Abstract
Purpose
Experimental evidence suggests that anticoagulants (ACs) may inhibit cancer growth and metastasis, but clinical data have been limited. We investigated whether use of ACs was associated with the risk of death from prostate cancer.
Patients and Methods
This study comprised 5,955 men in the Cancer of the Prostate Strategic Urologic Research Endeavor database with localized adenocarcinoma of the prostate treated with radical prostatectomy (RP) or radiotherapy (RT). Of them, 2,175 (37%) were receiving ACs (warfarin, clopidogrel, enoxaparin, and/or aspirin). The risk of prostate cancer–specific mortality (PCSM) was compared between the AC and non-AC groups.
Results
After a median follow-up of 70 months, risk of PCSM was significantly lower in the AC group compared with the non-AC group (3% v 8% at 10 years; P < .01). The risks of disease recurrence and bone metastasis were also significantly lower. In a subgroup analysis by clinical risk category, the reduction in PCSM was most prominent in patients with high-risk disease (4% v 19% at 10 years; P < .01). The benefit from AC was present across treatment modalities (RT or RP). Analysis by type of AC medication suggested that the PCSM reduction was primarily associated with aspirin. Multivariable analysis indicated that aspirin use was independently associated with a lower risk of PCSM (adjusted hazard ratio, 0.43; 95% CI, 0.21 to 0.87; P = .02).
Conclusion
AC therapy, particularly aspirin, was associated with a reduced risk of PCSM in men treated with RT or RP for prostate cancer. The association was most prominent in patients with high-risk disease.
INTRODUCTION
The association between cancer and the coagulation system is widely recognized. Patients with cancer are prone to develop thromboembolism,1,2 and patients diagnosed with idiopathic venous thrombosis are at increased risk of developing cancer.3,4 In addition to the correlative evidence, there are substantial experimental data that suggest that the coagulation system is implicated in multiple cancer pathways, including tumor proliferation, angiogenesis, apoptosis, and metastasis.5–12 Several clinical studies have been conducted to test whether cancer development and treatment outcomes can be modified by medications interfering with the coagulation pathway.13–16 However, perhaps because of the heterogeneity in cancer types and stages in these trials, findings have been conflicting and inconclusive.
For prostate cancer, the potential use of anticoagulants (ACs) for anticancer purposes is of particular interest. Prostate cancer is the most common noncutaneous malignancy in men, and because prostate cancer is typically diagnosed in the elderly, a significant proportion of patients have comorbidities that require AC therapy. It is important to understand whether such therapy can influence disease outcome and the health care costs related to prostate cancer treatment and recurrence. Several epidemiologic and prospective studies have suggested that AC medications reduce the incidence of prostate cancer development.17–19 A recent meta-analysis, which included 10 case-control and 14 cohort studies, also concluded that aspirin had a significant chemopreventive effect on prostate cancer, particularly for advanced prostate cancer.20
In addition to the possible chemopreventive effect on prostate cancer, there may be a therapeutic effect on preexisting prostate cancer as well. In a previous study of patients with prostate cancer treated with radiation therapy (RT), those undergoing AC therapy (warfarin, clopidogrel, or aspirin) demonstrated improved biochemical control rates compared with the men not receiving those medications.21 The rate of distant metastasis was also lower for men receiving ACs. Although suggestive of an antineoplastic effect of these medications, this single-institution study was limited by a relatively small number of patients who were all treated with RT only.
The objective of the current study was to explore the association between ACs and cancer outcome using a multi-institutional registry of men treated for prostate cancer with either RT or radical prostatectomy (RP). The confirmation of such an association in a larger data set would add significantly to the existing body of data and support a case to prospectively test the hypothesis that a fairly well-tolerated medication such as aspirin could improve disease outcomes for men undergoing therapy for prostate cancer.
PATIENTS AND METHODS
Study participants are enrolled in the Cancer of the Prostate Strategic Urologic Research Endeavor (CaPSURE) Study, a longitudinal, observational registry of men with biopsy-proven prostate cancer. Patients have been enrolled from 41 institutions nationwide since 1995 and treated according to the usual practices of their physicians. The database contains diagnostic, clinical, and pathologic information, as well as patient-reported data from questionnaires, administered at diagnosis and at regular intervals. Details of the CaPSURE methodology and database have been previously published.22
Of 13,821 men in the database at the time of analysis, 8,292 men had medication information included in the registry. Of these men, 8,084 had localized adenocarcinoma of the prostate (node-negative, nonmetastatic). A total of 5,955 patients were treated with primary RT or RP. Radiotherapy included brachytherapy, external-beam radiotherapy, or a combination of the two.
The primary end point was prostate cancer–specific mortality (PCSM). The sources for dates and causes of death include state-issued death certificates, National Death Index, and other sources, such as data reports from local study coordinators. Disease recurrence (biochemical failure or receipt of salvage treatment) and bone metastasis were also analyzed. Biochemical failure was defined as prostate-specific antigen (PSA) nadir plus 2 ng/mL for patients treated with RT23 and two consecutive PSAs ≥ 0.2 ng/mL at least 8 weeks after surgery for patients who received RP. For PCSM, both survivors and those who died as a result of other causes were censored. Vital status and/or cause of death data were available for 99% of patients in the current study. Life-table product limit estimates of 7- and 10-year rates were computed for each end point.
The independent variable of interest in this study was the use of AC medications. Patients reported medication use at study entry and at approximately 1-year intervals for a median of four cycles (interquartile range, two to six). Given the often chronic nature of AC use, for univariate two-group comparisons (Figs 1 and 2), we examined AC use as ever versus never usage. Patients were categorized into the AC group if their medication list included warfarin, enoxaparin, clopidogrel, and/or aspirin at baseline or at any time during follow-up. Patients not taking any of these medications were in the reference group.
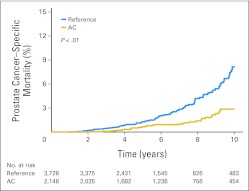
Fig 1.
Prostate cancer–specific mortality (PCSM) by anticoagulant (AC) use. Men undergoing AC therapy had a 10-year PCSM of 3% compared with 8% for men not taking an AC (P < .01 by log-rank test).
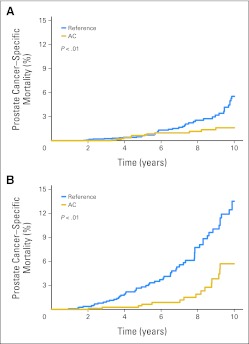
Fig 2.
Prostate cancer–specific mortality (PCSM) by the treatment modality. The improved PCSM in the anticoagulant (AC) group was observed whether patients were treated with (A) radical prostatectomy or (B) radiotherapy.
We also used multivariable Cox proportional hazards regression models and 95% CIs to estimate the hazard ratio (HR) of PCSM associated with AC use. In these models, we examined aspirin and nonaspirin AC use as time-dependent exposure variables and updated use during each individual questionnaire cycle to consider changes in usage over time. To minimize the potential for reverse causation to bias the results, the value for the primary exposure for the last reporting cycle was set to the value of the previous cycle. Because many patients have comorbid conditions that may influence survival, a separate multivariable analysis was included using the Fine & Gray competing risk model. The medication usage status was again considered a time-dependent covariate. Covariables potentially prognostic for PCSM were selected a priori and included in all models. Data were analyzed with SAS 9.1 for Windows software (SAS Institute, Cary, NC) and STATA/IC 11.2 for Windows (Statacorp, College Station, TX).
RESULTS
Patient Characteristics
The study included 5,955 men who were treated with either RP (n = 4,028, 68%) or RT (n = 1,927, 32%). Among them, 2,175 (37%) were identified as AC users. The majority of the patients reported their medication usage within the first 2 years (58%), with the median time to report of 14.0 months. The distribution of the medication type is shown in Table 1. Aspirin was the most commonly used AC (84%), followed by warfarin, clopidogrel, and enoxaparin. Four hundred thirty-nine patients (20%) were treated with multiple AC medications, mostly aspirin and another AC medication. The median age was 64 years (range, 39 to 86 years). The proportions of patients with low-, intermediate-, and high-risk disease were 42%, 36%, and 22%, respectively, according to the National Comprehensive Cancer Network criteria.24 Androgen deprivation therapy was administered in 27% of patients treated with RT, typically consisting of short-term androgen deprivation therapy (≤ 6 months).
Table 1.
Distribution of Anticoagulant Medication Types (n = 2,175)
| Medication | No. | % |
|---|---|---|
| Aspirin | 1,817 | 84 |
| Warfarin | 461 | 21 |
| Clopidogrel | 309 | 14 |
| Enoxaparin | 27 | 1 |
| Combination | 439 | 20 |
| No combination | 1,736 | 80 |
Patient, disease, and treatment characteristics stratified by the medication groups are shown in Table 2. The two groups had similar distributions of clinical T stage and Gleason scores. Although mean PSA was slightly lower in the AC group (8.4 v 9.5 ng/mL; P < .01), median values were similar (5.9 v 6.0 ng/mL). Fewer men receiving ACs presented with high-risk disease (19% v 23%; P < .01). Men on AC were slightly older (66 v 63 years; P < .01) and had longer median follow-up (79 v 63 months; P < .01). More patients were treated with RT in the AC group than in the reference group (37% v 30%; P < .01).
Table 2.
Patient, Disease, and Treatment Characteristics (n = 5,955)
| Characteristics | AC |
Reference |
P | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| No. of patients | 2,175 | 37 | 3,780 | 63 | |
| Age, years | < .01 | ||||
| Median | 66 | 63 | |||
| Range | 42-86 | 39-85 | |||
| Follow-up time, months | < .01 | ||||
| Median | 79.0 | 63.0 | |||
| Range | 2.0-330.0 | 1.0-352.0 | |||
| Gleason score, n = 5,676 | .24 | ||||
| 2-6 | 1,430 | 69 | 2,389 | 67 | |
| 7 | 507 | 24 | 912 | 25 | |
| 8+ | 149 | 7 | 289 | 8 | |
| Initial PSA, ng/mL | < .01 | ||||
| Median | 5.9 | 6.0 | |||
| Interquartile range | 4.5-8.7 | 4.5-9.4 | |||
| Clinical T stage, n = 5,659 | .08 | ||||
| T1-T2a | 1,498 | 72 | 2,578 | 72 | |
| T2b-c | 535 | 26 | 904 | 25 | |
| T3a-T4 | 40 | 2 | 104 | 3 | |
| Risk category, n = 5,533 | < .01 | ||||
| Low | 857 | 42 | 1,464 | 42 | |
| Intermediate | 782 | 39 | 1,229 | 35 | |
| High | 395 | 19 | 806 | 23 | |
| Treatment modality | < .01 | ||||
| RT + no ADT | 566 | 26 | 833 | 22 | |
| RT + ADT ≤ 6 months | 165 | 8 | 228 | 6 | |
| RT + ADT > 6 months | 68 | 3 | 67 | 2 | |
| RP | 1,376 | 63 | 2,652 | 70 | |
| Salvage therapy | .12 | ||||
| None | 1,821 | 84 | 3,099 | 82 | |
| RT | 78 | 4 | 129 | 3 | |
| Other | 276 | 13 | 552 | 15 | |
Abbreviations: AC, anticoagulant; ADT, androgen deprivation therapy; PSA, prostate-specific antigen; RP, radical prostatectomy; RT, radiotherapy.
After a median follow-up time of 70 months, 779 patients died and had cause of death documented. Among them 193 (25%) died of prostate cancer and 586 (75%) died of other causes. Within the 779 deceased patients, most of the patients with documented metastasis died of prostate cancer (92 dead of prostate cancer among 119 with metastases).
Comparison of Unadjusted Outcomes by Anticoagulant Use
When PCSM was compared between the two groups, the AC group had a significantly lower risk of PCSM. The 7- and 10-year actuarial PCSM was 1% and 3% in the AC group, compared with 3% and 8% in the reference group (log-rank P < .01). Kaplan-Meier curves for PCSM are shown in Figure 1. The use of AC was also associated with lower risks of disease recurrence and bone metastasis. The risk of disease recurrence was 24% and 28% at 7 and 10 years in the AC group, as compared with 28% and 36% in the reference group (P < .01). Similarly, the risk of bone metastasis was lower in the AC group (7- and 10-year risk of 1% and 3% compared with 3% and 6%, P < .01).
Analysis of Prostate Cancer–Specific Mortality by Subgroups
To identify a group of patients who may potentially benefit the most from AC therapy, an exploratory subgroup analysis was performed. When the entire cohort was divided into three risk categories, the difference in PCSM between the AC and reference groups was most prominent in patients with high-risk disease (10-year PCSM 4% v 19%; log-rank P < .01). Men with intermediate-risk disease had a modest difference in PCSM with AC (3% v 6%; P = .01). The difference was not statistically significant in low-risk patients (10-year PCSM of 2% v 4%, P = .12). When patients were analyzed by treatment modality (RP or RT), the improvement in PCSM in the AC group was observed in both treatment modalities (Fig 2).
Next, we analyzed the types of AC medications for their associations with PCSM. As shown in Table 3, those taking aspirin, either alone or with another AC medication, had a significantly lower PCSM, compared with the reference group. For those on nonaspirin AC medications (AC, no aspirin), the difference in PCSM was not as prominent. These results suggested that aspirin may offer more benefit than other AC medications.
Table 3.
Unadjusted Rates for PCSM by Type of Medication (n = 5,872)
| Medication Type | 7 Year (%) | 10 Year (%) | HR | 95% CI | P |
|---|---|---|---|---|---|
| None, n = 3,726 | 3 | 8 | 1.00 | — | |
| AC, no aspirin, n = 350 | 2 | 6 | 0.56 | 0.31 to 1.03 | .06 |
| Aspirin, n = 1,796 | 1 | 2 | 0.28 | 0.19 to 0.41 | < .01 |
Abbreviations: AC, anticoagulant; HR, hazard ratio; PCSM, prostate cancer–specific mortality.
Multivariable Analysis of Anticoagulant Use and Prostate Cancer–Specific Mortality
The association between AC medications and PCSM was further examined in a Cox proportional hazards regression model with covariates of aspirin use, nonaspirin AC use, initial PSA, Gleason score, T stage, and treatment modality (Table 4). The multivariable analysis confirmed that use of aspirin was independently associated with lower PCSM (HR, 0.43; 95% CI, 0.21 to 0.87). The use of other nonaspirin AC medications was not significantly associated with PCSM. Gleason score, clinical T stage, and treatment modality were also significantly associated with PCSM.
Table 4.
Multivariable HRs for AC Use and Prostate Cancer–Specific Mortality Using Cox Proportional Hazards Regression
| Covariate | Parameter | HR | 95% CI | P |
|---|---|---|---|---|
| Aspirin* | Yes v no (referent) | 0.43 | 0.21 to 0.87 | .02 |
| AC, no aspirin* | Yes v no (referent) | 1.30 | 0.55 to 3.06 | .55 |
| Initial PSA (log) | Per ng/mL | 1.28 | 0.96 to 1.70 | .10 |
| Gleason score | 2-6 (referent) | 1.00 | — | |
| 7 | 2.53 | 1.43 to 4.48 | < .01 | |
| 8-10 | 5.89 | 3.23 to 10.7 | < .01 | |
| Clinical T stage | T1-T2a (referent) | 1.00 | — | |
| T2b-c | 3.20 | 1.91 to 5.35 | < .01 | |
| T3a-T4 | 4.25 | 1.76 to 10.2 | < .01 | |
| Treatment modality | RP (referent) | 1.00 | — | |
| RT alone | 2.12 | 1.27 to 3.53 | < .01 | |
| RT + ADT | 0.97 | 0.44 to 2.12 | .93 |
Abbreviations: AC, anticoagulant; ADT, androgen deprivation therapy; HR, hazard ratio; PSA, prostate-specific antigen; RP, radical prostatectomy; RT, radiotherapy.
Updated as time-dependent variables.
Cumulative incidence analysis was also performed for PCSM, treating death from other causes as a competing risk (Appendix Table A1, online only). Fine and Gray's competing risk regression model continued to demonstrate a significant inverse association between aspirin use and PCSM (P < .01).
DISCUSSION
Medications that interfere with the coagulation pathway, or ACs, are frequently used among patients with prostate cancer, as these patients are often elderly and have cardiovascular comorbidities. Understanding the potential interaction between prostate cancer and such commonly prescribed medications is of significant interest.
We analyzed a large registry of 5,955 patients with prostate cancer to investigate the potential antineoplastic effect of ACs. When PCSM was compared between the group taking AC medications and those who were not, PCSM was significantly reduced in the AC group (10-year PCSM of 3% v 8%; P < .01), particularly in those with high-risk disease (10-year PCSM of 4% v 19%; P < .01). When different types of AC medications were analyzed, the reduction in PCSM was primarily associated with the use of aspirin, more than other AC medications. In multivariable analysis, use of aspirin was significantly associated with improved PCSM, independently of other prognostic factors.
Our findings corroborate and strengthen the hypothesis that aspirin may have chemopreventive and antineoplastic effects. The existing evidence is most convincing for colorectal cancer,25–27 but prostate cancer has been implicated as well.28 For example, a large cohort study of 146,113 patients showed that daily use of aspirin was associated with a reduced incidence of any malignancy, and specifically, prostate cancer among men.29 In addition to the chemopreventive effect on prostate cancer development, our results suggest that aspirin may have an antineoplastic effect on preexisting cancer.
The potential mechanism responsible for this finding is not clear. Carcinogenesis is a complex process involving initial transformation, progression, and metastasis.30 Abundant preclinical evidence suggests that the coagulation pathway plays a role in many of the steps during this process.7–12 For example, in metastasis, as cancer cells spread hematogenously, they are surrounded by platelets, which may promote survival and colonization at distant sites.31 Studies in mice have shown that deficiency in platelet aggregation substantially attenuates metastasis.5,6 Aspirin effectively reduces platelet aggregation, and this may confer protection against metastasis. Intriguingly, our study showed that the reduction in PCSM was most prominent among patients with high-risk disease, who have the highest risk of developing metastasis. Moreover, the rate of bone metastasis was significantly lower in patients taking anticoagulants. Similar to our results, a recent study in patients with breast cancer showed a reduction in risk of distant recurrence and breast cancer deaths with aspirin use.32 Elucidating the underlying biology and mechanism will help guide optimal aspirin use in the clinic. For example, there are currently conflicting data with regard to the timing of aspirin use and whether its use early versus late in carcinogenesis has a differential effect on prostate cancer outcomes.33,34 Further studies are warranted.
Although the findings from this study may potentially affect many patients diagnosed with prostate cancer, there are several caveats that need to be considered. First, it is possible that men receiving aspirin or other ACs had more significant comorbidities, and this competing mortality lowered the chance to observe PCSM in this group. In an attempt to address this possibility, cumulative incidence analysis was performed, and the association between the use of aspirin and PCSM remained significant. On the other hand, patients who live longer may have a greater chance to start on AC medications, which could lead to an overestimation of the effect of these medications on PCSM. We used time-dependent covariate analysis to minimize this potential bias. In addition, because of the observational nature of this study, unforeseen interactions with other variables may have contributed to the results. For example, it has been suggested that statins might be associated with improved biochemical control after RT in prostate cancer,35 and statins are commonly prescribed together with anticoagulants (1,459 of 2,175 patients in the AC group of the current study). Of note, when the use of a statin was included in the multivariable analysis, aspirin use continued to be associated with a lower risk of PCSM (Appendix Table A2, online only). Finally, in this study, the dosage, duration, and timing of aspirin use were not addressed in detail. The optimal usage of aspirin, as well as the potential toxicity, should be addressed in a prospective study.
In a large cohort of men with prostate cancer treated with radiation or surgery, use of aspirin was significantly associated with a reduction in PCSM. A randomized comparison would be valuable to corroborate this association and justify a routine recommendation of aspirin in patients with prostate cancer.
Appendix
Table A1.
Multivariable HRs for AC Use and Prostate Cancer–Specific Mortality Using Fine and Gray's Competing Risks Model
| Covariate | Parameter | HR | 95% CI | P |
|---|---|---|---|---|
| Aspirin* | Yes v no (referent) | 0.14 | 0.05 to 0.44 | < .01 |
| AC, no aspirin* | Yes v no (referent) | 0.40 | 0.12 to 1.35 | .14 |
| Initial PSA (log) | Per ng/mL | 1.72 | 1.38 to 2.14 | < .01 |
| Gleason score | 2-6 (referent) | 1.00 | — | |
| 7 | 1.31 | 0.81 to 2.11 | .28 | |
| 8-10 | 4.78 | 2.96 to 7.73 | < .01 | |
| Clinical T stage | T1-T2a (referent) | 1.00 | — | |
| T2b-c | 2.52 | 1.69 to 3.76 | < .01 | |
| T3a-T4 | 3.64 | 1.70 to 7.79 | < .01 | |
| Treatment modality | RP (referent) | 1.00 | — | |
| RT alone | 2.11 | 1.40 to 3.18 | < .01 | |
| RT + ADT | 1.12 | 0.62 to 2.00 | .71 |
Abbreviations: AC, anticoagulant; ADT, androgen deprivation therapy; HR, hazard ratio; PSA, prostate-specific antigen; RP, radical prostatectomy; RT, radiotherapy.
Updated as time-dependent variables.
Table A2.
Multivariable HRs for AC Use and Prostate Cancer–Specific Mortality by Fine and Gray's Competing Risks Model With Adjustment for Statin Use
| Covariate | Parameter | HR | 95% CI | P |
|---|---|---|---|---|
| Aspirin* | Yes v no (referent) | 0.18 | 0.06 to 0.57 | < .01 |
| AC, no aspirin* | Yes v no (referent) | 0.49 | 0.15 to 1.64 | .25 |
| Statin* | Yes v no (referent) | 0.43 | 0.22 to 0.83 | .01 |
| Initial PSA (log) | Per ng/mL | 1.62 | 1.29 to 2.02 | < .01 |
| Gleason score | 2-6 (referent) | 1.00 | — | |
| 7 | 1.34 | 0.82 to 2.18 | .24 | |
| 8-10 | 4.70 | 2.90 to 7.61 | < .01 | |
| Clinical T stage | T1-T2a (referent) | 1.00 | — | |
| T2b-c | 2.45 | 1.64 to 3.66 | < .01 | |
| T3a-T4 | 3.41 | 1.58 to 7.37 | < .01 | |
| Treatment modality | RP (referent) | 1.00 | — | |
| RT alone | 2.12 | 1.41 to 3.19 | < .01 | |
| RT + ADT | 1.13 | 0.63 to 2.02 | .69 |
Abbreviations: AC, anticoagulant; ADT, androgen deprivation therapy; HR, hazard ratio; PSA, prostate-specific antigen; RP, radical prostatectomy; RT, radiotherapy.
Updated as time-dependent variables.
Footnotes
The Cancer of the Prostate Strategic Urologic Research Endeavor (CaPSURE) is supported by an unrestricted educational gift from Abbott Laboratories (Chicago, IL), by the National Institutes of Health/National Cancer Institute (Grant No. 5RC1CA146596), and by the Agency for Healthcare Research and Quality (Grant No. 1U01CA88160).
Presented in part at the 52nd Annual Meeting of the American Society for Therapeutic Radiology and Oncology, October 31-November 4, 2010, San Diego, CA.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Kevin S. Choe, Janet E. Cowan, Peter R. Carroll, Anthony V. D'Amico, Stanley L. Liauw
Administrative support: Peter R. Carroll
Collection and assembly of data: Janet E. Cowan, Peter R. Carroll
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Heit JA, Silverstein MD, Mohr DN, et al. Risk factors for deep vein thrombosis and pulmonary embolism: A population-based case-control study. Arch Intern Med. 2000;160:809–815. doi: 10.1001/archinte.160.6.809. [DOI] [PubMed] [Google Scholar]
- 2.White RH, Chew HK, Zhou H, et al. Incidence of venous thromboembolism in the year before the diagnosis of cancer in 528,693 adults. Arch Intern Med. 2005;165:1782–1787. doi: 10.1001/archinte.165.15.1782. [DOI] [PubMed] [Google Scholar]
- 3.Prandoni P, Lensing AW, Büller HR, et al. Deep-vein thrombosis and the incidence of subsequent symptomatic cancer. N Engl J Med. 1992;327:1128–1133. doi: 10.1056/NEJM199210153271604. [DOI] [PubMed] [Google Scholar]
- 4.Sørensen HT, Mellemkjaer L, Steffensen FH, et al. The risk of a diagnosis of cancer after primary deep venous thrombosis or pulmonary embolism. N Engl J Med. 1998;338:1169–1173. doi: 10.1056/NEJM199804233381701. [DOI] [PubMed] [Google Scholar]
- 5.Bakewell SJ, Nestor P, Prasad S, et al. Platelet and osteoclast beta3 integrins are critical for bone metastasis. Proc Natl Acad Sci U S A. 2003;100:14205–14210. doi: 10.1073/pnas.2234372100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Camerer E, Qazi AA, Duong DN, et al. Platelets, protease-activated receptors, and fibrinogen in hematogenous metastasis. Blood. 2004;104:397–401. doi: 10.1182/blood-2004-02-0434. [DOI] [PubMed] [Google Scholar]
- 7.Chan TA, Morin PJ, Vogelstein B, et al. Mechanisms underlying nonsteroidal antiinflammatory drug-mediated apoptosis. Proc Natl Acad Sci U S A. 1998;95:681–686. doi: 10.1073/pnas.95.2.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Falanga A, Marchetti M, Vignoli A, et al. Clotting mechanisms and cancer: Implications in thrombus formation and tumor progression. Clin Adv Hematol Oncol. 2003;1:673–678. [PubMed] [Google Scholar]
- 9.Folkman J, Langer R, Linhardt RJ, et al. Angiogenesis inhibition and tumor regression caused by heparin or a heparin fragment in the presence of cortisone. Science. 1983;221:719–725. doi: 10.1126/science.6192498. [DOI] [PubMed] [Google Scholar]
- 10.Hejna M, Raderer M, Zielinski CC. Inhibition of metastases by anticoagulants. J Natl Cancer Inst. 1999;91:22–36. doi: 10.1093/jnci/91.1.22. [DOI] [PubMed] [Google Scholar]
- 11.Mahdi JG, Alkarrawi MA, Mahdi AJ, et al. Calcium salicylate-mediated apoptosis in human HT-1080 fibrosarcoma cells. Cell Prolif. 2006;39:249–260. doi: 10.1111/j.1365-2184.2006.00390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCulloch P, George WD. Warfarin inhibition of metastasis: The role of anticoagulation. Br J Surg. 1987;74:879–883. doi: 10.1002/bjs.1800741005. [DOI] [PubMed] [Google Scholar]
- 13.Bosetti C, Gallus S, La Vecchia C. Aspirin and cancer risk: A summary review to 2007. Recent Results Cancer Res. 2009;181:231–251. doi: 10.1007/978-3-540-69297-3_22. [DOI] [PubMed] [Google Scholar]
- 14.Maurer LH, Herndon JE, 2nd, Hollis DR, et al. Randomized trial of chemotherapy and radiation therapy with or without warfarin for limited-stage small-cell lung cancer: A Cancer and Leukemia Group B study. J Clin Oncol. 1997;15:3378–3387. doi: 10.1200/JCO.1997.15.11.3378. [DOI] [PubMed] [Google Scholar]
- 15.Thun MJ, Namboodiri MM, Heath CW., Jr Aspirin use and reduced risk of fatal colon cancer. N Engl J Med. 1991;325:1593–1596. doi: 10.1056/NEJM199112053252301. [DOI] [PubMed] [Google Scholar]
- 16.Zacharski LR, Henderson WG, Rickles FR, et al. Effect of warfarin anticoagulation on survival in carcinoma of the lung, colon, head and neck, and prostate: Final report of VA Cooperative Study #75. Cancer. 1984;53:2046–2052. doi: 10.1002/1097-0142(19840515)53:10<2046::aid-cncr2820531007>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 17.Dasgupta K, Di Cesar D, Ghosn J, et al. Association between nonsteroidal anti-inflammatory drugs and prostate cancer occurrence. Cancer J. 2006;12:130–135. [PubMed] [Google Scholar]
- 18.Habel LA, Zhao W, Stanford JL. Daily aspirin use and prostate cancer risk in a large, multiracial cohort in the US. Cancer Causes Control. 2002;13:427–434. doi: 10.1023/a:1015788502099. [DOI] [PubMed] [Google Scholar]
- 19.Jacobs EJ, Rodriguez C, Mondul AM, et al. A large cohort study of aspirin and other nonsteroidal anti-inflammatory drugs and prostate cancer incidence. J Natl Cancer Inst. 2005;97:975–980. doi: 10.1093/jnci/dji173. [DOI] [PubMed] [Google Scholar]
- 20.Mahmud SM, Franco EL, Aprikian AG. Use of nonsteroidal anti-inflammatory drugs and prostate cancer risk: A meta-analysis. Int J Cancer. 2010;127:1680–1691. doi: 10.1002/ijc.25186. [DOI] [PubMed] [Google Scholar]
- 21.Choe KS, Correa D, Jani AB, et al. The use of anticoagulants improves biochemical control of localized prostate cancer treated with radiotherapy. Cancer. 2010;116:1820–1826. doi: 10.1002/cncr.24890. [DOI] [PubMed] [Google Scholar]
- 22.Lubeck DP, Litwin MS, Henning JM, et al. The CaPSURE database: A methodology for clinical practice and research in prostate cancer—CaPSURE Research Panel. Cancer of the Prostate Strategic Urologic Research Endeavor. Urology. 1996;48:773–777. doi: 10.1016/s0090-4295(96)00226-9. [DOI] [PubMed] [Google Scholar]
- 23.Roach M, 3rd, Hanks G, Thames H, Jr, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: Recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 2006;65:965–974. doi: 10.1016/j.ijrobp.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 24.Kawachi MH, Bahnson RR, Barry M, et al. NCCN clinical practice guidelines in oncology: Prostate cancer early detection. J Natl Compr Canc Netw. 2010;8:240–262. doi: 10.6004/jnccn.2010.0016. [DOI] [PubMed] [Google Scholar]
- 25.Baron JA, Cole BF, Sandler RS, et al. A randomized trial of aspirin to prevent colorectal adenomas. N Engl J Med. 2003;348:891–899. doi: 10.1056/NEJMoa021735. [DOI] [PubMed] [Google Scholar]
- 26.Chan AT, Giovannucci EL, Meyerhardt JA, et al. Long-term use of aspirin and nonsteroidal anti-inflammatory drugs and risk of colorectal cancer. JAMA. 2005;294:914–923. doi: 10.1001/jama.294.8.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sandler RS, Halabi S, Baron JA, et al. A randomized trial of aspirin to prevent colorectal adenomas in patients with previous colorectal cancer. N Engl J Med. 2003;348:883–890. doi: 10.1056/NEJMoa021633. [DOI] [PubMed] [Google Scholar]
- 28.Cuzick J, Otto F, Baron JA, et al. Aspirin and non-steroidal anti-inflammatory drugs for cancer prevention: An international consensus statement. Lancet Oncol. 2009;10:501–507. doi: 10.1016/S1470-2045(09)70035-X. [DOI] [PubMed] [Google Scholar]
- 29.Jacobs EJ, Thun MJ, Bain EB, et al. A large cohort study of long-term daily use of adult-strength aspirin and cancer incidence. J Natl Cancer Inst. 2007;99:608–615. doi: 10.1093/jnci/djk132. [DOI] [PubMed] [Google Scholar]
- 30.Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 31.Uluçkan O, Eagleton MC, Floyd DH, et al. APT102, a novel adpase, cooperates with aspirin to disrupt bone metastasis in mice. J Cell Biochem. 2008;104:1311–1323. doi: 10.1002/jcb.21709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holmes MD, Chen WY, Li L, et al. Aspirin intake and survival after breast cancer. J Clin Oncol. 2010;28:1467–1472. doi: 10.1200/JCO.2009.22.7918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dhillon PK, Kenfield SA, Stampfer MJ, et al. Long-term aspirin use and the risk of total, high-grade, regionally advanced and lethal prostate cancer in a prospective cohort of health professionals, 1988-2006. Int J Cancer. 2011;128:2444–2452. doi: 10.1002/ijc.25811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dhillon PK, Kenfield S, Stampfer MJ, et al. Aspirin use after a prostate cancer diagnosis and cancer survival in a prospective cohort. Cancer Prev Res. doi: 10.1158/1940-6207.CAPR-12-0171. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gutt R, Tonlaar N, Kunnavakkam R, et al. Statin use and risk of prostate cancer recurrence in men treated with radiation therapy. J Clin Oncol. 2010;28:2653–2659. doi: 10.1200/JCO.2009.27.3003. [DOI] [PubMed] [Google Scholar]