Abstract
This report describes a case of one-level total disc replacement (TDR) of L5-S1 requiring revision at 9.5 years following the index surgery due to polyethylene failure caused by high oxidation. Primary revision strategies for TDR include instrumented posterolateral fusion, or 360° fusion with replacement of the prosthesis with cages or allograft bone. A revision of a TDR with a similar prosthesis has not been described in the literature. An active 42-year-old female underwent TDR with a Charité artificial disc. She remained active and pain free for 9.5 years before presenting with moderate low-back pain and sciatica. Radiographic studies confirmed a fragmented polyethylene core. The failed prosthesis was revised to a new Charité disc with the patient again active and pain free for 6 months following surgery. Chemical and physical analysis of the core indicated high oxidation due to gamma sterilization in air; a process changed to gamma sterilization in nitrogen in 1998 to meet industry standards. No evidence of wear debris was noted. Revision of an artificial disc with an artificial disc can be performed safely and adequately with the Charité disc prosthesis as an alternative to fusion necessitated by a device failure. An anterior revision approach carries significant risk and should only be performed by surgeons experienced in anterior lumbar surgery.
Keywords: Lumbar spine, Artificial disc, Revision
Introduction
Prior long-term studies of total disc replacement (TDR) in the lumbar spine have included few reports of prosthesis failure and/or continued pain necessitating revision with surgical arthrodesis [4–6, 8, 9]. The primary revision technique to address a failed TDR or a patient with continued residual pain following TDR is instrumented posterolateral fusion with transpedicular fixation. Posterior instrumented fusion immobilizes the prosthesis, causing it to act as an inactive spacer. An alternative technique is 360° instrumented fusion with removal of the prosthesis and replacement with interbody cages or femoral ring allograft. In a patient properly indicated for TDR who was previously pain-free, neither revision with fusion option is an optimal solution. To date, no reports in the literature describe the revision of a TDR prosthesis in vivo for an extended period of time following the index surgery with a new TDR prosthesis.
The third-generation Charité artificial disc has been implanted outside the United States since 1987. The device consists of two CoCrMo alloy plates and a freestanding core made of ultra-high-molecular-weight polyethylene (UHMWPE). Six “teeth” are forcefully implanted into the cranial and caudal vertebral endplates for initial stability.
The purpose of this report is to describe a case of revision of a Charité artificial disc, 9.5 years in vivo, with a Charité artificial disc; and to describe the method of failure, analysis of the explant, and the factors contributing to device failure.
Case report
In 1993, an active 42-year-old woman, with a secretarial occupation, presented with progressive intractable back pain caused by degenerative disc disease (DDD) at L5-S1 with normal facet joints (indicated for TDR), as confirmed by diagnostic radiological studies. She failed non-operative management and subsequently underwent TDR at L5-S1 with a third-generation Charité artificial disc (DePuy Spine, Raynham, Mass., USA) utilizing first-generation instrumentation. The uncoated prosthesis endplates were size 1—the smallest available, with 5° lordotic angles. The height of the polyethylene core was 9.5 mm. A standard left-sided anterior retroperitoneal approach was performed. A window annulotomy technique was used which included suturing of the anterior longitudinal ligament (ALL) and annulus following implantation of the prosthesis. This served to protect the area of implantation from scar formation. The patient tolerated the surgery well and encountered no early postoperative complications. Immediate postoperative radiographs confirmed somewhat sub-optimal placement of the superior endplate, which was too far anterior. Radiographic evaluation at 1 year (Fig. 1) showed an intact implant with the superior endplate noted to be too far anterior, but not migrated from its original position. However, the patient was pain-free, returned to work and was able to perform all of her prior daily activities.
Fig. 1.
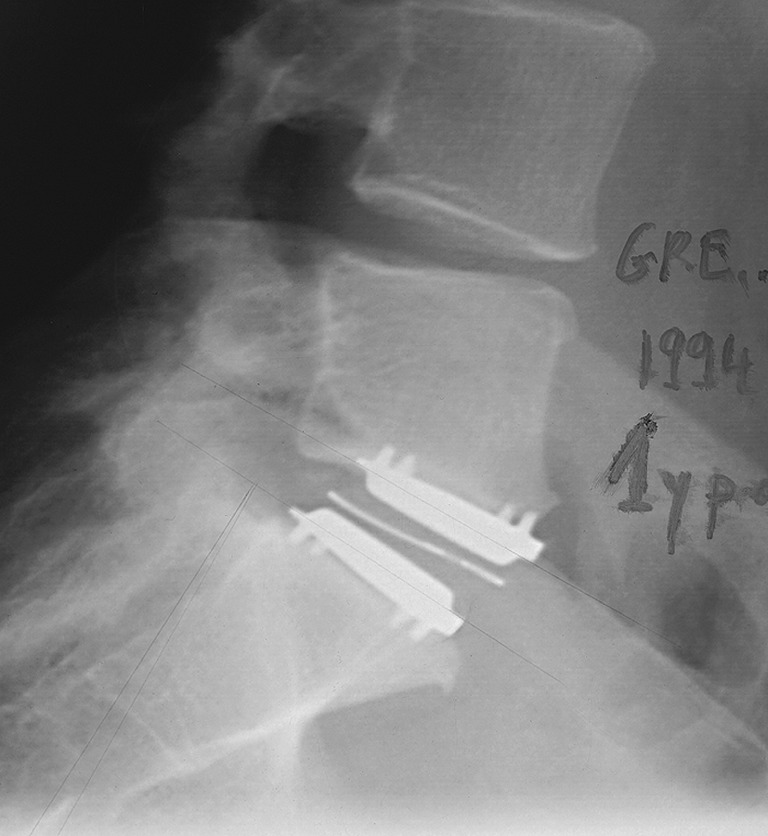
Lateral radiograph at 1 year following index surgery showing the X-ray wire in the polyethylene core appears normal (not expanded outwards), though the placement of the inferior endplate is too anterior
The patient remained pain-free with an excellent functional and clinical result until 9.5 years following surgery (age = 52 years). At that time, the patient presented with moderate low-back pain and sciatica. Plain lateral flexion and extension radiographs demonstrated 8° of flexion/extension motion as well as an expansion of the X-ray wire around the polyethylene core (Fig. 2). CT scans confirmed a fragmented core, with the outer ring expanded outward radially, but with no intrusion into the spinal canal (Fig. 3).
Fig. 2.
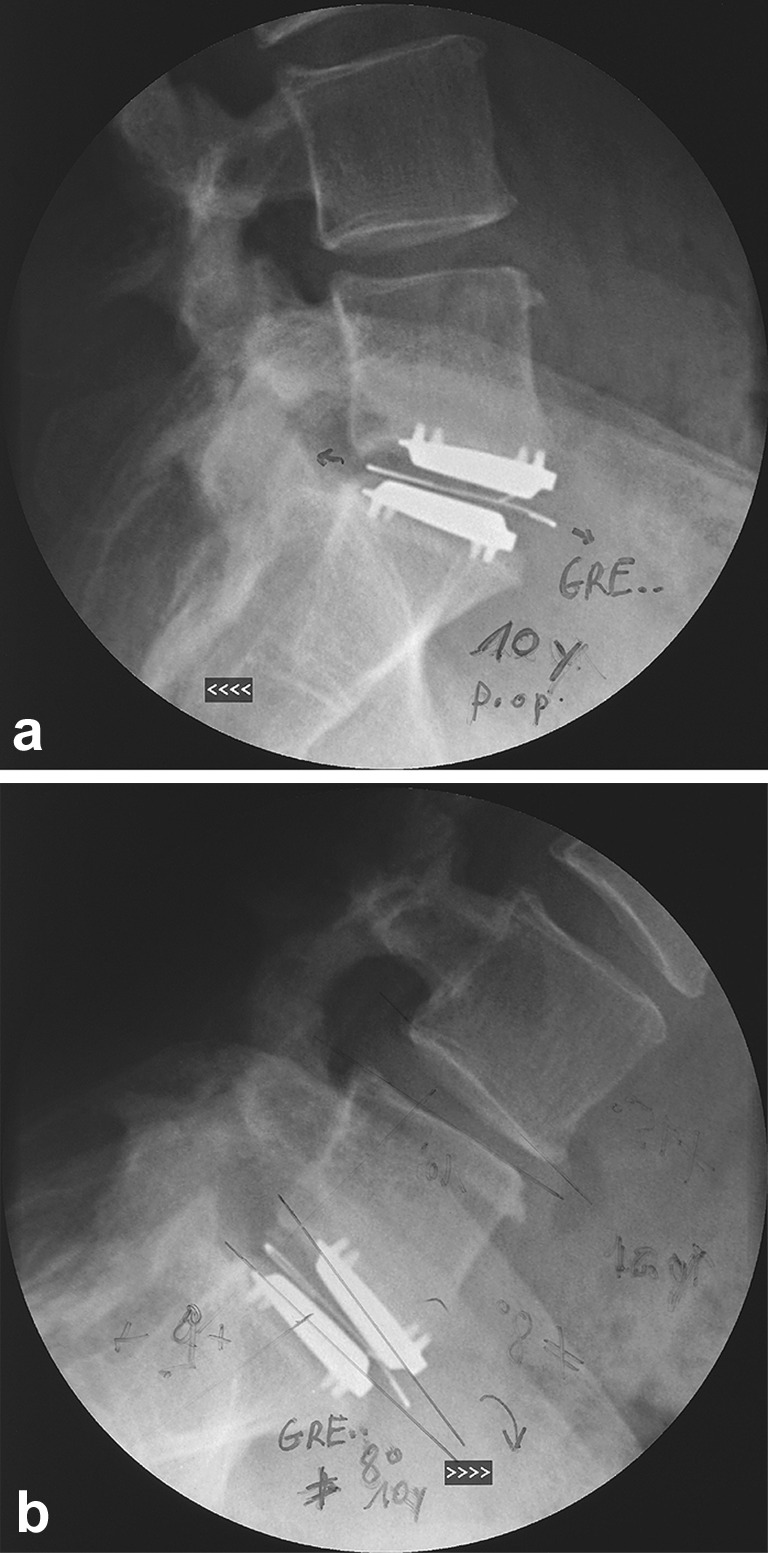
a Lateral extension radiograph at 9.5 years following index surgery, showing the expanded X-ray wire. b Lateral flexion radiography at the same time point. Despite the failed polyethylene core, the patient maintained 8° of range of motion on flexion/extension
Fig. 3.

a, b Coronal CT scan slices at 9.5 years following index surgery, confirming a fragmented core with the outer ring expanded outward radially but with no intrusion into the spinal canal. The facets appear normal
A revision surgery was performed via a retroperitoneal approach to replace the failed prosthesis. During the procedure, to prevent intrusion or laceration, a catheter was placed in the left ureter. The ALL and annulus were found to be intact and fully healed with minimal scar formation. The great vessels were easily mobilized. The X-ray wire was found to be expanded outward and separated from the core. The core was removed in six pieces with visual confirmation of no intrusion into the spinal canal. It should be noted that the sutured ALL and annulus served to contain the prosthesis following failure. The prosthesis endplates were found to be secured to the vertebral endplates in their original position and were removed. No evidence of infection, wear debris, or heterotopic ossification was identified. A new, third-generation Charité prosthesis with size 2 endplates and an 8.5 mm core was implanted with second generation instrumentation without complication slightly more posterior in the disc space than the original implant. The ALL and annulus were again sutured prior to closing the wound. At 6 months following the revision procedure, placement of the new prosthesis was satisfactory (Fig. 4) and the patient was pain-free, leading an active lifestyle, and returned to work. The endplates and core (Fig. 5) were sent to DePuy spine for analysis.
Fig. 4.
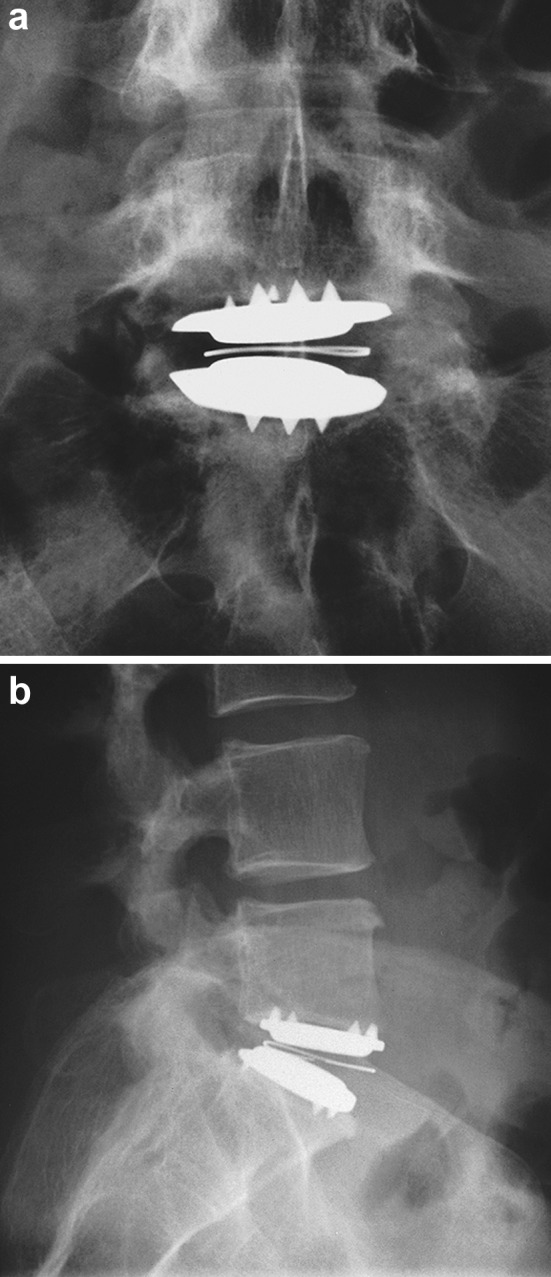
a Anteroposterior radiograph at 6 months following revision surgery with a new Charité artificial disc. b Lateral radiograph at the same time point. The patient was pain free and had excellent function
Fig. 5.

The explanted Charité artificial disc with no evidence of polyethylene or cobalt chromium wear debris
Physical and chemical analysis
Physical and chemical analyses were performed on the explant. The articulating surfaces of the endplates were in excellent condition, with no visible marring, scratches, or nicks. Due to the absence of visible wear debris, lack of local tissue inflammation, or osteolysis at the time of revision, no histological analysis was performed. There was no evidence of contact between the endplates. There did not appear to be any material (protein, polyethylene, etc.) or film on the articulating faces of the endplates. The surface finish of the articulating faces was measured and found to be less than 0.2 µm, which is equivalent to that of a new implant.
Fragments of the core are shown in Fig. 6, with fragment “C” as the bi-convex portion of the core that articulates with the endplates. The original height of the core was 9.5 mm. The explanted height was measured as 7.7 mm. The failure mode of the spherical portion of the core indicated a shearing force was applied between the superior and inferior hemispheres, causing them to shift by 2.9 mm.
Fig. 6.
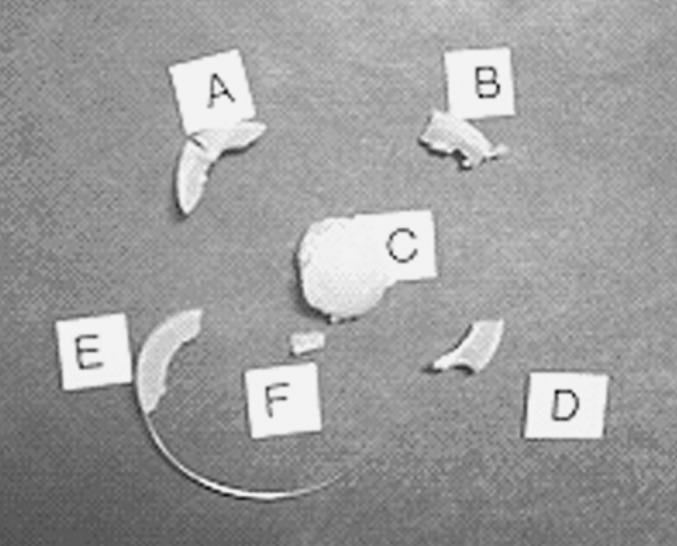
Core fragments from the explanted prosthesis
Fourier transform infra-red (FTIR) analysis is a discrete spectral analysis that yields oxidation data points across the thickness of a test sample. FTIR analysis by an independent lab revealed oxidative indices as high as 5.4 at the surface of the explanted core, with values has high as 3.0 in the central part of the core. A fresh core would be expected to have an oxidative index of much less than 1.
Discussion
Despite damage to the core, reduced functionality of the device was maintained. The core height was reduced by less than 3 mm. The damaged core assumed a height that was slightly larger than the smallest core size (7.5 mm) meaning the core maintained separation of the endplates following failure. This is further supported by the lack of damage to the articulating surfaces, and the patient’s ability to achieve 8° of motion on flexion/extension 9.5 years postoperatively.
The failure mode of the spherical portion of the core indicates that a shearing force was applied, in combination with an anterior placement of the device, and perhaps insufficient lordosis in the segment. The ideal floating center of rotation (FCR) is 2 mm posterior to the geometric center of the disc space in the sagittal plane [3]. Ideal placement of the center of the Charité prosthesis is within 3 mm of this point, as described by McAfee et al. [7]. A larger lordotic angle on the inferior implant endplate minimizes the shearing force applied to the core. In 1999, a greater range of lordotic angles (7.5° and 10°) were introduced to the market allowing for improved implant configurations and minimization of shear forces acting on the core.
The chemical analysis of the core indicated that the core was heavily oxidized. Oxidation of the polyethylene core can result from aging, gamma sterilization (especially in air), and other factors. An oxidative index greater than 1.0 generally indicates highly oxidized polyethylene. The explant measured in excess of 3.0. The manufacturing process was changed in 1997 to incorporate “nitrogen-vacuum” packaging of the cores, consistent with industry practice. Since that time, the cores were packaged and sterilized in a nitrogen-vacuum environment. Gamma sterilization of polyethylene in nitrogen is known to substantially reduce oxidation relative to sterilization in air [1]. The device explanted in this case was gamma sterilized in air and this is the likely source of the high (>1.0) oxidative index measured in this core. As a result of this high level of oxidation, this core became more brittle, and thus more susceptible to the shear forces established by sub-optimal endplate positioning. This would not be expected in a normal non-oxidized core, and has not been shown before in the literature. Published long-term results with Charité by David and Lemaire have shown no similar failures. In comparison, reported results for unicompartmental knee replacement describe 10-year survivorship rates of 95–97% [2]. This type of polyethylene failure is a rare occurrence. A change in sterilization practices has reduced the likelihood of oxidation of the core. Maximizing the angle of the inferior endplate by utilizing currently available lordotic endplates may minimize the shear stresses applied to the prostheses during normal use. Ongoing instrument and technique refinements may improve placement accuracy. As a result, this type of core failure is less likely to occur in the future.
Conclusions
Revision of an artificial disc with an artificial disc can be performed safely and adequately with the Charité disc prosthesis as an alternative to fusion necessitated by a device failure. However, a revision necessitating an anterior approach carries significant risk to the vascular structures, the ureter, and neurological elements. It should only be performed by surgeons with a high degree of skill and experience in anterior lumbar surgery. Suturing of the ALL following the primary surgery reduced scarring, “boxed” the failed prosthesis in place, and allowed for uncomplicated removal of the prosthesis.
References
- 1.Bargmann LS, Bargmann BC, Collier JP, Currier BH, Mayor MB. Current sterilization and packaging methods for polyethylene. Clin Orthop. 1999;369:49–58. doi: 10.1097/00003086-199912000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Callaghan JJ. Mobile-bearing knee replacement: clinical results: a review of the literature. Clin Orthop. 2001;392:221–225. doi: 10.1097/00003086-200111000-00027. [DOI] [PubMed] [Google Scholar]
- 3.Cunningham BW, Godron JD, Dmitriev AE, Hu N, McAfee PC. Biomechanical evaluation of total disc replacement arthroplasty: an in vitro human cadaveric model. Spine. 2003;28:S110–S117. doi: 10.1097/01.BRS.0000092209.27573.90. [DOI] [PubMed] [Google Scholar]
- 4.David T. Lumbar disc prosthesis: five years follow-up study on 147 patients with 163 SB Charité prosthesis. Eur Spine J. 2002;11(Suppl 1):S18. [Google Scholar]
- 5.Huang RC, Girardi FP, Cammisa FP, Jr, Tropiano P, Marnay T. Long-term flexion-extension range of motion of the ProDisc total disc replacement. J Spinal Disord Tech. 2003;2(16):435–440. doi: 10.1097/00024720-200310000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Lemaire JP. SB Charité III intervertebral disc prosthesis: biomechanical, clinical, and radiological correlations with a series of 100 cases over a follow-up of more than 10 years. Rachis. 2002;14:271–285. [Google Scholar]
- 7.McAfee PC, Cunningham BW, Geisler FH, Regan JJ, Guyer RD, Blumenthal SL, Maxwell J (2004) A prospective randomized FDA study of the Charité disc replacement—a radiographic outcome analysis of 276 consecutive patients. Proceedings of the 31st annual meeting of the international society for the study of the lumbar spine (ISSLS), Porto, 5 June 2004
- 8.Marnay T. Lumbar disc replacement, 7–10 years results with Prodisc. Eur Spine J. 2001;10(Suppl 1):S67. [Google Scholar]
- 9.Ooij A, Oner FC, Verbout AbJ. Complications of artificial disc replacement. J Spinal Disord Tech. 2003;16:369–383. doi: 10.1097/00024720-200308000-00009. [DOI] [PubMed] [Google Scholar]


