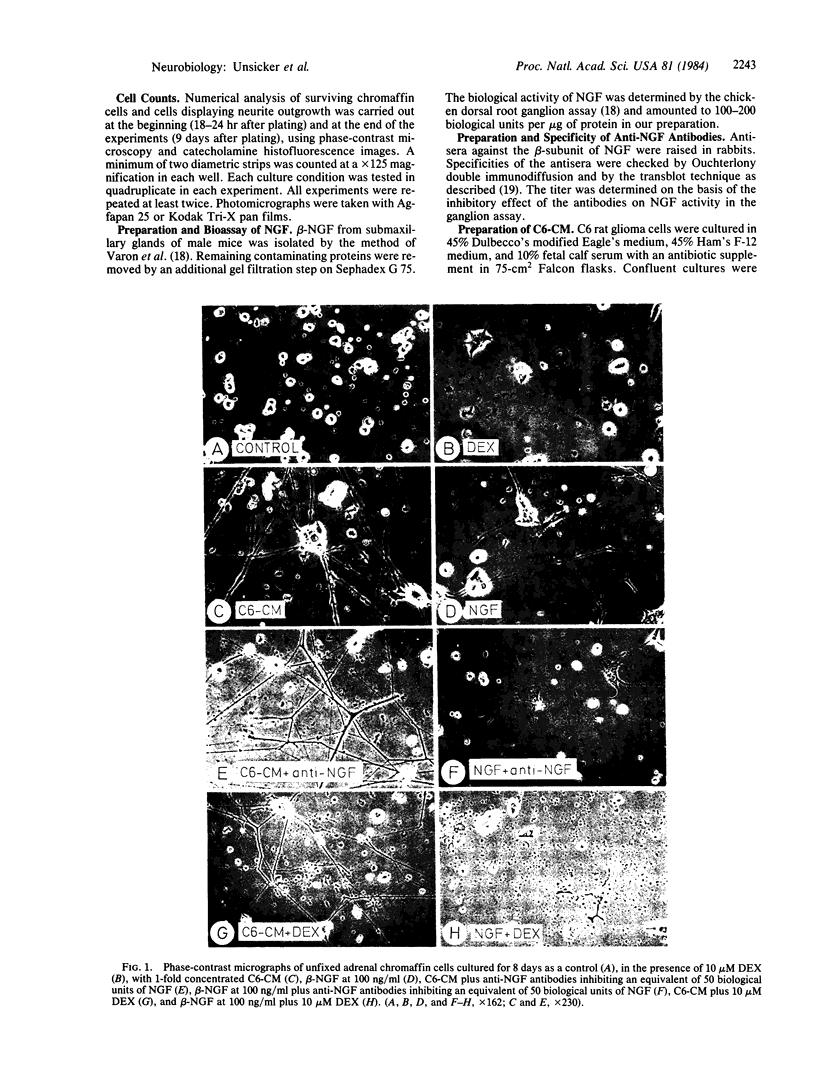
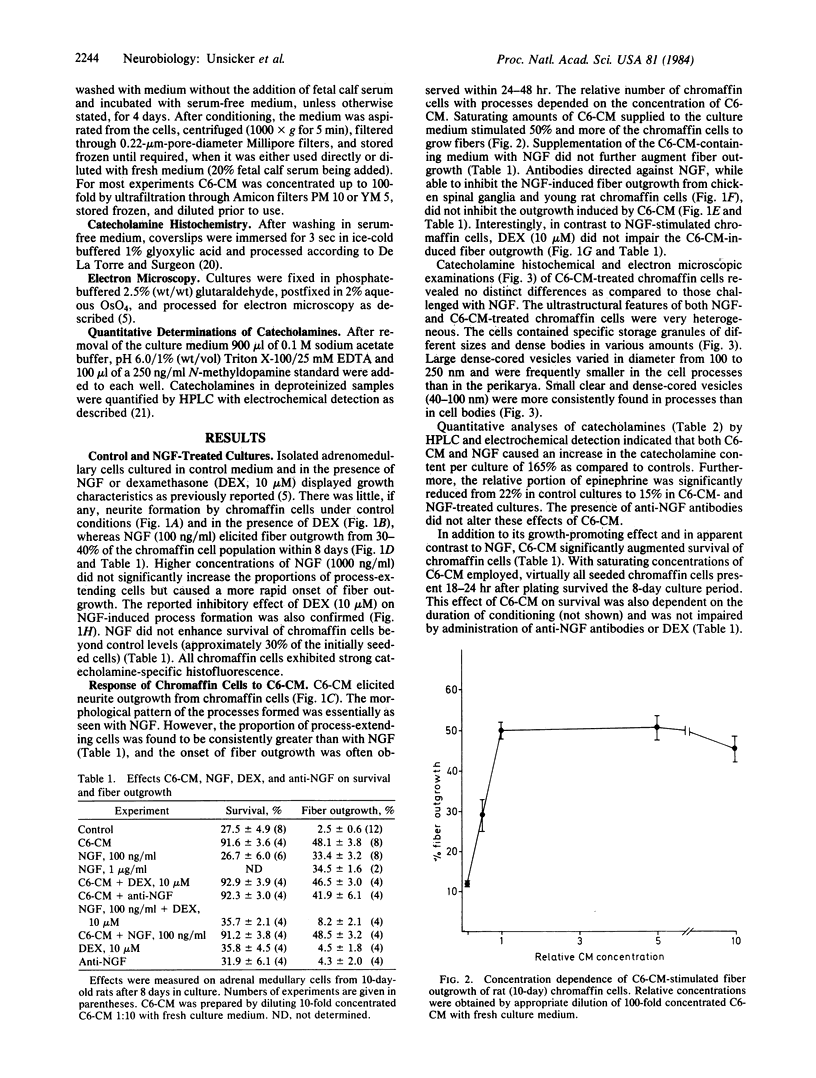
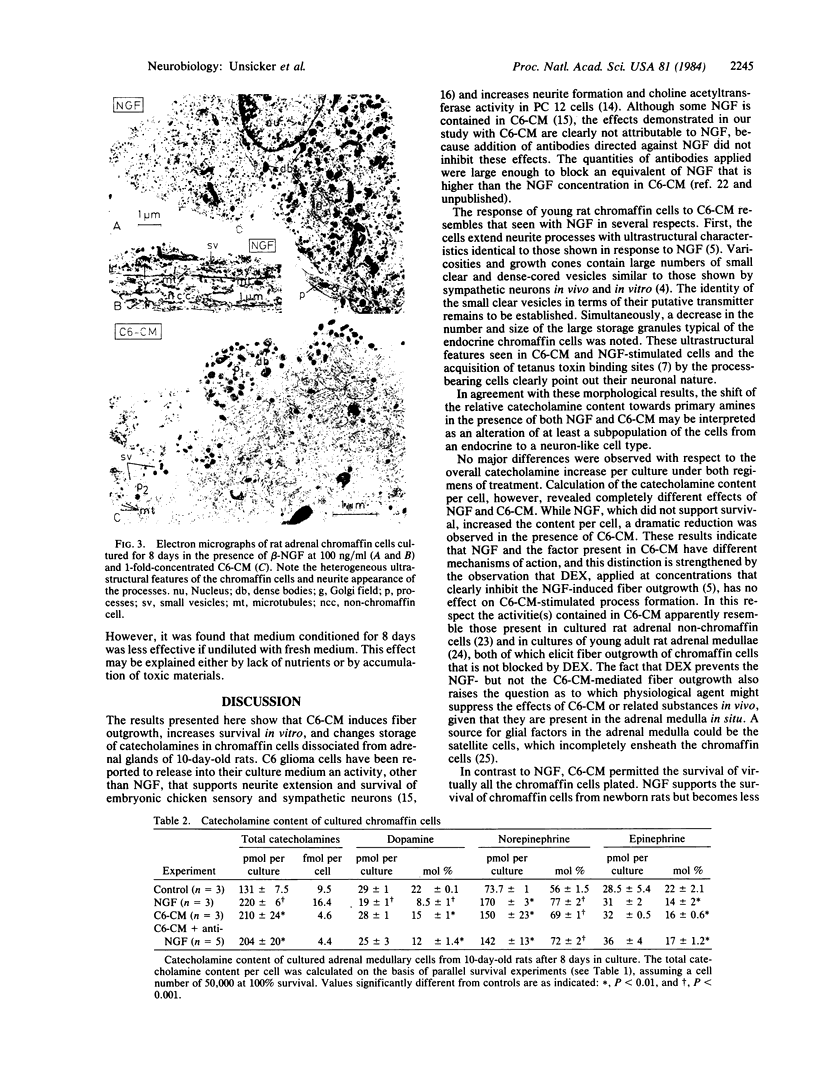
Abstract
The effects of medium conditioned by rat C6 glioma cells (C6-CM) on the survival, neurite formation, and catecholamine content of adrenal medullary cells in culture were investigated and compared with the effects of nerve growth factor (NGF). Adrenal medullary cells were isolated from 10-day-old rats and the proportions of surviving and neurite-extending cells were determined after 8 days in culture. In the presence of C6-CM virtually all seeded cells survived and 50% developed neuritic processes. In contrast, NGF did not support survival above control levels (30%) and induced neurite formation from approximately one-third of the surviving cells. C6-CM and NGF had no additive effects on neurite outgrowth. C6-CM-mediated fiber outgrowth was not inhibited by physiological concentrations of glucocorticoids which abolished the NGF-induced neurite formation. Both C6-CM and NGF increased the catecholamine content of the cultures and reduced the relative content of epinephrine. However, in view of its substantial effect on cell survival as compared to NGF, C6-CM caused a reduction of the catecholamine content per cell. We conclude that adrenal medullary cells, like other members of the sensory-sympathetic cell lineage of the neural crest, respond to glial-conditioned medium. This response differs both quantitatively and qualitatively from that mediated by NGF.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barde Y. A., Edgar D., Thoenen H. Sensory neurons in culture: changing requirements for survival factors during embryonic development. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1199–1203. doi: 10.1073/pnas.77.2.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barde Y. A., Lindsay R. M., Monard D., Thoenen H. New factor released by cultured glioma cells supporting survival and growth of sensory neurones. Nature. 1978 Aug 24;274(5673):818–818. doi: 10.1038/274818a0. [DOI] [PubMed] [Google Scholar]
- Cocchia D., Michetti F. S-100 antigen in satellite cells of the adrenal medulla and the superior cervical ganglion of the rat. An immunochemical and immunocytochemical study. Cell Tissue Res. 1981;215(1):103–112. doi: 10.1007/BF00236252. [DOI] [PubMed] [Google Scholar]
- Edgar D., Barde Y. A., Thoenen H. Induction of fibre outgrowth and choline acetyltransferase in PC12 pheochromocytoma cells by conditioned media from glial cells and organ extracts. Exp Cell Res. 1979 Jul;121(2):353–361. doi: 10.1016/0014-4827(79)90015-6. [DOI] [PubMed] [Google Scholar]
- Edgar D., Barde Y. A., Thoenen H. Subpopulations of cultured chick sympathetic neurones differ in their requirements for survival factors. Nature. 1981 Jan 22;289(5795):294–295. doi: 10.1038/289294a0. [DOI] [PubMed] [Google Scholar]
- Hawrot E., Patterson P. H. Long-term culture of dissociated sympathetic neurons. Methods Enzymol. 1979;58:574–584. doi: 10.1016/s0076-6879(79)58174-9. [DOI] [PubMed] [Google Scholar]
- Hofmann H. D., Unsicker K. The seminal vesicle of the bull: a new and very rich source of nerve growth factor. Eur J Biochem. 1982 Nov 15;128(2-3):421–426. [PubMed] [Google Scholar]
- Lietzke R., Unsicker K. Tetanus toxin binding to different morphological phenotypes of cultured rat and bovine adrenal medullary cells. Neurosci Lett. 1983 Aug 8;38(3):233–238. doi: 10.1016/0304-3940(83)90374-9. [DOI] [PubMed] [Google Scholar]
- Müller T. H., Unsicker K. High-performance liquid chromatography with electrochemical detection as a highly efficient tool for studying catecholaminergic systems. I. Quantification of noradrenaline, adrenaline and dopamine in cultured adrenal medullary cells. J Neurosci Methods. 1981 Jun;4(1):39–52. doi: 10.1016/0165-0270(81)90017-0. [DOI] [PubMed] [Google Scholar]
- Schwartz J. P., Chuang D. M., Costa E. Increase in nerve growth factor content of C6 glioma cells by the activation of a beta-adrenergic receptor. Brain Res. 1977 Dec 2;137(2):369–375. doi: 10.1016/0006-8993(77)90349-3. [DOI] [PubMed] [Google Scholar]
- Tischler A. S., Perlman R. L., Nunnemacher G., Morse G. M., DeLellis R. A., Wolfe H. J., Sheard B. E. Long-term effects of dexamethasone and nerve growth factor on adrenal medullary cells cultured from young adult rats. Cell Tissue Res. 1982;225(3):525–542. doi: 10.1007/BF00214802. [DOI] [PubMed] [Google Scholar]
- Torre J. C., Surgeon J. W. A methodological approach to rapid and sensitive monoamine histofluorescence using a modified glyoxylic acid technique: the SPG method. Histochemistry. 1976 Oct 22;49(2):81–93. doi: 10.1007/BF00495672. [DOI] [PubMed] [Google Scholar]
- Unsicker K., Krisch B., Otten U., Thoenen H. Nerve growth factor-induced fiber outgrowth from isolated rat adrenal chromaffin cells: impairment by glucocorticoids. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3498–3502. doi: 10.1073/pnas.75.7.3498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unsicker K., Millar T. J., Hofmann H. D. Nerve growth factor requirement of postnatal rat adrenal medullary cells in vitro for survival, aggregate formation and maintenance of extended neurites. Dev Neurosci. 1982;5(5-6):412–417. doi: 10.1159/000112701. [DOI] [PubMed] [Google Scholar]
- Unsicker K., Tschechne B., Tschechne D. Differentiation and transdifferentiation of adrenal chromaffin cells of the guinea-pig. I. Transplants to the anterior chamber of the eye. Cell Tissue Res. 1981;215(2):341–367. doi: 10.1007/BF00239120. [DOI] [PubMed] [Google Scholar]
- Unsicker K., Zwarg U., Habura-Flüh O. Differentiation and transdifferentiation of adrenal chromaffin cells of the guinea pig. III. Transplants under the kidney capsule. Cell Tissue Res. 1983;229(2):299–308. doi: 10.1007/BF00214977. [DOI] [PubMed] [Google Scholar]
- Varon S., Adler R. Nerve growth factors and control of nerve growth. Curr Top Dev Biol. 1980;16:207–252. doi: 10.1016/s0070-2153(08)60157-x. [DOI] [PubMed] [Google Scholar]
- Weston J. A. The migration and differentiation of neural crest cells. Adv Morphog. 1970;8:41–114. doi: 10.1016/b978-0-12-028608-9.50006-5. [DOI] [PubMed] [Google Scholar]
- Ziegler W., Hofmann H. D., Unsicker K. Rat adrenal non-chromaffin cells contain a neurite outgrowth-promoting factor immunologically different from nerve growth factor. Brain Res. 1983 Apr;283(2-3):353–357. doi: 10.1016/0165-3806(83)90193-1. [DOI] [PubMed] [Google Scholar]