Abstract
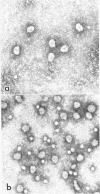
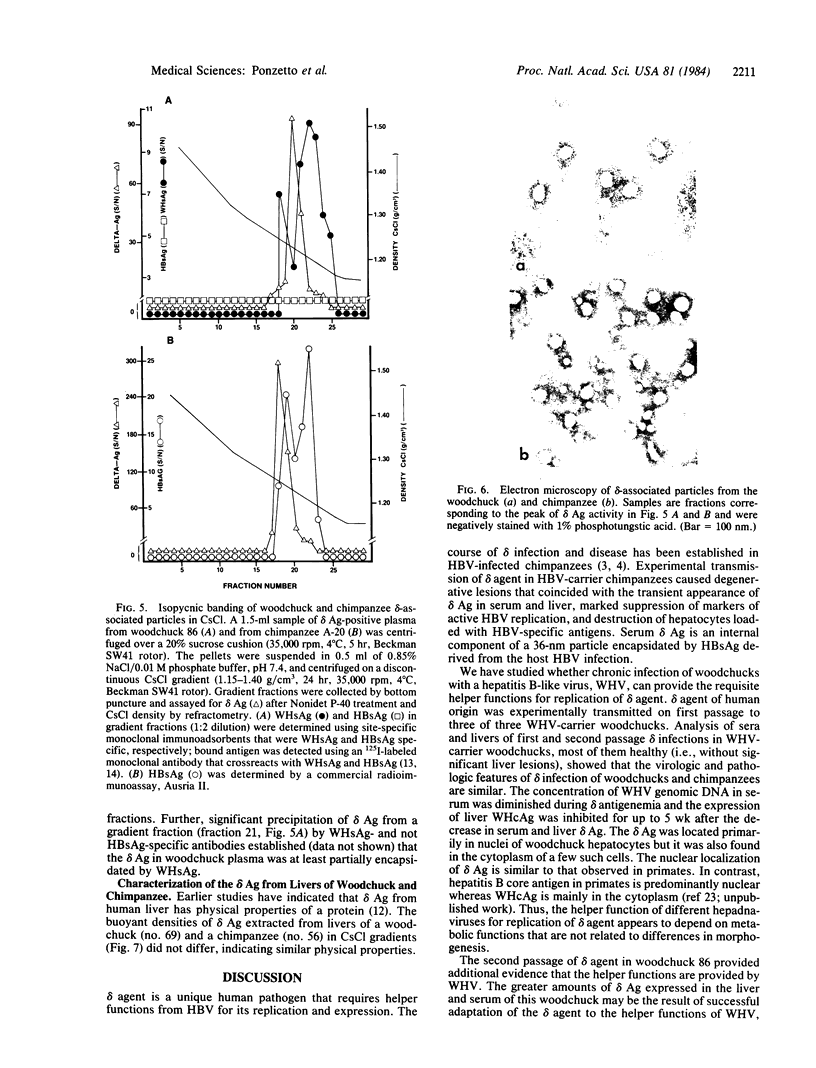
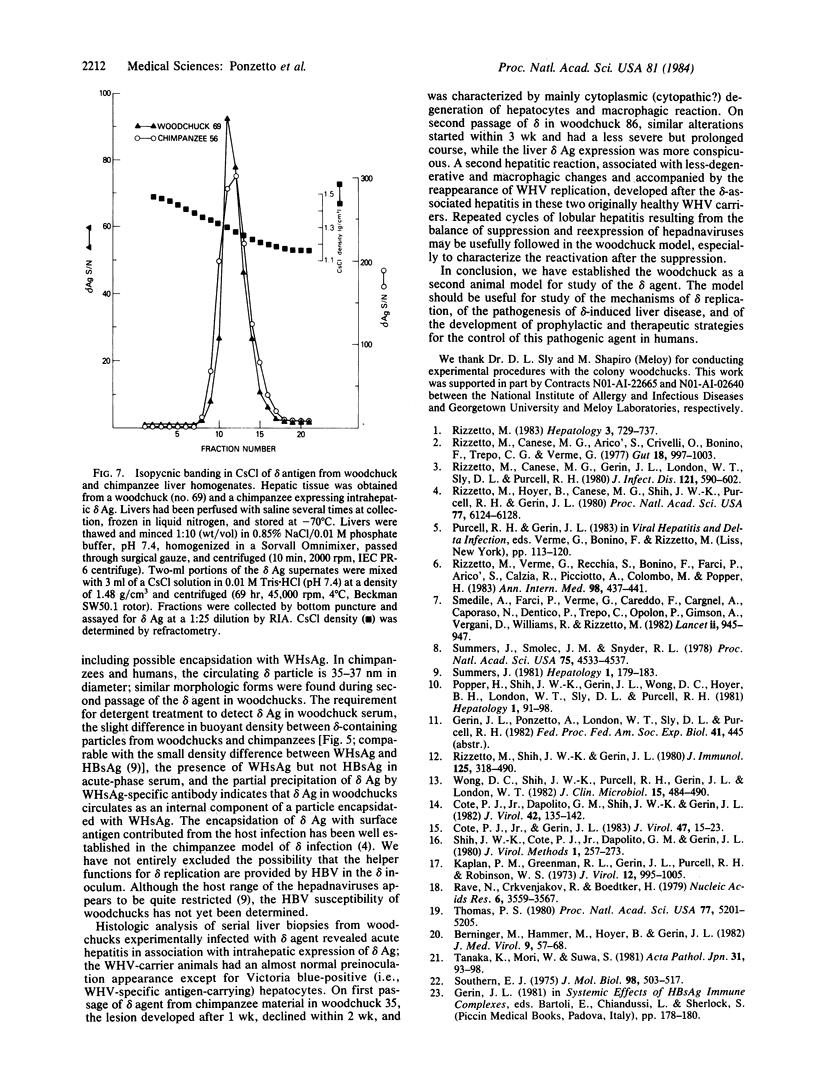
delta agent of human origin was inoculated into four woodchucks chronically infected with woodchuck hepatitis virus (WHV). The animals developed delta infections with serologic patterns similar to those previously observed in human and chimpanzee infections. delta antigen was detected transiently in serum and liver and was followed by seroconversion to anti-delta antibody. Analogous to the chimpanzee model of delta infection, serum and hepatocyte markers of WHV were suppressed in the woodchuck during acute delta infection. The suppression of WHV DNA in serum was evident only during the time of delta-antigen positivity, while the inhibition of other WHV markers was more protracted. The delta antigen in woodchuck sera circulated as an internal component of a particle similar in size to the human delta particle (36-nm diameter) and was encapsidated by the woodchuck hepatitis virus surface antigen; delta antigen from infected woodchuck and chimpanzee livers had similar biophysical properties. Histologic analysis showed that experimental delta infection is associated with a transient acute hepatitis in woodchucks and loss of hepatocytes carrying WHV antigens. The lesions differed from the conspicuous hepatitis associated with reappearance of WHV replication. Hepatitis B-like viruses, therefore, appear to provide the requisite helper functions for delta replication and the woodchuck represents a useful model for study of the virology and pathology of the delta agent.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berninger M., Hammer M., Hoyer B., Gerin J. L. An assay for the detection of the DNA genome of hepatitis B virus in serum. J Med Virol. 1982;9(1):57–68. doi: 10.1002/jmv.1890090109. [DOI] [PubMed] [Google Scholar]
- Cote P. J., Jr, Dapolito G. M., Shih J. W., Gerin J. L. Surface antigenic determinants of mammalian "hepadnaviruses" defined by group- and class-specific monoclonal antibodies. J Virol. 1982 Apr;42(1):135–142. doi: 10.1128/jvi.42.1.135-142.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cote P. J., Jr, Gerin J. L. Nonoverlapping antigenic sites of woodchuck hepatitis virus surface antigen and their cross-reactivity with ground squirrel hepatitis virus and hepatitis B virus surface antigens. J Virol. 1983 Jul;47(1):15–23. doi: 10.1128/jvi.47.1.15-23.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan P. M., Greenman R. L., Gerin J. L., Purcell R. H., Robinson W. S. DNA polymerase associated with human hepatitis B antigen. J Virol. 1973 Nov;12(5):995–1005. doi: 10.1128/jvi.12.5.995-1005.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popper H., Shih J. W., Gerin J. L., Wong D. C., Hoyer B. H., London W. T., Sly D. L., Purcell R. H. Woodchuck hepatitis and hepatocellular carcinoma: correlation of histologic with virologic observations. Hepatology. 1981 Mar-Apr;1(2):91–98. doi: 10.1002/hep.1840010202. [DOI] [PubMed] [Google Scholar]
- Rave N., Crkvenjakov R., Boedtker H. Identification of procollagen mRNAs transferred to diazobenzyloxymethyl paper from formaldehyde agarose gels. Nucleic Acids Res. 1979 Aug 10;6(11):3559–3567. doi: 10.1093/nar/6.11.3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzetto M., Canese M. G., Aricò S., Crivelli O., Trepo C., Bonino F., Verme G. Immunofluorescence detection of new antigen-antibody system (delta/anti-delta) associated to hepatitis B virus in liver and in serum of HBsAg carriers. Gut. 1977 Dec;18(12):997–1003. doi: 10.1136/gut.18.12.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzetto M., Canese M. G., Gerin J. L., London W. T., Sly D. L., Purcell R. H. Transmission of the hepatitis B virus-associated delta antigen to chimpanzees. J Infect Dis. 1980 May;141(5):590–602. doi: 10.1093/infdis/141.5.590. [DOI] [PubMed] [Google Scholar]
- Rizzetto M., Hoyer B., Canese M. G., Shih J. W., Purcell R. H., Gerin J. L. delta Agent: association of delta antigen with hepatitis B surface antigen and RNA in serum of delta-infected chimpanzees. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6124–6128. doi: 10.1073/pnas.77.10.6124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzetto M., Shih J. W., Gerin J. L. The hepatitis B virus-associated delta antigen: isolation from liver, development of solid-phase radioimmunoassays for delta antigen and anti-delta and partial characterization of delta antigen. J Immunol. 1980 Jul;125(1):318–324. [PubMed] [Google Scholar]
- Rizzetto M. The delta agent. Hepatology. 1983 Sep-Oct;3(5):729–737. doi: 10.1002/hep.1840030518. [DOI] [PubMed] [Google Scholar]
- Rizzetto M., Verme G., Recchia S., Bonino F., Farci P., Aricò S., Calzia R., Picciotto A., Colombo M., Popper H. Chronic hepatitis in carriers of hepatitis B surface antigen, with intrahepatic expression of the delta antigen. An active and progressive disease unresponsive to immunosuppressive treatment. Ann Intern Med. 1983 Apr;98(4):437–441. doi: 10.7326/0003-4819-98-4-437. [DOI] [PubMed] [Google Scholar]
- Shih J. W., Cote P. J., Jr, Dapolito G. M., Gerin J. L. Production of monoclonal antibodies against hepatitis B surface antigen (HBsAg) by somatic cell hybrids. J Virol Methods. 1980;1(5):257–273. doi: 10.1016/0166-0934(80)90023-3. [DOI] [PubMed] [Google Scholar]
- Smedile A., Farci P., Verme G., Caredda F., Cargnel A., Caporaso N., Dentico P., Trepo C., Opolon P., Gimson A. Influence of delta infection on severity of hepatitis B. Lancet. 1982 Oct 30;2(8305):945–947. doi: 10.1016/s0140-6736(82)90156-8. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Summers J., Smolec J. M., Snyder R. A virus similar to human hepatitis B virus associated with hepatitis and hepatoma in woodchucks. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4533–4537. doi: 10.1073/pnas.75.9.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers J. Three recently described animal virus models for human hepatitis B virus. Hepatology. 1981 Mar-Apr;1(2):179–183. doi: 10.1002/hep.1840010215. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Mori W., Suwa K. Victoria blue-nuclear fast red stain for HBs antigen detection in paraffin section. Acta Pathol Jpn. 1981 Jan;31(1):93–98. doi: 10.1111/j.1440-1827.1981.tb00987.x. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong D. C., Shih J. W., Purcell R. H., Gerin J. L., London W. T. Natural and experimental infection of woodchucks with woodchuck hepatitis virus, as measured by new, specific assays for woodchuck surface antigen and antibody. J Clin Microbiol. 1982 Mar;15(3):484–490. doi: 10.1128/jcm.15.3.484-490.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]