Abstract
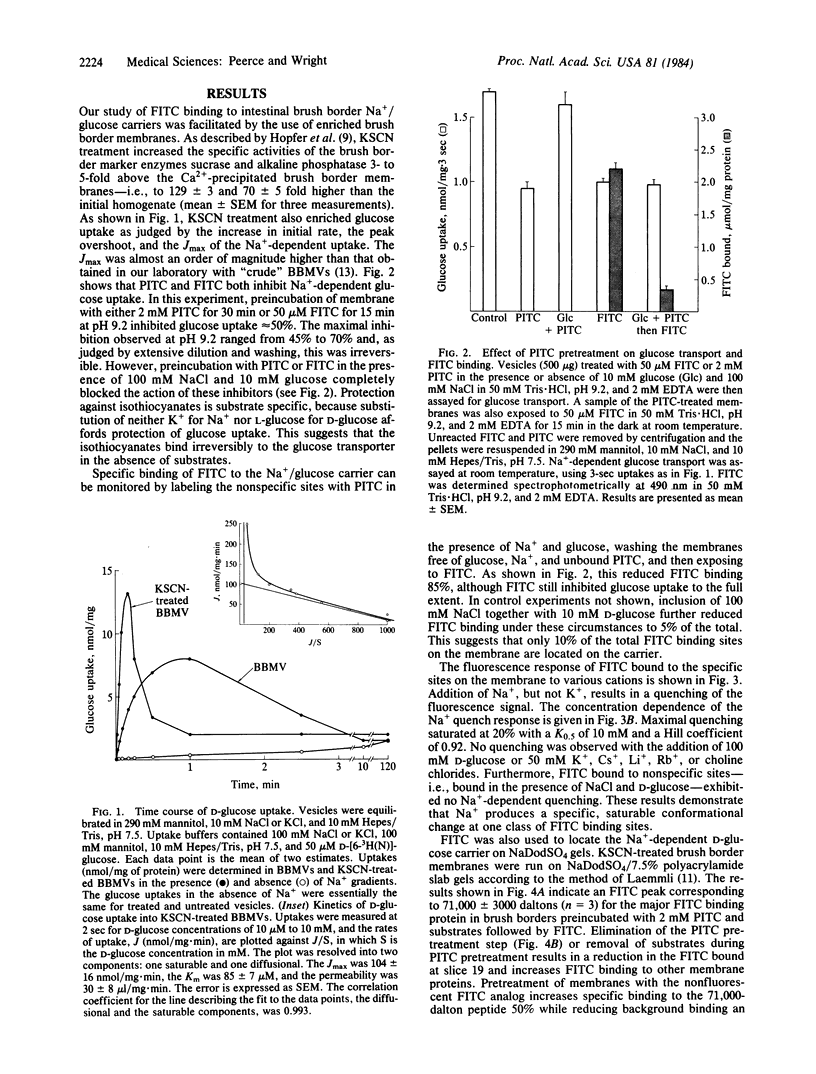
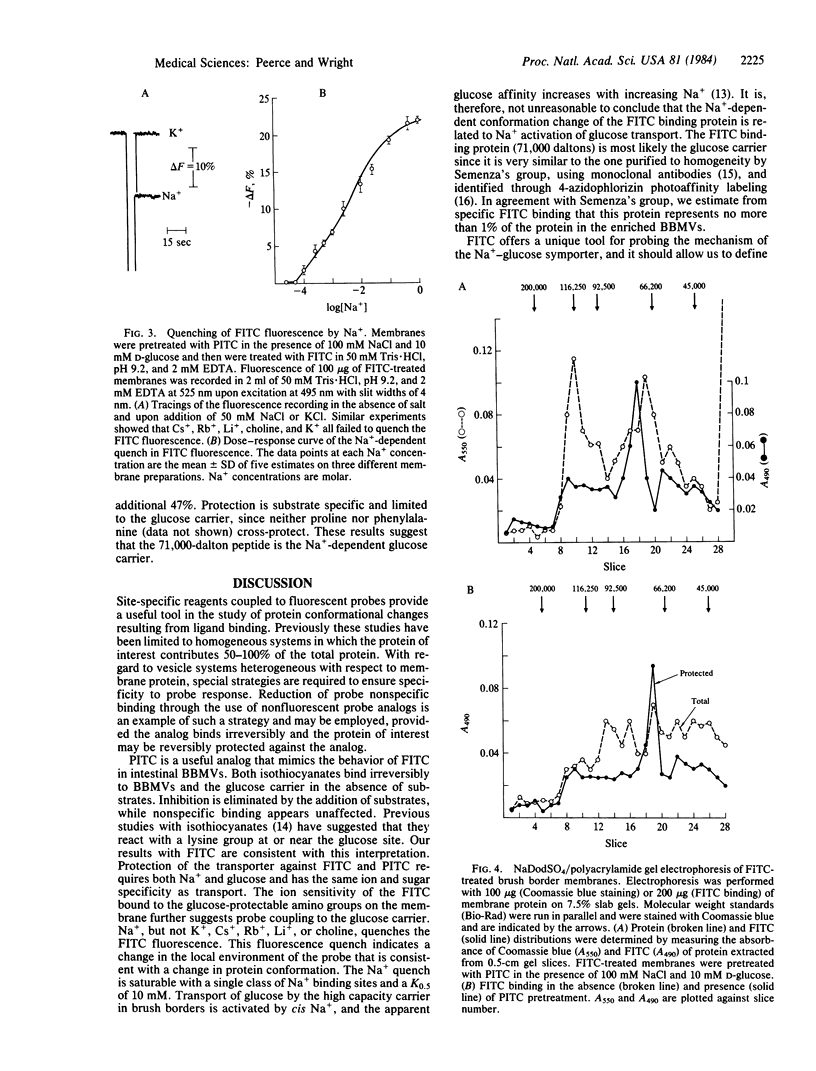
Fluorescein isothiocyanate (FITC) was used to label the rabbit intestinal brush border Na+-glucose carrier, identify the carrier protein on sodium dodecyl sulfate/polyacrylamide gel electrophoresis, and monitor the effect of ions and substrates on fluorescence quenching. Enriched brush border preparations were employed to study both glucose transport and FITC binding. FITC and a nonfluorescent analog (phenyl isothiocyanate, PITC) both inhibited Na+-dependent D-glucose transport irreversibly. Inhibition was blocked completely by the presence of Na+ and D-glucose during labeling. PITC was used to label nonspecific amino groups in the presence of glucose and Na+, and then the glucose carrier was labeled with FITC in the absence of substrates. Fluorescence of FITC bound to the carrier was quenched specifically with Na+ in a saturable fashion, and this indicates a Na+-dependent conformational change in the carrier. Sodium dodecyl sulfate/polyacrylamide gel electrophoresis of FITC-labeled membranes revealed specific labeling of a 71,000-dalton peptide. We conclude that Na+ induces a conformational shift in the 71,000-dalton glucose carrier, and this is quite consistent with the kinetics of Na+-dependent glucose transport in these membranes.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carilli C. T., Farley R. A., Perlman D. M., Cantley L. C. The active site structure of Na+- and K+-stimulated ATPase. Location of a specific fluorescein isothiocyanate reactive site. J Biol Chem. 1982 May 25;257(10):5601–5606. [PubMed] [Google Scholar]
- Hopfer U., Crowe T. D., Tandler B. Purification of brush border membrane by thiocyanate treatment. Anal Biochem. 1983 Jun;131(2):447–452. doi: 10.1016/0003-2697(83)90197-5. [DOI] [PubMed] [Google Scholar]
- Hosang M., Gibbs E. M., Diedrich D. F., Semenza G. Photoaffinity labeling and identification of (a component of) the small-intestinal Na+,D-glucose transporter using 4-azidophlorizin. FEBS Lett. 1981 Aug 3;130(2):244–248. doi: 10.1016/0014-5793(81)81130-1. [DOI] [PubMed] [Google Scholar]
- Jackson R. J., Mendlein J., Sachs G. Interaction of fluorescein isothiocyanate with the (H+ + K+)-ATPase. Biochim Biophys Acta. 1983 May 26;731(1):9–15. doi: 10.1016/0005-2736(83)90391-7. [DOI] [PubMed] [Google Scholar]
- Karlish S. J. Characterization of conformational changes in (Na,K) ATPase labeled with fluorescein at the active site. J Bioenerg Biomembr. 1980 Aug;12(3-4):111–136. doi: 10.1007/BF00744678. [DOI] [PubMed] [Google Scholar]
- Kaunitz J. D., Gunther R., Wright E. M. Involvement of multiple sodium ions in intestinal d-glucose transport. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2315–2318. doi: 10.1073/pnas.79.7.2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaunitz J. D., Wright E. M. Kinetics of sodium D-glucose cotransport in bovine intestinal brush border vesicles. J Membr Biol. 1984;79(1):41–51. doi: 10.1007/BF01868525. [DOI] [PubMed] [Google Scholar]
- Kessler M., Acuto O., Storelli C., Murer H., Müller M., Semenza G. A modified procedure for the rapid preparation of efficiently transporting vesicles from small intestinal brush border membranes. Their use in investigating some properties of D-glucose and choline transport systems. Biochim Biophys Acta. 1978 Jan 4;506(1):136–154. doi: 10.1016/0005-2736(78)90440-6. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mircheff A. K., Wright E. M. Analytical isolation of plasma membranes of intestinal epithelial cells: identification of Na, K-ATPase rich membranes and the distribution of enzyme activities. J Membr Biol. 1976 Sep 17;28(4):309–333. doi: 10.1007/BF01869703. [DOI] [PubMed] [Google Scholar]
- Nigg E. A., Cherry R. J. Influence of temperature and cholesterol on the rotational diffusion of band 3 in the human erythrocyte membrane. Biochemistry. 1979 Aug 7;18(16):3457–3465. doi: 10.1021/bi00583a004. [DOI] [PubMed] [Google Scholar]
- Pick U., Bassilian S. Modification of the ATP binding site of the Ca2+ -ATPase from sarcoplasmic reticulum by fluorescein isothiocyanate. FEBS Lett. 1981 Jan 12;123(1):127–130. doi: 10.1016/0014-5793(81)80035-x. [DOI] [PubMed] [Google Scholar]
- Pick U. Dynamic interconversions of phosphorylated and non-phosphorylated intermediates of the Ca-ATPase from sarcoplasmic reticulum followed in a fluorescein-labeled enzyme. FEBS Lett. 1981 Jan 12;123(1):131–136. doi: 10.1016/0014-5793(81)80036-1. [DOI] [PubMed] [Google Scholar]
- Schmidt U. M., Eddy B., Fraser C. M., Venter J. C., Semenza G. Isolation of (a subunit of) the Na+/D-glucose cotransporter(s) of rabbit intestinal brush border membranes using monoclonal antibodies. FEBS Lett. 1983 Sep 19;161(2):279–283. doi: 10.1016/0014-5793(83)81025-4. [DOI] [PubMed] [Google Scholar]
- Stevens B. R., Kaunitz J. D., Wright E. M. Intestinal transport of amino acids and sugars: advances using membrane vesicles. Annu Rev Physiol. 1984;46:417–433. doi: 10.1146/annurev.ph.46.030184.002221. [DOI] [PubMed] [Google Scholar]
- Weber J., Semenza G. Chemical modification of the small intestinal Na+/D-glucose cotransporter by amino group reagents. Evidence for a role of amino group(s) in the binding of the sugar. Biochim Biophys Acta. 1983 Jun 23;731(3):437–447. doi: 10.1016/0005-2736(83)90039-1. [DOI] [PubMed] [Google Scholar]



