Abstract
Purpose
Studies in Asia have questioned the dictum that signet ring cell carcinoma (SRC) has a worse prognosis than other forms of gastric cancer. Our study determined differences in presentation and outcomes between SRC and gastric adenocarcinoma (AC) in the United States.
Patients and Methods
The National Cancer Institute Surveillance, Epidemiology, and End Results database was reviewed for SRC and AC from 2004 to 2007.
Results
We reviewed 10,246 cases of patients with gastric cancer, including 2,666 of SRC and 7,580 of AC. SRC presented in younger patients (61.9 v 68.7 years; P < .001) and less often in men (52.7% v 68.7%; P < .001). SRC patients were more frequently black (11.3% v 10.9%), Asian (16.4% v 13.2%), American Indian/Alaska Native (0.9% v 0.8%), or Hispanic (23.3% v 14.0%; P < .001). SRC was more likely to be stage T3-4 (45.8% v 33.3%), have lymph node spread (59.7% v 51.8%), and distant metastases (40.2% v 37.6%; P < .001). SRC was more likely to be found in the lower (30.7% v 24.2%) and middle stomach (30.6% v 20.7%; P < .001). Median survival was not different between the two (AC, 14.0 months v SRC, 13.0 months; P = .073). Multivariable analyses demonstrated SRC was not associated with mortality (hazard ratio [HR], 1.05; 95% CI, 0.96 to 1.11; P = .150). Mortality was associated with age (HR, 1.01; 95% CI, 1.01 to 1.02; P < .001), black race (HR, 1.10; 95% CI, 1.01 to 1.20; P = .026), and tumor grade. Variables associated with lower mortality risk included Asian race (HR, 0.83; 95% CI, 0.77 to 0.91; P < .001) and surgery (HR, 0.37; 95% CI, 0.34 to 0.39; P < .001).
Conclusion
In the United States, SRC significantly differs from AC in extent of disease at presentation. However, when adjusted for stage, SRC does not portend a worse prognosis.
INTRODUCTION
Signet ring cell gastric carcinoma is a histologic diagnosis based on microscopic characteristics as described by the World Health Organization.1 It has long been thought to have a worse prognosis than other forms of gastric cancer.2,3 Recently, studies in Asia have begun to question this idea.4–8 No studies have analyzed signet ring cell carcinoma (SRC) versus gastric adenocarcinoma in a large national database in the United States. The aim of our study was to determine differences in presentation and outcomes between signet ring cell gastric carcinoma and gastric adenocarcinoma in order to determine whether signet ring cell histology conveys worse prognosis in the United States.
PATIENTS AND METHODS
Data Source
After approval from the Temple University institutional review board, data from the 17 Surveillance, Epidemiology, and End Results (SEER) cancer registries of the National Cancer Institute were obtained. Information collected by SEER is obtained through participating cancer registries. The database comprises 28% of the United States population. Information evaluated from SEER included: patient age, sex, race, primary tumor site, stage at diagnosis, number of lymph nodes, surgery, radiation, and follow-up vital status. The public-use data files were converted into Stata data sets for statistical analysis (Stata 12, StataCorp, College Station, TX).
Study Sample
Records from 2004 to 2007 were analyzed for this study. Records from this time period were correlated with the American Joint Committee on Cancer (AJCC) staging manual (6th edition). Signet ring cell gastric cancer is a histologic diagnosis based on microscopic characteristics. These cells contain a large amount of mucin, which pushes the nucleus to the cell periphery.1 The International Classification of Diseases code 8490 was used to identify patients with signet ring cell gastric carcinoma, whereas code 8140 was used for adenocarcinoma, not otherwise specified.
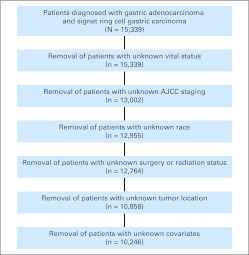
Of 15,339 patients with gastric adenocarcinomas and signet ring cell gastric carcinomas, we excluded individuals from our analytic sample using the following criteria: vital status unknown (n = 0), staging unknown (n = 2,337), race unknown (n = 47), surgery status unknown (n = 0), radiation status unknown (n = 191), tumor location unknown (n = 1,906), and missing data on study covariates (n = 612), leaving us with a final analytic sample of 10,246 patients. Characteristics of patients included and excluded were compared using multivariable analysis. Variables in which differences were observed were included in our multivariable models.
Statistical Analysis
To examine unadjusted (bivariate) associations, t tests and χ2 tests were used. Survival curves were generated using the Kaplan-Meier method and then compared with the log-rank test. Survival was determined using cause-specific mortality. Cox proportional regression analysis was performed for multivariable analysis of prognostic factors, including age at diagnosis, race, surgery, radiation, grade, and stage. Stage and radiation were stratification variables. Data was analyzed using Stata statistical software.
RESULTS
Patient Demographics
Patient demographics are listed in Table 1. Of the 10,246 patients included in the study, 2,666 patients (26.0%) had signet ring cell carcinoma and 7,580 patients (74.0%) had adenocarcinoma. Signet ring cell carcinoma presented at a younger age (61.9 v 68.7 years; t test P < .001). Both groups were predominantly male; however, a smaller proportion of signet ring cell patients were male (52.7% v 68.7%; χ2 P < .001). Patients with adenocarcinoma were more frequently white (75.1% v 71.4%; χ2 P < .001). Signet ring cell carcinoma patients were more frequently black (11.3% v 10.9%; χ2 P < .001) and Asian/Pacific Islander (16.4% v 13.2%; χ2 P < .001). Patients who reported Hispanic ethnicity were also more frequently represented among signet ring cell carcinoma patients (23.3% v 14.0%; χ2 P < .001).
Table 1.
Demographics of Entire Cohort (N = 10,246)
| Variable | Signet Ring Cell Carcinoma (n = 2,666) |
Adenocarcinoma (n = 7,580) |
P | ||
|---|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | ||
| Age, years | |||||
| Mean | 61.9 | 68.7 | < .001 | ||
| SD | 14.8 | 13.2 | |||
| Men | 1,405 | 52.7 | 5,207 | 68.7 | < .001 |
| Race/ethnicity | |||||
| White | 1,904 | 71.4 | 5,690 | 75.1 | < .001 |
| Black | 302 | 11.3 | 825 | 10.9 | < .001 |
| API* | 436 | 16.4 | 1,003 | 13.2 | < .001 |
| AIA† | 24 | 0.9 | 62 | 0.8 | < .001 |
| Hispanic | 620 | 23.3 | 1,062 | 14.0 | < .001 |
Abbreviation: AIA, American Indian/Alaska Native; API, Asian/Pacific Islander; SD, standard deviation.
We compared characteristics of individuals included to those excluded from this sample using a multivariable logistic regression model. Results indicated that there were no differences between groups on distribution of reported SRC frequency, sex, race, Hispanic ethnicity, tumor grade, tumor stage, or tumor location. Individuals included in the sample were younger than those excluded (mean difference, 3.8 years younger; multivariable Cox HR, 0.99; 95% CI, 0.98 to 0.99; P < .001) and were diagnosed at an earlier date (mean difference, 21 days earlier; multivariable Cox HR, 0.72; 95% CI, 0.66 to 0.78; P < .001). Patients included in the study were more likely to have received surgery (multivariable Cox HR, 3.36; 95% CI, 2.71 to 4.17; P < .001) and were more likely to have received radiation (multivariable Cox HR, 3.12; 95% CI, 2.33 to 4.18; P < .001). Thus, we conclude that our analytic sample broadly represents the demographic and clinical presentation characteristics of all identified gastric adenocarcinomas and signet ring cell carcinomas, with the above caveats. All characteristics on which differences were observed were included in our multivariable models.
Tumor Presentation
Signet ring cell carcinoma was more likely to present at AJCC stage 4 (50.0% v 42.8%; χ2 P < .001) as listed in Table 2. A higher proportion of patients with signet ring cell carcinoma presented with tumor stages T3 (24.4% v 16.7%; χ2 P < .001) or T4 (21.3% v 16.6%; χ2 P < .001). More patients with signet ring cell carcinoma presented with node stages N2 (15.2% v 8.4%; χ2 P < .001) or N3 (8.4% v 2.8%; χ2 P < .001) or with distant metastases (40.2% v 37.6%; χ2 P < .001).
Table 2.
Tumor Characteristics at Presentation
| Variable | Signet Ring Cell Carcinoma |
Adenocarcinoma |
P | ||
|---|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | ||
| AJCC stage | 2,666 | 7,580 | — | ||
| 1A | 317 | 11.9 | 1,366 | 18.0 | < .001 |
| 1B | 249 | 9.3 | 897 | 11.8 | < .001 |
| 2 | 332 | 12.5 | 1,081 | 14.3 | < .001 |
| 3A | 335 | 12.6 | 843 | 11.1 | < .001 |
| 3B | 109 | 4.1 | 148 | 2.0 | < .001 |
| 4 | 1,324 | 50.0 | 3,245 | 42.8 | < .001 |
| Tumor stage | 2,346 | 6,523 | — | ||
| T1 | 510 | 21.7 | 2,098 | 32.2 | < .001 |
| T2a | 189 | 8.1 | 655 | 10.0 | < .001 |
| T2b | 572 | 24.4 | 1,599 | 24.5 | < .001 |
| T3 | 574 | 24.5 | 1,089 | 16.7 | < .001 |
| T4 | 501 | 21.3 | 1,082 | 16.6 | < .001 |
| Node stage | 2,365 | 6,763 | — | ||
| N0 | 962 | 40.3 | 3,258 | 48.2 | < .001 |
| N1 | 870 | 36.1 | 2,743 | 40.6 | < .001 |
| N2 | 351 | 15.2 | 571 | 8.4 | < .001 |
| N3 | 182 | 8.4 | 191 | 2.8 | < .001 |
| Metastases stage | 2,644 | 7,564 | — | ||
| M0 | 1,581 | 59.8 | 4,722 | 62.4 | .018 |
| M1 | 1,063 | 40.2 | 2,842 | 37.6 | .018 |
| Tumor grade | 2,298 | 6,739 | — | ||
| 1 | 7 | 0.3 | 342 | 5.1 | < .001 |
| 2 | 61 | 2.7 | 2,211 | 31.8 | < .001 |
| 3 | 2,150 | 93.6 | 4,060 | 61.3 | < .001 |
| 4 | 80 | 3.4 | 126 | 1.8 | < .001 |
Abbreviation: AJCC, American Joint Committee on Cancer, 6th edition.
Patients with signet ring cell carcinoma were also more likely to present with tumor grades T3 (93.6% v 61.3%; χ2 P < .001) or T4 (3.4% v 1.8%; χ2 P < .001). The distribution of anatomic location of the two cancers is listed in Table 3. Signet ring cell carcinoma was more likely to be found in the middle stomach (30.6% v 20.7%; χ2 P < .001), defined as the body, greater, and lesser curvature, and the lower stomach (30.7% v 24,2%; χ2 P < .001), defined as the antrum or pylorus. Adenocarcinoma was more frequently found in the upper stomach (48.5% v 24.9%; χ2 P < .001), defined as the cardia or fundus. Signet ring cell carcinoma was more common in overlapping locations (13.8% v 6.6%; χ2 P < .001).
Table 3.
Anatomic Location of Tumor in the Stomach
| Variable | Signet Ring Cell Carcinoma (n = 2,666) |
Adenocarcinoma (n = 7,580) |
P | ||
|---|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | ||
| Upper stomach | 665 | 24.9 | 3,680 | 48.5 | < .001 |
| Middle stomach | 816 | 30.6 | 1,569 | 20.7 | < .001 |
| Lower stomach | 818 | 30.7 | 1,828 | 24.2 | < .001 |
| Overlapping | 367 | 13.8 | 503 | 6.6 | < .001 |
Survival
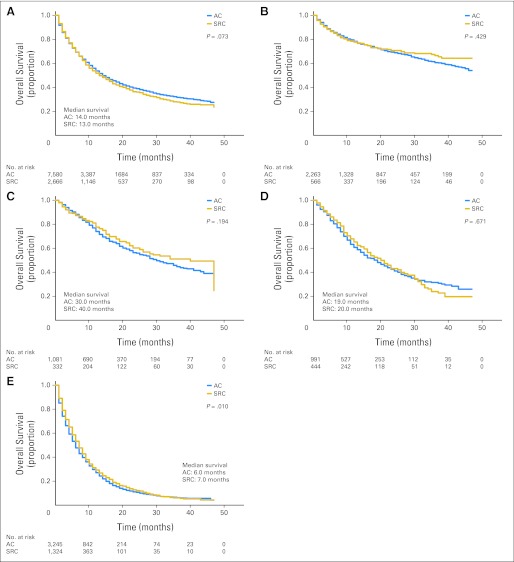
Kaplan-Meier (KM) survival curves are shown in Figure 2. Disease-specific survival for all stages of signet ring cell carcinoma and adenocarcinoma was not significantly different (14.0 v 13.0 months; KM P = .073). Of 7,580 patients with gastric adenocarcinoma, 3,920 patients (51.7%) died. Of 2,666 patients with signet ring cell carcinoma, 1,432 patients (53.7%) died. When comparing stage 1 signet ring cell carcinoma with adenocarcinoma, survival was not significantly different (KM P = .429). Median survival for SRC versus adenocarcinoma was not significantly different for stage 2 (40.0 v 30.0 months; KM P = .194) and stage 3 (20.0 v 19.0 months; KM P = .671) cancers. When comparing stage 4 cancers, signet ring cell carcinoma had longer median survival (7.0 v 6.0 months; KM P = .010).
Fig 2.
Kaplan-Meier survival curves comparing months of survival in gastric adenocarcinoma (AC) and signet ring cell carcinoma (SRC) are shown for (A) all stages, (B) American Joint Committee on Cancer, 6th edition (AJCC) stage 1 tumors, (C) AJCC stage 2 tumors, (D) AJCC stage 3 tumors, and (E) AJCC stage 4 tumors. Median survival for AJCC stage 1 tumors is not shown because more than 50% of patients had censored data for survival.
Fig 1.
CONSORT diagram. AJCC, American Joint Committee on Cancer, 6th edition.
Predictors of Mortality
Unadjusted (bivariate) associations with mortality are listed in Table 4. Signet ring cell carcinoma added no additional risk of mortality (bivariate Cox HR, 1.06; 95% CI, 1.00 to 1.12; P = .070). Factors associated with increased mortality included age at diagnosis (bivariate Cox HR, 1.01; 95% CI, 1.00 to 1.01; P < .001, per year increased age at diagnosis), Alaska/American Indian ethnicity (bivariate Cox HR, 1.33; 95% CI, 1.02 to 1.72; P = .032), Hispanic ethnicity (bivariate Cox HR, 1.09; 95% CI, 1.02 to 1.17; P = .013), increasing AJCC stage, increasing tumor stage, increasing node stage, presence of distant metastases, and increasing tumor grade (Table 4). Unadjusted (bivariate) associations with survival included Asian/Pacific Islander race (bivariate Cox HR, 0.74; 95% CI, 0.69 to 0.81; P < .001), surgical resection (bivariate Cox HR, 0.25; 95% CI, 0.24 to 0.26; P < .001), radiation therapy (bivariate Cox HR, 0.43; 95% CI, 0.37 to 0.49; P < .001), radiation before surgery (bivariate Cox HR, 0.38; 95% CI, 0.35 to 0.41; P < .001), and radiation after surgery (bivariate Cox HR, 0.45; 95% CI, 0.19 to 0.66; P < .001).
Table 4.
Unadjusted Associations With Mortality
| Characteristic | Hazard Ratio | 95% CI | P |
|---|---|---|---|
| Signet ring cell histology | 1.06 | 1.00 to 1.12 | .070 |
| Age at diagnosis | 1.01 | 1.00 to 1.01 | < .001 |
| Female sex | 1.04 | 0.99 to 1.10 | .135 |
| Race/ethnicity, % | |||
| Black | 1.04 | 0.96 to 1.14 | .317 |
| API | 0.74 | 0.69 to 0.81 | < .001 |
| AIA | 1.33 | 1.02 to 1.72 | .032 |
| Hispanic ethnicity | 1.09 | 1.02 to 1.17 | .013 |
| Surgical resection | 0.25 | 0.24 to 0.26 | < .001 |
| AJCC stage | |||
| 1B | 0.98 | 0.85 to 1.13 | .766 |
| 2 | 1.35 | 1.19 to 1.53 | < .001 |
| 3A | 2.13 | 1.88 to 2.41 | < .001 |
| 3B | 1.86 | 1.54 to 2.26 | < .001 |
| 4 | 5.36 | 4.82 to 5.95 | < .001 |
| Tumor stage | |||
| 2A | 0.70 | 0.61 to 0.81 | < .001 |
| 2B | 1.02 | 0.92 to 1.12 | .740 |
| 3 | 1.30 | 1.18 to 1.43 | < .001 |
| 4A | 2.73 | 2.48 to 2.99 | < .001 |
| 4B | 4.11 | 3.73 to 4.54 | < .001 |
| Node stage | |||
| 1 | 1.33 | 1.24 to 1.41 | < .001 |
| 2 | 1.19 | 1.08 to 1.30 | < .001 |
| 3 | 1.63 | 1.45 to 1.84 | < .001 |
| Metastases stage 1 | 4.10 | 3.88 to 4.33 | < .001 |
| Tumor grade | |||
| 2 | 1.55 | 1.28 to 1.89 | < .001 |
| 3 | 2.06 | 1.71 to 2.49 | < .001 |
| 4 | 2.04 | 1.59 to 2.60 | < .001 |
| Radiation therapy administered | 0.43 | 0.37 to 0.49 | < .001 |
| Radiation before surgery | 0.38 | 0.35 to 0.41 | < .001 |
| Radiation after surgery | 0.45 | 0.19 to 0.66 | < .001 |
Abbreviations: AIA, American Indian/Alaska Native; AJCC, American Joint Committee on Cancer, 6th edition; API, Asian/Pacific Islander.
Multivariable results from Cox proportional hazards regression analysis are listed in Table 5. Signet ring cell carcinoma was not an independent predictor of mortality (multivariable Cox HR, 1.05; 95% CI, 0.96 to 1.11; P = .150). Age at diagnosis (multivariable Cox HR, 1.01; 95% CI, 1.01 to 1.02; P < .001), black race (multivariable Cox HR, 1.10; 95% CI, 1.01 to 1.20; P = .026), and increasing tumor grade (Table 4) were independently associated with mortality. Factors independently associated with survival include Asian/Pacific Islander ethnicity (multivariable Cox HR, 0.83; 95% CI, 0.77 to 0.91; P < .001) and surgical resection (multivariable Cox HR, 0.37; 95% CI, 0.34 to 0.39; P < .001).
Table 5.
Multiple Variable Model Predicting Risk of Mortality (stratified by radiation and AJCC stage)
| Characteristic | Hazard Ratio | 95% CI | P |
|---|---|---|---|
| Signet ring cell histology | 1.05 | 0.96 to 1.11 | .150 |
| Age at diagnosis | 1.01 | 1.01 to 1.02 | < .001 |
| Female sex | 1.01 | 0.95 to 1.07 | .790 |
| Race/ethnicity, % | |||
| Black | 1.10 | 1.01 to 1.20 | .026 |
| API | 0.83 | 0.77 to 0.91 | < .001 |
| AIA | 1.01 | 0.73 to 1.39 | .972 |
| Hispanic ethnicity | 1.01 | 0.94 to 1.09 | .847 |
| Surgical resection | 0.37 | 0.34 to 0.39 | < .001 |
| Tumor grade | |||
| 2 | 1.20 | 0.98 to 1.45 | .071 |
| 3 | 1.47 | 1.22 to 1.78 | < .001 |
| 4 | 1.49 | 1.03 to 1.55 | .022 |
Abbreviations: AIA, American Indian/Alaska Native; AJCC, American Joint Committee on Cancer, 6th edition; API, Asian/Pacific Islander.
To determine whether the outcomes of signet ring cell gastric cancer were influenced by tumor location, differences in anatomic location between signet ring cell carcinoma and adenocarcinoma were evaluated in three different ways. First, we estimated models excluding proximal tumors, and the conclusions were identical. Second, we tested interactions between tumor location and signet ring cell carcinoma to assess potential effect modification. All interaction coefficients were nonsignificant. Finally, we tested proportionality of odds for signet ring cell carcinoma by stage, adjusting for location, and there was no evidence that the odds were not proportional. Each of these evaluations offers support that the association between signet ring cell carcinoma and survival is not dependent on anatomic location.
DISCUSSION
Overall survival for gastric cancer in the United States remains poor, with no marked improvement over the last 20 years.9,10 Greater characterization of the various histologic subtypes of gastric cancer in the United States is merited. This is especially true for signet ring cell carcinoma, as few studies have examined its presentation and prognosis in the United States.
Recent studies from Asia4–6,8,11 demonstrate that when adjusting for stage, patients with signet ring cell gastric carcinoma do not have worse outcomes than patients with adenocarcinoma. Gastric cancer in Asia is known to behave differently than gastric cancer in Europe and the United States.12,13 It has a different prognosis and patients do better with extensive lymph node resection.14,15
A study carried out using the California Cancer Registry in 1999 determined that signet ring cell carcinoma did not impact survival in advanced gastric cancer cases. That study found that signet ring cell cancers were more likely to be found in women and in the distal stomach.16 However, a literature review found no similar study has been carried out using a national database in the United States.
Our findings indicate that signet ring cell carcinoma has a distinct presentation when compared with gastric adenocarcinoma. It presents in younger patients. The majority of signet ring cell carcinoma patients are men, and more women have signet ring cell carcinoma than adenocarcinoma. This is similar to what was seen in the study carried out using the California Cancer Registry by Theuer et al,16 and has also been demonstrated in Asian studies.11,17 Signet ring cell gastric carcinoma also has a different ethnic distribution, as it is more common among black, Asian/Pacific Islander, American Indian/Alaska Native, and Hispanic ethnic groups, which has not been described previously.
Signet ring cell carcinoma appears to present at later stages, with a greater proportion of patients presenting at AJCC stage 4, with more advanced TNM stage, and higher tumor grade. Interestingly, studies from Asia have found that signet ring cell gastric cancers do not demonstrate more frequent lymph node metastases than other types of gastric cancer.18 The two tumors also appear to present at different anatomic locations. Whereas adenocarcinoma presents more proximally, signet ring cell was more likely to present in the body or lower stomach. Signet ring cell carcinoma is also more likely to present with an overlapping location. This was also observed in the study by Theuer et al.16
The differences in presentation of signet ring cell carcinoma that we have identified in our study may support an emerging concept that signet ring cell carcinoma might actually be a disease distinct from gastric adenocarcinoma.19,20 Studies have shown that subtypes of gastric cancer with histologic and epidemiologic distinctions can also be distinguished by gene expression data, which suggests that signet ring cell carcinoma may be a completely distinct entity. This gene expression data may allow for a new classification of gastric cancers that improves our understanding of the disease19 and improves our ability to predict prognosis.20 It may also allow for the identification of molecular markers that are unique to each individual gastric subtype.19,20 In addition, it may improve our ability to predict patient response to chemotherapy.20
The primary finding of our study is that signet ring cell carcinoma is not independently associated with mortality, compared with adenocarcinoma when stratifying for radiation and AJCC stage. Signet ring cell carcinoma has long been thought to confer worse prognosis. However, our results indicate that it is not a negative prognostic indicator. Overall median survival did not differ between the two tumor types. Patients with AJCC stage 4 signet ring cell carcinoma had better survival than those with gastric adenocarcinoma (7.0 v 6.0 months). Although this increased survival of 1 month was statistically significant, its clinical significance is of modest magnitude. Of note, the years analyzed in this study correspond to the AJCC staging manual (6th edition).
Interestingly, studies in Asia have reported improved survival with early stages of signet ring cell carcinoma compared with adenocarcinoma,4,6 and relatively worse survival in later stages of the disease.7,21 This could reflect differences in staging systems. In addition, it may indicate that the signet ring cell carcinoma subtype behaves differently in patients in the United States. Such differences in outcomes could also reflect the aggressive screening and resection strategies seen in Asian countries.
In this study, Asian ethnicity was found to bestow a survival advantage to patients with signet ring cell carcinoma and adenocarcinoma. Numerous studies have shown superior survival rates in gastric cancer patients treated in Asian countries compared with those in the United States.22,23 In addition, patients of Asian descent, living in the United States, have higher survival rates than other ethnicities.24 Numerous hypotheses have been proposed to explain these findings, such as differences in tumor biology and more aggressive screening and treatment regimens; however, the reasons are likely multifactorial.25 The survival advantage observed in the Asian population, whether in the United States or in Asia, merits further investigation.
There are some limitations to our study, including those related to retrospective analysis and use of large databases. SEER data are obtained from cancer registries, thus, adding some degree of selection bias. However, SEER is a large, population-based sample that is representative of the United States population, which can reduce the effect of bias and allow examination of the main variables of interest in our study. In our multiple-variable Cox regression model, we did stratify for AJCC stage and receipt of radiation therapy. Future research could extend these analyses using SEER-Medicare data, which would allow further adjustment for chemotherapy and comorbidities.
Finally, one must consider that classification of gastric carcinoma subtypes may differ among pathologists. For this reason, we compared only those cases that have been clearly distinguished as adenocarcinoma to those distinguished as signet ring cell carcinoma. Future research could examine other gastric subtypes, for which histologic diagnosis is not as clearly defined.
In conclusion, our study shows that signet ring cell gastric carcinoma presents with more advanced disease; however, on multivariable analysis it does not independently bestow additional risk of mortality. Although the histologic subtype of signet ring cell carcinoma was once thought to convey additional risk of mortality, our study demonstrates that, stage for stage, signet ring cell carcinoma does not have worse prognosis.
Footnotes
Supported in part by Grants No. R01HD069769, R21CA158877, R01AG13180, and R01CA158361 from the National Institutes of Health and Grants No. PENR-2010-04643 and PENR-2011-04489 from the US Department of Agriculture.
Presented as a poster at the 9th Annual Gastrointestinal Cancers Symposium of the American Society of Clinical Oncology, San Francisco, CA, January 19-21, 2012, and the 65th Annual Cancer Symposium of the Society of Surgical Oncology, Orlando, FL, March 21-24, 2012.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Sharven Taghavi, Alliric I. Willis
Collection and assembly of data: Sharven Taghavi, Senthil N. Jayarajan, Alliric I. Willis
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Watanabe H, Jass JR, Sobin LH, et al. ed 2. Berlin, Germany: Springer; 1990. Histological Typing of Oesophageal and Gastric Tumours: WHO International Histological Classification of Tumours No. 18. [Google Scholar]
- 2.Lauren P. The two histological main types of gastric carcinoma: Diffuse and so-called intestinal-type carcinoma—An attempt at a histo-clinical classification. Acta Pathol Microbiol Scand. 1965;64:31–49. doi: 10.1111/apm.1965.64.1.31. [DOI] [PubMed] [Google Scholar]
- 3.Ribeiro MM, Sarmento JA, Sobrino Simões MA, et al. Prognostic significance of Lauren and Ming classifications and other pathologic parameters in gastric carcinoma. Cancer. 1981;47:780–784. doi: 10.1002/1097-0142(19810215)47:4<780::aid-cncr2820470424>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 4.Hyung WJ, Noh SH, Lee JH, et al. Early gastric carcinoma with signet ring cell histology. Cancer. 2002;94:78–83. doi: 10.1002/cncr.10120. [DOI] [PubMed] [Google Scholar]
- 5.Kim DY, Park YK, Joo JK, et al. Clinicopathological characteristics of signet ring cell carcinoma of the stomach. Asian J Surg. 2004;74:1060–1064. doi: 10.1111/j.1445-1433.2004.03268.x. [DOI] [PubMed] [Google Scholar]
- 6.Kunisaki C, Shimada H, Nomura M, et al. Therapeutic strategy for signet ring cell carcinoma of the stomach. Br J Surg. 2004;91:1319–1324. doi: 10.1002/bjs.4637. [DOI] [PubMed] [Google Scholar]
- 7.Li C, Kim S, Lai JF, et al. Advanced gastric carcinoma with signet ring cell histology. Oncology. 2007;72:64–68. doi: 10.1159/000111096. [DOI] [PubMed] [Google Scholar]
- 8.Zhang M, Zhu G, Zhang H, et al. Clinicopathologic features of gastric carcinoma with signet ring cell histology. J Gastrointest Surg. 2010;14:601–606. doi: 10.1007/s11605-009-1127-9. [DOI] [PubMed] [Google Scholar]
- 9.Lau M, Le AT, El-Serag HB. Noncardia gastric cancer remains an important and deadly cancer in the United States: Secular trends in incidence and survival. Am J Gastroenterol. 2006;101:2485–2492. doi: 10.1111/j.1572-0241.2006.00778.x. [DOI] [PubMed] [Google Scholar]
- 10.Le A, Berger D, Lau M, et al. Secular trends in the use, quality, and outcomes of gastrectomy for noncardia gastric cancer in the United States. Ann Surg Oncol. 2007;14:2519–2527. doi: 10.1245/s10434-007-9386-8. [DOI] [PubMed] [Google Scholar]
- 11.Yokota T, Kunii Y, Teshima S, et al. Signet ring cell carcinoma of the stomach: A clinicopathological comparison with the other histological types. Tohoku J Exp Med. 1998;186:121–130. doi: 10.1620/tjem.186.121. [DOI] [PubMed] [Google Scholar]
- 12.Crew KD, Neugut AI. Epidemiology of gastric cancer. World J Gastroenterol. 2006;12:354–362. doi: 10.3748/wjg.v12.i3.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bonenkamp JJ, Hermans J, Sasako M, et al. Extended lymph-node dissection for gastric cancer. N Engl J Med. 1999;340:908–914. doi: 10.1056/NEJM199903253401202. [DOI] [PubMed] [Google Scholar]
- 14.Brennan MF. Current status of surgery for gastric cancer: A review. Gastric Cancer. 2005;8:64–70. doi: 10.1007/s10120-005-0319-6. [DOI] [PubMed] [Google Scholar]
- 15.Sasako M, Sano T, Yamamoto S, et al. D2 lymphadenectomy alone or with para-aortic nodal dissection for gastric cancer. N Engl J Med. 2008;359:453–462. doi: 10.1056/NEJMoa0707035. [DOI] [PubMed] [Google Scholar]
- 16.Theuer CP, Nastanski F, Brewster WR, et al. Signet ring cell histology is associated with unique clinical features but does not affect gastric cancer survival. Am Surg. 1999;65:915–921. [PubMed] [Google Scholar]
- 17.Maehara Y, Sakaguchi Y, Moriguchi S, et al. Signet ring cell carcinoma of the stomach. Cancer. 1992;69:1645–1650. doi: 10.1002/1097-0142(19920401)69:7<1645::aid-cncr2820690702>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 18.Lee JH, Choi IJ, Kook MC, et al. Risk factors for lymph node metastasis in patients with early gastric cancer and signet ring cell histology. Br J Surg. 2010;97:732–736. doi: 10.1002/bjs.6941. [DOI] [PubMed] [Google Scholar]
- 19.Shah MA, Khanin R, Tang L, et al. Molecular classification of gastric cancer: A new paradigm. Clin Cancer Res. 2011;17:2693–2701. doi: 10.1158/1078-0432.CCR-10-2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tan IB, Ivanova T, Lim KH, et al. Intrinsic subtypes of gastric cancer, based on gene expression pattern, predict survival and respond differently to chemotherapy. Gastroenterology. 2011;141(suppl e1-e11):476–485. doi: 10.1053/j.gastro.2011.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Otsuji E, Yamaguchi T, Sawai K, et al. Characterization of signet ring cell carcinoma of the stomach. J Surg Oncol. 1998;67:216–220. doi: 10.1002/(sici)1096-9098(199804)67:4<216::aid-jso2>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 22.Wanebo HJ, Kennedy BJ, Chmiel J, et al. Cancer of the stomach: A patient care study by the American College of Surgeons. Ann Surg. 1993;218:583–592. doi: 10.1097/00000658-199321850-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maruyama K, Sasako M, Kinoshita T, et al. Surgical treatment for gastric cancer: The Japanese approach. Semin Oncol. 1996;23:360–368. [PubMed] [Google Scholar]
- 24.Theuer CP, Kurosaki T, Ziogas A, et al. Asian patients with gastric carcinoma in the United States exhibit unique clinical features and superior overall and cancer specific survival rates. Cancer. 2000;89:1883–1892. doi: 10.1002/1097-0142(20001101)89:9<1883::aid-cncr3>3.3.co;2-8. [DOI] [PubMed] [Google Scholar]
- 25.Gill S, Shah A, Le N, et al. Asian ethnicity-related differences in gastric cancer presentation and outcome among patients treated at a Canadian cancer center. J Clin Oncol. 2003;21:2070–2076. doi: 10.1200/JCO.2003.11.054. [DOI] [PubMed] [Google Scholar]