 10.1097/00007632-200210010-00022. [DOI] [PubMed] [Google Scholar]
- 17.Lieberman IH, Dudeney S, Reinhardt MK, Bell G. Initial outcome and efficacy of “kyphoplasty” in the treatment of painful osteoporotic vertebral compression fractures. Spine. 2001;26:1631–1638. doi: 10.1097/00007632-200107150-00026. [DOI] [PubMed] [Google Scholar]
- 18.Magerl F, Aebi M, Gertzbein SD, Harms J, Nazarian S. A comprehensive classification of thoracic and lumbar injuries. Eur Spine J. 1994;3:184–201. doi: 10.1007/BF02221591. [DOI] [PubMed] [Google Scholar]
- 19.Martin JB, Jean B, Sugiu K, SanMillan Ruiz D, Piotin M, Murphy K, Rufenacht B, Muster M, Rufenacht DA. Vertebroplasty clinical experience and follow-up results. Bone. 1999;25:11s–15s. doi: 10.1016/S8756-3282(99)00126-X. [DOI] [PubMed] [Google Scholar]
- 20.McGraw JK, Lippert JA, Minkus KD, Rami PM, Davis TM, Budzik RF. Prospective evaluation of pain relief in 100 patients undergoing percutaneous vertebroplasty: results and follow-up. J Vasc Interv Radiol. 2002;13:883–886. doi: 10.1016/s1051-0443(07)61770-9. [DOI] [PubMed] [Google Scholar]
- 21.Moreland DB, Landi MK, Grand W. Vertebroplasty: techniques to avoid complications. Spine J. 2001;1:66–71. doi: 10.1016/S1529-9430(01)00013-4. [DOI] [PubMed] [Google Scholar]
- 22.Padovani B, Kasriel O, Brunner P, Peretti Viton P. Pulmonary embolism caused by acrylic cement: a rare complication of percutaneous vertebroplasty. AJNR Am J Neuroradiol. 1999;20:375–377. [PMC free article] [PubMed] [Google Scholar]
- 23.Phillips FM, Todd Wetzel F, Lieberman I, Campbell Hupp M. An in vivo comparison of the potential for extravertebral cement leak after vertebroplasty and kyphoplasty. Spine. 2002;27:2173–2178. doi: 10.1097/00007632-200210010-00018. [DOI] [PubMed] [Google Scholar]
- 24.Ratliff J, Nguyen T, Heiss J. Root and spinal cord compression from methylmethacrylate vertebroplasty. Spine. 2001;26:E300–E302. doi: 10.1097/00007632-200107010-00021. [DOI] [PubMed] [Google Scholar]
- 25.Ryu KS, Park CK, Kim MC, Kang JK. Dose-dependent epidural leakage of polymethylmethacrylate after percutaneous vertebroplasty in patients with osteoporotic vertebral compression fractures. J Neurosurg. 2002;96:56–61. doi: 10.3171/spi.2002.96.1.0056. [DOI] [PubMed] [Google Scholar]
- 26.Shapiro S, Abel T, Purvines S. Surgical removal of epidural and intradural polymethylmethacrylate extravasation complicating percutaneous vertebroplasty for an osteoporotic lumbar compression fracture. Case report. J Neurosurg. 2003;98:90–92. doi: 10.3171/spi.2003.98.1.0090. [DOI] [PubMed] [Google Scholar]
- 27.Watts NB, Harris ST, Genant HK. Treatment of painful osteoporotic vertebral fractures with percutaneous vertebroplasty or kyphoplasty. Osteoporos Int. 2001;12:429–437. doi: 10.1007/s001980170086. [DOI] [PubMed] [Google Scholar]
- 28.Wenger M, Markwalder TM. Surgically controlled, transpedicular methyl methacrylate vertebroplasty with fluoroscopic guidance. Acta Neurochir (Wien) 1999;141:625–631. doi: 10.1007/s007010050352. [DOI] [PubMed] [Google Scholar]
- 29.Wiltse LL, Bateman JG, Hutchinson RH, Nelson WE. The Paraspinal sacrospinalis—splitting approach to the lumbar spine. J Bone Joint Surg Am. 1968;50:919–926. [PubMed] [Google Scholar]
- 30.Wong W, Reiley MA, Garfin S. Vertebroplasty/kyphoplasty. J Womens Imag. 2000;2:117–124. [Google Scholar]
- 31.Yeom JS, Kim WJ, Choy WS, Lee CK, Chang BS, Kang JW. Leakage of cement in percutaneous transpedicular vertebroplasty for painful osteoporotic compression fractures. J Bone Joint Surg Br. 2003;85:83–89. doi: 10.1302/0301-620X.85B1.13026. [DOI] [PubMed] [Google Scholar]