Abstract
Significant progress has been made in stem cell biology, regenerative medicine, and stem cell-based tissue engineering. Such scientific strides highlight the potential of replacing or repairing damaged tissues in congenital abnormalities, diseases, or injuries, as well as constructing functional tissue or organs in vivo. Since mesenchymal stem cells (MSCs) are capable of differentiating into bone-forming cells, they constitute an appropriate cell source to repair damaged bone tissues. In addition, the immunoregulatory property of MSCs provides a foundation for their use in treating a variety of autoimmune diseases. However, the interaction between MSCs and immune cells in cell-based tissue regeneration is largely unknown. In this review, we will discuss the current understanding of MSC-based tissue regeneration, emphasizing the role of the immune microenvironment in bone regeneration.
Introduction
Tissue engineering is commonly defined as “an interdisciplinary field that applies the principles of engineering and life sciences toward the development of biological substitutes that restore, maintain, or improve tissue function or a whole organ” (Langer et al., 1993). Tissue engineering involves donor cells, synthetic materials (scaffolds), soluble or bound growth factors, and recipient microenvironments. Experimental regenerative medicine is currently investigating virtually every type of tissue and organ within the human body. Pilot studies in a variety of systems, such as urethral, bladder, blood vessel, and tracheal replacement, showed great prospects for cell-based tissue regeneration, either in clinics or laboratories (Bianco et al., 2001; Zandonella 2003; Laflamme et al., 2011; Koike et al., 2004).
Despite the significant demand for repairing severe bone defects caused by congenital malformations, oncologic resection, pathologic degenerative bone destruction, and post-traumatic loss, current therapeutic approaches have often resulted in unsatisfactory clinical outcomes. Therefore, newly developed biotechniques, such as MSC-based bone tissue engineering, should be investigated to overcome current clinical challenges. For MSC-based bone regeneration, the most popular cell source is derived from bone marrow MSCs (BMMSCs). However, the quality and quantity of MSC-mediated bone regeneration may not meet clinical needs, essentially because the mechanisms underlying MSC-mediated tissue regeneration are not fully understood. Furthermore, we still do not understand how donor cells interact with recipient immune cells in vivo, making it difficult to significantly improve MSC-based tissue regeneration. In this review, we will discuss the current knowledge of MSC-based tissue regeneration, emphasizing the crosstalk between MSCs and receipt immune cells, with the aims of 1) highlighting the critical role of the recipient immune system to MSC-based tissue regeneration and 2) providing appropriate approaches to rigorously assess engineered tissue function.
Stem Cell Property of MSCs
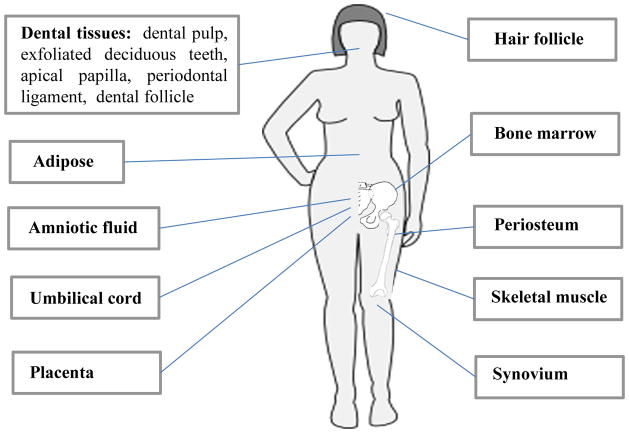
MSCs can be derived from a variety of tissues, including amniotic fluid, bone marrow, adipose and dental tissues, etc. (Fig. 1). Each type of tissue-specific MSC possesses advantage and disadvantage biological characteristics as listed in Table 1. BMMSCs were reported to be nonhematopoietic multipotent stem cells and an adherent fibroblast-like population capable of differentiating into osteogenic cells (Friedenstein et al., 1970). MSCs have been confirmed to express CD73, CD90, CD146, CD105, Stro-1, stem cell antigen-1 (Sca-1), octamer-binding transcription factor-4 (Oct-4), pericyte-associated antigen (3G5) and whereas in the absence of CD34, CD45, CD14 or CD11b, CD79a, or CD19 surface molecules (Dominici et al., 2006; Shi et al., 2006). MSCs are also capable of differentiating into both mesenchymal and nonmesenchymal cell types, including, for example, osteoblasts, adipocytes and chondrocytes (Bianco et al., 2001; Friedenstein et al., 1974; Owen et al., 1988; Pittenger et al., 1999; Prockop,1997).
Figure 1.
Possible sources of mesenchymal stem cells for tissue engineering
Table 1.
Advantages and disadvantages of different MSC sources for tissue engineering.
| Source | Advantages | Disadvantages | References |
|---|---|---|---|
| Adipose | Rich source, easy isolation and expansion | Trauma during harvesting; limited differentiation potentials | Locke et al., 2011 |
| Amniotic Fluid | Broadly multipotent | Limited accessibility | De Coppi et al., 2007 |
| Bone marrow | As a rich resource; broadly multipotent and well characterized | Trauma during harvesting | |
| Dental tissues | Easy isolation and expansion; broadly multipotent; ideal for orofacial tissues | Some dental stem cells show limited accessibility | Zheng et al., 2009 |
| Hair Follicle | Easy accessibility, isolation and expansion | Limited differentiation potentials | Peng et al., 2011 |
| Periosteum | Committed osteogenic differentiation | Trauma during harvesting; limited differentiation potentials | Ferretti et al., 2012 |
| Skeletal muscle | Rich source | Trauma during harvesting; limited differentiation potentials | Chen et al., 2012 |
| Synovium | Superiority in cartilage formation | Limited accessibility; limited differentiation potentials | Jones et al., 2012 |
| Umbilical cord and placenta | Highly proliferative, broad differentiation potentials including myogenic | Limited accessibility | Kadam et al., 2012 |
MSCs are generally considered to be poorly immunogenic. Immunological characterization of MSCs revealed that they showed only intermediate expression levels of major histocompatibility complex (MHC) class I and no, or very low, expression of MHC class II antigen and co-stimulatory molecules CD40, CD80 and CD86 (Le Blanc et al., 2003; Zhang et al., 2009; Majumdar et al., 2003). Expression of MHC class I prevented MSCs from behaving like natural killer cells, whereas the absence of co-stimulatory molecules causes a state of anergy in T cells (Ryan et al., 2005; Nauta et al., 2007). Several studies indicated neither differentiated nor undifferentiated MSCs elicit proliferation of allogeneic lymphocytes (Li et al., 2005; Le Blanc, 2003; Djouad et al., 2003). To date, a variety of preclinical and clinical studies have shown that exogenously added autologous and allogenic MSCs could give rise to the generation of new bone and bone-associated tissues to replace damaged and diseased tissues by assisting the regenerative capacities of endogenous cells in the affected areas (García-Gómez et al., 2010; Tasso et al., 2010; Bueno et al., 2009).
Regeneration property of MSCs
There is a great deal of scientific and clinical interest in the potential of using MSC to regeneration the damaged tissues, including bone, skin and cartilage, cardiac, etc (Quarto, et al., 2001; Bianco et al., 2001; Fuster et al., 2001). The most popular cell types include bone marrow-derived MSC, adipose tissue-derived MSCs, peripheral blood MSCs; MSCs from perioplacenta and umbilical cord blood. MSC-based regeneration was associated with following functional properties (Keating et al., 2012; Wu et al., 2007; Battiwalla et al., 2012; Rodríguez et al., 2012): (1) their capacity to differentiate into several cell lineages; (2) their ability to secrete soluble factors which regulate crucial biological functions, such as proliferation and differentiation over a broad spectrum of target cells; and (3) their ability to home to damaged tissues. Based on these properties MSCs are being exploited worldwide for a wide range of potential clinical applications. Until now, MSCs are being used in many clinical trials as therapeutic agents. The major clinical trials for the use of MSC as regenerative medicine purpose are summarized in Table 2 and 3. Although it is indisputable that MSC-based regeneration has offered promising clinical outcome, unstable therapeutic effects and uncleared mechanism limited the advanced application of MSCs in tissue regeneration.
Table 2.
A summary of the major clinical trials for the use of MSC as regenerative medicine and therapy for autoimmune diseases
| Disease/Target tissues or organs | Methods of MSC administration | References |
|---|---|---|
| Bone defects/tibia. humerus and ulna | Autologous BMMSC+ hydroxyapatite Scaffolds | Quarto et al., 2001 |
| Skin defects/cutaneous wounds | Autologous BMMSC+ fibrin | Falanga et al., 2007 |
| Cartilage defects/trachea | Autologous MSC and epithelial cells + acellular trachea matrix | Macchiarini et al., 2008 |
| Cardio vascular structures/heart valves | Autologous umbilical cord MSC + porous polymer | Sodian et al., 2006 |
| Urinary structures/bladder | Autologous bladder cells + collagen scaffold | Atala et al., 2006 |
| Graft versus host disease (GVHD)/gut and liver | Systemic infusion of BMMSC | Le Blanc et al., 2004 |
| Systemic lupus erythematosus (SLE)/bone and kidney | Systemic infusion of BMMSC or SHED | Sun et al., 2009; Yamaza et al., 2010 |
| Systemic sclerosis (SS)/skin | Systemic infusion of BMMSC | Akiyama et al., 2012 |
Table 3.
Current distribution of clinical trials using MSCs (clinicaltrials.gov)
| Study | Clinical Trials | Autologous/Allogeneic | Completed | Recruiting | Other Status |
|---|---|---|---|---|---|
| Phase I | 139 | 23/116 | 32 | 61 | 46 |
| Phase II | 160 | 27/133 | 24 | 74 | 62 |
| Phase III | 12 | 2/10 | 4 | 5 | 3 |
| Phase IV | 0 | 0 | 0 | 0 | 0 |
Immunomodulatory property of MSCs
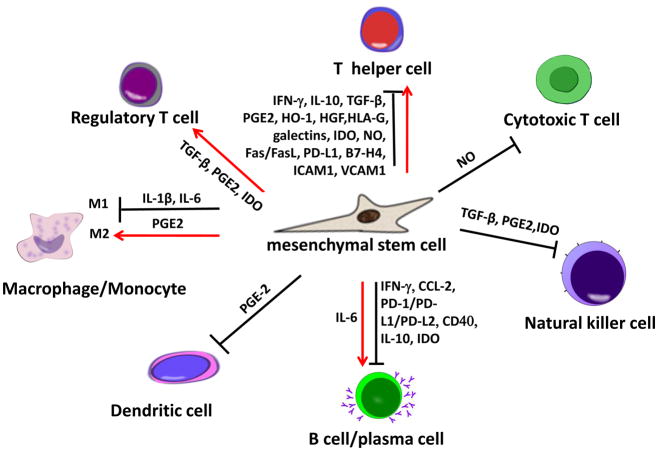
It is well known that MSCs have immunosuppressive and immunomodulatory properties in vitro and in vivo. Most in vitro studies showed that MSCs were able to interact with almost all subsets of lymphocytes, including T cells, B cells, natural killer cells, monocyte/macrophages, dendritic cells, and neutrophils (Fig 2) (Krampera et al., 2003; Corcione et al., 2006; Jiang et al., 2005). MSCs could efficiently suppress the proliferation of Th1 and Th17 cells (Di Nicola et al., 2002; Krampera et al., 2006), as well as the production of interferon γ (IFN-γ) by Th1 cells and interleukin-17 (IL17) by Th17 cells, whereas MSCs could enhance IL-4 secretion by Th2 cells (Aggarwal et al., 2005; Zhao et al., 2010). MSCs have been demonstrated to inhibit cytotoxic T lymphocyte (CTL) formation, thereby downregulating CTL-mediated cytotoxicity. MSCs have also been reported to directly or indirectly induce the proliferation of Tregs and promote their immunomodulatory capacity (Aggarwal et al., 2005; Di Ianni et al., 2008). Moreover, MSCs inhibit B cell proliferation, differentiation and antibody secretion in both in vitro coculture system and such animal models as multiple sclerosis mice (Asari et al., 2009; Augello et al., 2005; Corcione et al., 2006; Gerdoni et al., 2007). Additionally, MSCs are capable of inhibiting proliferation, cytokine production, and cytotoxic activity of both resting and preactivated NK cells (Sotiropoulou et al., 2006; Spaggiari et al., 2006; Spaggiari et al., 2008). Although the immunomodulatory mechanism of MSCs remains largely unknown, several soluble factors, or cell contact-dependent mediators, have been proved to play an important role (Aggarwal et al., 2005; Ren et al., 2008; Sheng et al., 2008; Yang et al., 2009; Selmani et al., 2008, Choi, H. et al., 2011; Chiesa et al., 2011; Du Rocher et al., 2012; Giuliani et al., 2011; Jia et al., 2012; Maby-El Hajjami et al., 2009; Nicolaidou et al., 2012; Qu et al., 2012; Sato et al., 2007; Schena et al., 2010; Spaggiari et al., 2009; Tabera et al., 2008) (Fig 2). Recently, our group demonstrates that the transfusion of bone marrow MSCs induces apoptosis in T cell via the Fas/Fas ligand pathway as a novel mechanism for MSC-mediated immune tolerance and immune therapies (Akiyama et al., 2012). Indeed, MSCs express Fas to control their secretion of monocyte chemotactic protein 1 (MCP-1), which attracts T cell migration, facilitating Fas ligand-mediated apoptosis of T cells by MSCs in a cell-cell contact manner. In systemic sclerosis (SS) mouse models and dextran-sulfate-sodium-induced experimental colitis, the apoptotic T cells induced by MSC infusion trigger macrophages to produce TGFβ, leading to a Treg upregulation-associated immune tolerance and eventually ameliorates disease phenotype, respectively (Akiyama et al., 2012). Moreover, a newly identified immunoregulatory property of MSCs provides a foundation for the clinical treatment of a variety of autoimmune diseases, such as acute graft-versus-host disease (GVHD), encephalomyelitis (EAE), multiple sclerosis and systemic lupus (SLE) (Sun et al., 2009; English et al., 2010). The major clinical trials for using MSCs to treat autoimmune diseases are summarized in Table 2 and 3. Interestingly, our group demonstrated that allogenic MSCs effectively ameliorated disease activity in patients with SLE (Sun et al., 2009), whereas another group showed that autologous MSC infusion failed to ameliorate disease activity in SLE patients (Carrion et al., 2010). This may be attributed to diseased-induced impairment of bone marrow MSCs, as observed in SLE patients and SLE-like MRL/lpr mice (Sun et al., 2009). Therefore, health status of the donor from whom MSC are derived may be crucial for cell-based therapies.
Figure 2.
A summary of the immunomodulatory property of MSCs
Crosstalk between Immune Cells and MSCs
Several studies indicated that priming by inflammatory cytokines is essential for MSC-mediated immunosuppression. That is, no immunosuppression was observed unless MSCs were pretreated with interferon (IFN)-γ, together with TNF-α or IL-1 (Ren et al., 2009; Meisel et al., 2004;Polchert et al., 2008; Mougiakakos et al., 2011; Djouad et al., 2003; Chan et al., 2006). MSCs can participate in antigen presentation if exposed to a narrow window of low levels of IFN-γ through upregulation of MHC-II, whereas high concentrations of IFN-γ and other inflammatory factors, such as TGF-β, suppress MHC-II expression. MHC molecules can be upregulated by IFN-γ treatment (Tang et al., 2008; Romieu-Mourez et al., 2007; Rasmusson et al., 2007). Pretreatment with IFN-γ increased the expression level of MHC class I molecules in MSCs, but failed to restore CTL-mediated killing response (Romieu-Mourez et al., 2007; Zhang et al., 2004), suggesting that MSCs inhibit, but are not the target of, CTL activity. Recent experimental evidence further revealed that stimulated NK cells could efficiently lyse autologous and allogenic MSCs (Crop et al., 2011; Poggi et al., 2006; Chan et al., 2008). Activating NK cell receptors NKp30, NKG2D, and DNAM-1 contributed to NK cell-mediated cytotoxicity against MSCs. IFN-γ-exposed MSCs were less susceptible to NK cell lysis as a consequence of the upregulation of MHC class I molecules at the MSC surface (Chan et al., 2008). Additional studies revealed that IFN-γ-stimulated MSCs present exogenous antigens through MHC class II molecules, resulting in the activation of CD4+ T cells and implying that MSCs may be similar to APCs by possessing antigen presenting function (Stagg et al., 2006; François et al., 2009). MSCs were also able to cross-present exogenous antigens, leading to the induction of CD8+ T-cell proliferation (Liotta et al., 2008). Further studies showed that MSCs express high levels of Toll-like receptors (TLR) 3 and 4. TLR-mediated signaling resulted in the production of proinflammatory mediators, such as IL-1β, IL-6, and IL-8 (Aksu et al., 2008; Spaggiari et al., 2006). In addition, MSCs support the proliferation and stimulation of antibody secretion in B cells (Rasmusson et al., 2007; Traggiai et al., 2008).
Local inflammatory microenvironment affects MSC-based tissue regeneration
It was recently reported that the host immune system, especially T lymphocytes, could affect MSC-mediated bone regeneration (Liu et al., 2011). When bone marrow MSCs were implanted subcutaneously using hydroxyapatite tricalcium phosphate as a carrier, autologous MSCs failed to regenerate bone in C57BL6 mice. However, both human and mouse bone marrow MSCs can form bone and bone-associated hematopoietic marrow components in immunocompromised mice. These data suggest that the recipient immune system may play an inhibitory role in regulating MSC-based tissue regeneration. Further study confirmed that Pan T, CD4+ or CD4+CD25− T cell infusion totally blocked MSC-mediated bone formation and that CD8+ T cells partially blocked MSC-mediated bone formation in immunocompromised mice. However, administration of CD4+CD25+Foxp3+ regulatory T cells (Treg cells) had no inhibitory effect on MSC-mediated bone formation (Liu et al., 2011). It has been reported that interleukin-2 (IL-2)–activated natural killer (NK) cells and CD3/CD28-activated T cells can induce MSC apoptosis through the Fas–Fas ligand (FasL) pathway (Yamaza et al., 2008; Kogianni et al., 2004). In addition, T cells can induce MSC and osteoblast apoptosis through the CD40-CD40L pathway, as observed in some models of bone-related diseases (Hess et al., 1996; Li et al., 2011; Ahuja et al., 2003; Schrum et al., 2003).
Increase in the concentrations of such inflammatory factors as IFN-γ and TNF-α is also negatively correlated with the function of MSCs (Suzawa et al., 2003). A high concentration of IFN-γ inhibits osteogenic differentiation of implanted MSCs by inducing upregulation of smad 6, thereby inhibiting Runt-related transcription factor 2 (Runx2), a key transcription factor associated with osteoblast differentiation. TNF-α is able to induce MSC apoptosis in a dose-dependent manner. However, the combination of IFN-γ and TNF-α significantly accelerates MSC apoptosis through internalization of Fas receptor, which is a death receptor known as tumor necrosis factor receptor superfamily member 6, with reduction of the antiapoptotic factors nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), X-linked inhibitor of apoptosis protein (XIAP), and FLICE-like inhibitory protein (FLIP) (Liu et al., 2011). Therefore, the crosstalk between implanted donor MSCs and recipient immune cells plays a key role in determining the success of MSC-mediated bone regeneration.
MSC-based bone regeneration is regulated by recipient T cells
Since the recipient immune system plays a critical role in MSC-based tissue engineering, it is reasonable to assume that MSC-based tissue regeneration could be improved by modulating recipient T cells. Indeed, reduction of Th1 cytokines IFN-γand TNF-α by systemic infusion of Foxp3+ regulatory T cells, a subpopulation of T cells capable of inducing immune tolerance and ameliorating autoimmune disorders (Aggarwal et al., 2005; Di Ianni et al., 2008; Selmani et al., 2008), markedly improved MSC-based bone regeneration and calvarial defect repair in C57BL/6 mice (Liu et al., 2011). Furthermore, systemically infused MSCs are able to directly or indirectly upregulate Foxp3+ regulatory T cells and home to injury and diseased sites, which may contribute to the tissue repair process (Suzawa et al., 2005; Horwitz et al., 2002; Herrera et al., 2004). The contribution of MSCs to tissue repair is also mediated by secretion of paracrine factors with angiogenic and antiapoptotic properties (Rüster et al., 2006; Kinnaird et al., 2004; Kinnaird et al., 2004). These paracrine factors not only attract endothelial cells and macrophages, but they are also likely to stimulate resident stem/progenitor cells to facilitate the process for tissue repair (Chen et al., 2008; Nakanishi et al., 2008). Therefore, systemic MSC infusion may improve cell-based tissue regeneration through upregulating Tregs, homing to injured sites, and modifying the microenvironment by paracrine factors. Moreover, site-specific pharmacological administration, such as using aspirin, was capable of improving MSC-based bone regeneration and calvarial defect repair via inhibition of the Th1 cytokines IFN-γ and TNF-α (Liu et al., 2011). The therapeutic effects of aspirin in preclinical tests and clinical trials for improving fracture healing may be the focus of future studies.
Conclusions
Recent progress in stem cell biology and tissue engineering suggests that MSC-based tissue regeneration may have an expanded clinical applicability in the future and may represent a viable therapeutic option for those who would benefit from the life-extending benefits of tissue replacement or repair. However, there are still several important issues linked to clinical use of MSCs. Since the health status of the donor from whom MSC are derived varies, it is important to establish effective and reliable protocols to characterize donor MSCs prior to clinical application. Moreover, it will be interesting to clarify the possible role of MSCs in promoting immunosuppression when they are locally implanted, and how these effects can be counterbalanced in order to maintain the homeostasis of the recipients. Properly understanding the characteristics of MSCs and the relationship between host immune system and donor MSCs will provide a foundation for improving the therapeutic effect on MSC-based tissue regeneration.
Acknowledgments
Some studies reported in this manuscript were supported by grants from National Institute of Dental and Craniofacial Research, National Institutes of Health, Department of Health and Human Services (R01DE017449 and R01 DE019932 to S.S.)
Footnotes
Author Disclosure Statement
No competing financial interests exist.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- Ahuja SS, Zhao S, Bellido T, Plotkin LI, Jimenez F, Bonewald LF. CD40 ligand blocks apoptosis induced by tumor necrosis factorα, glucocorticoids, and etoposide in osteoblasts and the osteocyte-like cell line murine long bone osteocyte-Y4. Endocrinology. 2003;144(5):1761–1769. doi: 10.1210/en.2002-221136. [DOI] [PubMed] [Google Scholar]
- Akiyama K, Chen C, Wang D, Xu X, Qu C, Yamaza T, et al. Mesenchymal-stem-cell-induced immunoregulation involves FAS-ligand-/FAS-mediated T cell apoptosis. Cell Stem Cell. 2012;10(5):544–555. doi: 10.1016/j.stem.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aksu AE, Horibe E, Sacks J, Ikeguchi R, Breitinger J, Scozio M, et al. Co-infusion of donor bone marrow with host mesenchymal stem cells treats GVHD and promotes vascularized skin allograft survival in rats. Clin Immunol. 2008;127(3):348–358. doi: 10.1016/j.clim.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Asari S, Itakura S, Ferreri K, Liu CP, Kuroda Y, Kandeel F, et al. Mesenchymal stem cells suppress B-cell terminal differentiation. Exp Hematol. 2009;37(5):604–615. doi: 10.1016/j.exphem.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atala A, Bauer SB, Soker S, Yoo JJ, Retik AB. Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet. 2006;367(9518):1241–6. doi: 10.1016/S0140-6736(06)68438-9. [DOI] [PubMed] [Google Scholar]
- Augello A, Tasso R, Negrini SM, Amateis A, Indiveri F, Cancedda R, et al. Bone marrow mesenchymal progenitor cells inhibit lymphocyte proliferation by activation of the programmed death 1 pathway. Eur J Immunol. 2005;35(5):1482–1490. doi: 10.1002/eji.200425405. [DOI] [PubMed] [Google Scholar]
- Battiwalla M, Hematti P. Mesenchymal stem cells in hematopoietic stem cell transplantation. Cytotherapy. 2009;11(5):503–515. doi: 10.1080/14653240903193806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco P, Robey PG. Stem cells in tissue engineering. Nature. 2001;414(6859):118–121. doi: 10.1038/35102181. [DOI] [PubMed] [Google Scholar]
- Bueno EM, Glowacki J. Cell-free and cell-based approaches for bone regeneration. Nat Rev Rheumatol. 2009;5:685–697. doi: 10.1038/nrrheum.2009.228. [DOI] [PubMed] [Google Scholar]
- Bianco P, Riminucci M, Gronthos S, Robey PG. Bone marrow stromal stem cells: nature, biology, and potential applications. Stem Cells. 2001;19:180–192. doi: 10.1634/stemcells.19-3-180. [DOI] [PubMed] [Google Scholar]
- Carrion F, Nova E, Ruiz C, Diaz F, Inostroza C, Rojo D, et al. Autologous mesenchymal stem cell treatment increased T regulatory cells with no effect on disease activity in two systemic lupus erythematosus patients. Lupus. 2010;19(3):317–322. doi: 10.1177/0961203309348983. [DOI] [PubMed] [Google Scholar]
- Chan WK, Lau AS, Li JC, Law HK, Lau YL, Chan GC. MHC expression kinetics and immunogenicity of mesenchymal stromal cells after short-term IFN-gamma challenge. Exp Hematol. 2008;36(11):1545–1555. doi: 10.1016/j.exphem.2008.06.008. [DOI] [PubMed] [Google Scholar]
- Chan JL, Tang KC, Patel AP, Bonilla LM, Pierobon N, Ponzio NM, et al. Antigen-presenting property of mesenchymal stem cells occurs during a narrow window at low levels of interferon gamma. Blood. 2006;107(12):4817–4824. doi: 10.1182/blood-2006-01-0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B, Wang B, Zhang WJ, Zhou G, Cao Y, Liu W. In vivo tendon engineering with skeletal muscle derived cells in a mouse model. Biomaterials. 2012 Jun 4; doi: 10.1016/j.biomaterials.2012.05.022. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Chen L, Tredget EE, Wu PY, Wu Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS ONE. 2008;3(4):e1886. doi: 10.1371/journal.pone.0001886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Xiang LX, Shao JZ, Pan RL, Wang YX, Dong XJ, et al. Recruitment of endogenous bone marrow mesenchymal stem cells towards injured liver. J Cell Mol Med. 2010;14(6B):1494–1508. doi: 10.1111/j.1582-4934.2009.00912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiesa S, Morbelli S, Morando S, Massollo M, Marini C, Bertoni A, et al. Mesenchymal stem cells impair in vivo T-cell priming by dendritic cells. Proc Natl Acad Sci U S A. 2011;108(42):17384–17389. doi: 10.1073/pnas.1103650108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi H, Lee RH, Bazhanov N, Oh JY, Prockop DJ. Anti-inflammatory protein TSG-6 secreted by activated MSCs attenuates zymosan-induced mouse peritonitis by decreasing TLR2/NF-κB signaling in resident macrophages. Blood. 2011;118(2):330–338. doi: 10.1182/blood-2010-12-327353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcione A, Benvenuto F, Ferretti E, Giunti D, Cappiello V, Cazzanti F, et al. Human mesenchymal stem cells modulate B cell functions. Blood. 2006;107(1):367–372. doi: 10.1182/blood-2005-07-2657. [DOI] [PubMed] [Google Scholar]
- Crop MJ, Korevaar SS, de Kuiper R, Ijzermans JN, van Besouw NM, Baan CC, et al. Human mesenchymal stem cells are susceptible to lysis by CD8+ T-cells and NK cells. Cell Transplant. 2011 Mar 7; doi: 10.3727/096368910X564076. [DOI] [PubMed] [Google Scholar]
- De Coppi P, Bartsch G, Jr, Siddiqui MM, Xu T, Santos CC, Perin L, Mostoslavsky G, Serre AC, Snyder EY, Yoo JJ, Furth ME, Soker S, Atala A. Isolation of amniotic stem cell lines with potential for therapy. Nat Biotechnol. 2007;25(1):100–6. doi: 10.1038/nbt1274. [DOI] [PubMed] [Google Scholar]
- Di Ianni M, Del Papa B, De Ioanni M, Moretti L, Bonifacio E, Cecchini D, et al. Mesenchymal cells recruit and regulate T regulatory cells. Exp Hematol. 2008;36(3):309–318. doi: 10.1016/j.exphem.2007.11.007. [DOI] [PubMed] [Google Scholar]
- Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, et al. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99(10):3838–3843. doi: 10.1182/blood.v99.10.3838. [DOI] [PubMed] [Google Scholar]
- Djouad F, Plence P, Bony C, Tropel P, Apparailly F, Sany J, et al. Immunosuppressive effect of mesenchymal stem cells favors tumor growth in allogeneic animals. Blood. 2003;102(10):3837–3844. doi: 10.1182/blood-2003-04-1193. [DOI] [PubMed] [Google Scholar]
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- Du Rocher B, Mencalha AL, Gomes BE, Abdelhay E. Mesenchymal stromal cells impair the differentiation of CD14(++) CD16(−) CD64(+) classical monocytes into CD14(++) CD16(+) CD64(++) activate monocytes. Cytotherapy. 2012;14(1):12–25. doi: 10.3109/14653249.2011.594792. [DOI] [PubMed] [Google Scholar]
- Eming SA, Krieg T, Davidson JM. Inflammation in wound repair: molecular and cellular mechanisms. J Invest Dermatol. 2007;127(3):514–525. doi: 10.1038/sj.jid.5700701. [DOI] [PubMed] [Google Scholar]
- English K, French A, Wood KJ. Mesenchymal stromal cells: facilitators of successful transplantation? Cell Stem Cell. 2010;7:431–442. doi: 10.1016/j.stem.2010.09.009. [DOI] [PubMed] [Google Scholar]
- Falanga V, Iwamoto S, Chartier M, Yufit T, Butmarc J, Kouttab N, Shrayer D, Carson P. Autologous bone marrow-derived cultured mesenchymal stem cells delivered in a fibrin spray accelerate healing in murine and human cutaneous wounds. Tissue Eng. 2007;13(6):1299–312. doi: 10.1089/ten.2006.0278. [DOI] [PubMed] [Google Scholar]
- Ferretti C, Borsari V, Falconi M, Gigante A, Lazzarini R, Fini M, Di Primio R, Mattioli-Belmonte M. Human periosteum-derived stem cells for tissue engineering applications: the role of VEGF. Stem Cell Rev. 2012 May 24; doi: 10.1007/s12015-012-9374-7. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970;3(4):393–403. doi: 10.1111/j.1365-2184.1970.tb00347.x. [DOI] [PubMed] [Google Scholar]
- Friedenstein AJ, Chailakhyan RK, Latsinik NV, Panasyuk AF, Keiliss-Borok IV. Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues. Cloning in vitro and retransplantation in vivo. Transplantation. 1974;17:331–340. doi: 10.1097/00007890-197404000-00001. [DOI] [PubMed] [Google Scholar]
- García-Gómez I, Elvira G, Zapata AG, Lamana ML, Ramírez M, Castro JG, et al. Mesenchymal stem cells: biological properties and clinical applications. Expert Opin Biol Ther. 2010;10:1453–1468. doi: 10.1517/14712598.2010.519333. [DOI] [PubMed] [Google Scholar]
- Gerdoni E, Gallo B, Casazza S, Musio S, Bonanni I, Pedemonte E, et al. Mesenchymal stem cells effectively modulate pathogenic immune response in experimental autoimmune encephalomyelitis. Ann Neurol. 2007;61(3):219–227. doi: 10.1002/ana.21076. [DOI] [PubMed] [Google Scholar]
- Giuliani M, Oudrhiri N, Noman ZM, Vernochet A, Chouaib S, Azzarone B, et al. Human mesenchymal stem cells derived from induced pluripotent stem cells down-regulate NK-cell cytolytic machinery. Blood. 2011;118(12):3254–3262. doi: 10.1182/blood-2010-12-325324. [DOI] [PubMed] [Google Scholar]
- Herrera MB, Bussolati B, Bruno S, Fonsato V, Romanazzi GM, Camussi G. Mesenchymal stem cells contribute to the renal repair of acute tubular epithelial injury. Int J Mol Med. 2004;14(6):1035–1041. [PubMed] [Google Scholar]
- Hess S, Engelmann H. A novel function of CD40: induction of cell death in transformed cells. J Exp Med. 1996;183(1):159–167. doi: 10.1084/jem.183.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz EM, Gordon PL, Koo WK, Marx JC, Neel MD, McNall RY, et al. Isolated allogeneic bone marrow-derived mesenchymal stem cells engraft and stimulate growth in children with osteogenesis imperfecta: implications for cell therapy of bone. Proc Natl Acad Sci USA. 2002;99(13):8932–8937. doi: 10.1073/pnas.132252399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang XX, Zhang Y, Liu B, Zhang SX, Wu Y, Yu XD, et al. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood. 2005;105(10):4120–4126. doi: 10.1182/blood-2004-02-0586. [DOI] [PubMed] [Google Scholar]
- Jia Z, Jiao C, Zhao S, Li X, Ren X, Zhang L, et al. Immunomodulatory effects of mesenchymal stem cells in a rat corneal allograft rejection model. Exp Eye Res. 2012 Jul 16; doi: 10.1016/j.exer.2012.06.008. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Jones BA, Pei M. Synovium-Derived Stem Cells: A Tissue-Specific Stem Cell for Cartilage Engineering and Regeneration. Tissue Eng Part B Rev. 2012 Apr 19; doi: 10.1089/ten.TEB.2012.0002. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Kadam S, Govindasamy V, Bhonde R. Generation of functional islets from human umbilical cord and placenta derived mesenchymal stem cells. Methods Mol Biol. 2012;879:291–313. doi: 10.1007/978-1-61779-815-3_17. [DOI] [PubMed] [Google Scholar]
- Keating A. Mesenchymal stromal cells: new directions. Cell Stem Cell. 2012;10(6):709–716. doi: 10.1016/j.stem.2012.05.015. [DOI] [PubMed] [Google Scholar]
- Kinnaird T, Stabile E, Burnett MS, Shou M, Lee CW, Barr S, et al. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation. 2004;109(12):1543–1549. doi: 10.1161/01.CIR.0000124062.31102.57. [DOI] [PubMed] [Google Scholar]
- Kinnaird T, Stabile E, Burnett MS, Lee CW, Barr S, Fuchs S, et al. Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms. Circ Res. 2004;94(5):678–685. doi: 10.1161/01.RES.0000118601.37875.AC. [DOI] [PubMed] [Google Scholar]
- Kogianni G, Mann V, Ebetino F, Nuttall M, Nijweide P, Simpson H, et al. Fas/CD95 is associated with glucocorticoid-induced osteocyte apoptosis. Life Sci. 2004;75(24):2879–2895. doi: 10.1016/j.lfs.2004.04.048. [DOI] [PubMed] [Google Scholar]
- Koike N, Fukumura D, Gralla O, Au P, Schechner JS, Jain RK. Tissue engineering: creation of long-lasting blood vessels. Nature. 2004;428(6979):138–139. doi: 10.1038/428138a. [DOI] [PubMed] [Google Scholar]
- Krampera M, Cosmi L, Angeli R, Pasini A, Liotta F, Andreini A, et al. Role for interferon-gamma in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells. 2006;24(2):386–398. doi: 10.1634/stemcells.2005-0008. [DOI] [PubMed] [Google Scholar]
- Krampera M, Glennie S, Dyson J, Scott D, Laylor R, Simpson E, et al. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood. 2003;101:3722–3729. doi: 10.1182/blood-2002-07-2104. [DOI] [PubMed] [Google Scholar]
- Krysko DV, Denecker G, Festjens N, Gabriels S, Parthoens E, D’Herde K, et al. Macrophages use different internalization mechanisms to clear apoptotic and necrotic cells. Cell Death Differ. 2006;13(12):2011–2022. doi: 10.1038/sj.cdd.4401900. [DOI] [PubMed] [Google Scholar]
- François M, Romieu-Mourez R, Stock-Martineau S, Boivin MN, Bramson JL, Galipeau J. Mesenchymal stromal cells cross-present soluble exogenous antigens as part of their antigen-presenting cell properties. Blood. 2009;114(13):2632–2638. doi: 10.1182/blood-2009-02-207795. [DOI] [PubMed] [Google Scholar]
- Fuster V, Sanz J. Gene therapy and stem cell therapy for cardiovascular diseases today: a model for translational research. Nat Clin Pract Cardiovasc Med. 2007;4 (Suppl 1):S1–S8. doi: 10.1038/ncpcardio0737. [DOI] [PubMed] [Google Scholar]
- Laflamme MA, Murry CE. Heart regeneration. Nature. 2011;19,473(7347):326–335. doi: 10.1038/nature10147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer R, Vacanti JP. Tissue engineering. Science. 1993;260(5110):920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- Le Blanc K. Immunomodulatory effects of fetal and adult mesenchymal stem cells. Cytotherapy. 2003;5(6):485–489. doi: 10.1080/14653240310003611. [DOI] [PubMed] [Google Scholar]
- Le Blanc K, Tammik C, Rosendahl K, Zetterberg E, Ringdén O. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp Hematol. 2003;31(10):890–896. doi: 10.1016/s0301-472x(03)00110-3. [DOI] [PubMed] [Google Scholar]
- Li CD, Zhang WY, Li HL, Jiang XX, Zhang Y, Tang PH, et al. Mesenchymal stem cells derived from human placenta suppress allogeneic umbilical cord blood lymphocyte proliferation. Cell Res. 2005;15(7):539–547. doi: 10.1038/sj.cr.7290323. [DOI] [PubMed] [Google Scholar]
- Li JY, Tawfeek H, Bedi B, Yang X, Adams J, Gao KY, et al. Ovariectomy disregulates osteoblast and osteoclast formation through the T-cell receptor CD40 ligand. Proc Natl Acad Sci USA. 2011;108(2):768–773. doi: 10.1073/pnas.1013492108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Xu Q, Peng H, Cheng R, Sun Z, Ye Z. IFN-g enhances HOS and U2OS cell lines susceptibility to γδ T cell–mediated killing through the Fas/Fas ligand pathway. Int Immunopharmacol. 2011;11(4):496–503. doi: 10.1016/j.intimp.2011.01.001. [DOI] [PubMed] [Google Scholar]
- Liotta F, Angeli R, Cosmi L, Filì L, Manuelli C, Frosali F, et al. Toll-like receptors 3 and 4 are expressed by human bone marrow-derived mesenchymal stem cells and can inhibit their T-cell modulatory activity by impairing Notch signaling. Stem Cells. 2008;26(1):279–289. doi: 10.1634/stemcells.2007-0454. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wang L, Kikuiri T, Akiyama K, Chen C, Xu X, et al. Mesenchymal stem cell-based tissue regeneration is governed by recipient T lymphocytes via IFN-γ and TNF-α. Nat Med. 2011;17(12):1594–1601. doi: 10.1038/nm.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locke M, Feisst V, Dunbar PR. Concise review: human adipose-derived stem cells: separating promise from clinical need. Stem Cells. 2011;29(3):404–11. doi: 10.1002/stem.593. [DOI] [PubMed] [Google Scholar]
- Luster AD, Alon R, von Andrian UH. Immune cell migration in inflammation: present and future therapeutic targets. Nat Immunol. 2005;6(12):1182–1190. doi: 10.1038/ni1275. [DOI] [PubMed] [Google Scholar]
- Maby-El Hajjami H, Amé-Thomas P, Pangault C, Tribut O, DeVos J, Jean R, et al. Functional alteration of the lymphoma stromal cell niche by the cytokine context: role of indoleamine-2,3 dioxygenase. Cancer Res. 2009;69(7):3228–3237. doi: 10.1158/0008-5472.CAN-08-3000. [DOI] [PubMed] [Google Scholar]
- Macchiarini P, Jungebluth P, Go T, et al. Clinical transplantation of a tissue-engineered airway. Lancet. 2008;372:2023–30. doi: 10.1016/S0140-6736(08)61598-6. [DOI] [PubMed] [Google Scholar]
- Majumdar MK, Keane-Moore M, Buyaner D, Hardy WB, Moorman MA, McIntosh KR, et al. Characterization and functionality of cell surface molecules on human mesenchymal stem cells. J Biomed Sci. 2003;10:228–241. doi: 10.1007/BF02256058. [DOI] [PubMed] [Google Scholar]
- Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- Meisel R, Zibert A, Laryea M, Göbel U, Däubener W, Dilloo D. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood. 2004;103(12):4619–4621. doi: 10.1182/blood-2003-11-3909. [DOI] [PubMed] [Google Scholar]
- Mougiakakos D, Jitschin R, Johansson CC, Okita R, Kiessling R, Le Blanc K. The impact of inflammatory licensing on heme oxygenase-1-mediated induction of regulatory T cells by human mesenchymal stem cells. Blood. 2011;117(18):4826–4835. doi: 10.1182/blood-2010-12-324038. [DOI] [PubMed] [Google Scholar]
- Nakanishi C, Yamagishi M, Yamahara K, Hagino I, Mori H, Sawa Y, et al. Activation of cardiac progenitor cells through paracrine effects of mesenchymal stem cells. Biochem Biophys Res Commun. 2008;374(1):11–16. doi: 10.1016/j.bbrc.2008.06.074. [DOI] [PubMed] [Google Scholar]
- Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stem cells. Blood. 2007;110:3499–506. doi: 10.1182/blood-2007-02-069716. [DOI] [PubMed] [Google Scholar]
- Nicolaidou V, Wong MM, Redpath AN, Ersek A, Baban DF, Williams LM, et al. Monocytes induce STAT3 activation in human mesenchymal stem cells to promote osteoblast formation. PLoS ONE. 2012;7(7):e39871. doi: 10.1371/journal.pone.0039871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen M, Friedenstein AJ. Stromal stem cells: marrow-derived osteogenic precursors. Ciba Found Symp. 1988;136:42–60. doi: 10.1002/9780470513637.ch4. [DOI] [PubMed] [Google Scholar]
- Peng HF, Liu JY, Andreadis ST, Swartz DD. Hair follicle-derived smooth muscle cells and small intestinal submucosa for engineering mechanically robust and vasoreactive vascular media. Tissue Eng Part A. 2011;17(7–8):981–990. doi: 10.1089/ten.tea.2010.0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Rodríguez R, García-Castro J, Trigueros C, García Arranz M, Menéndez P. Multipotent mesenchymal stromal cells: clinical applications and cancer modeling. Adv Exp Med Biol. 2012;741:187–205. doi: 10.1007/978-1-4614-2098-9_13. [DOI] [PubMed] [Google Scholar]
- Polchert D, Sobinsky J, Douglas G, Kidd M, Moadsiri A, Reina E, et al. IFN-gamma activation of mesenchymal stem cells for treatment and prevention of graft versus host disease. Eur J Immunol. 2008;38(6):1745–1755. doi: 10.1002/eji.200738129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poggi A, Zocchi MR. Antigen presenting cells and stromal cells trigger human natural killer lymphocytes to autoreactivity: evidence for the involvement of natural cytotoxicity receptors (NCR) and NKG2D. Clin Dev Immunol. 2006;13(2–4):325–336. doi: 10.1080/17402520600578194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- Qu X, Liu X, Cheng K, Yang R, Zhao RC. Mesenchymal stem cells inhibit Th17 cell differentiation by IL-10 secretion. Exp Hematol. 2012 May 23; doi: 10.1016/j.exphem.2012.05.006. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Quarto R, Mastrogiacomo M, Cancedda R, Kutepov SM, Mukhachev V, Lavroukov A, Kon E, Marcacci M. Repair of large bone defects with the use of autologous bone marrow stromal cells. N Engl J Med. 2001;344(5):385–6. doi: 10.1056/NEJM200102013440516. [DOI] [PubMed] [Google Scholar]
- Rasmusson I, Le Blanc K, Sundberg B, Ringdén O. Mesenchymal stem cells stimulate antibody secretion in human B cells. Scand J Immunol. 2007;65(4):336–343. doi: 10.1111/j.1365-3083.2007.01905.x. [DOI] [PubMed] [Google Scholar]
- Rasmusson I, Uhlin M, Le Blanc K, Levitsky V. Mesenchymal stem cells fail to trigger effector functions of cytotoxic T lymphocytes. J Leukoc Biol. 2007;82:887–893. doi: 10.1189/jlb.0307140. [DOI] [PubMed] [Google Scholar]
- Ren G, Zhang L, Zhao X, Xu G, Zhang Y, Roberts AI, et al. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008;2(2):141–150. doi: 10.1016/j.stem.2007.11.014. [DOI] [PubMed] [Google Scholar]
- Ren G, Su J, Zhang L, Zhao X, Ling W, L’huillie A, et al. Species variation in the mechanisms of mesenchymal stem cell-mediated immunosuppression. Stem Cells. 2009;27(8):1954–1962. doi: 10.1002/stem.118. [DOI] [PubMed] [Google Scholar]
- Romieu-Mourez R, François M, Boivin MN, Stagg J, Galipeau J. Regulation of MHC class II expression and antigen processing in murine and human mesenchymal stromal cells by IFN-gamma, TGF-beta, and cell density. J Immunol. 2007;179(3):1549–1558. doi: 10.4049/jimmunol.179.3.1549. [DOI] [PubMed] [Google Scholar]
- Rüster B, Göttig S, Ludwig RJ, Bistrian R, Müller S, Seifried E, et al. Mesenchymal stem cells display coordinated rolling and adhesion behavior on endothelial cells. Blood. 2006;108(12):3938–3944. doi: 10.1182/blood-2006-05-025098. [DOI] [PubMed] [Google Scholar]
- Ryan JM, Barry FP, Murphy JM, Mahon BP. Mesenchymal stem cells avoid allogeneic rejection. J Inflamm. 2005;2:8, 54. doi: 10.1186/1476-9255-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki M, Abe R, Fujita Y, Ando S, Inokuma D, Shimizu H. Mesenchymal stem cells are recruited into wounded skin and contribute to wound repair by transdifferentiation into multiple skin cell type. J Immunol. 2008;180(4):2581–2587. doi: 10.4049/jimmunol.180.4.2581. [DOI] [PubMed] [Google Scholar]
- Sato K, Ozaki K, Oh I, Meguro A, Hatanaka K, Nagai T, et al. Nitric oxide plays a critical role in suppression of T-cell proliferation by mesenchymal stem cells. Blood. 2007;109(1):228–234. doi: 10.1182/blood-2006-02-002246. [DOI] [PubMed] [Google Scholar]
- Savill J, Dransfield I, Gregory C, Haslett C. A blast from the past: clearance of apoptotic cells regulates immune responses. Nat Rev Immunol. 2002;2(12):965–975. doi: 10.1038/nri957. [DOI] [PubMed] [Google Scholar]
- Schena F, Gambini C, Gregorio A, Mosconi M, Reverberi D, Gattorno M, et al. Interferon-γ-dependent inhibition of B cell activation by bone marrow-derived mesenchymal stem cells in a murine model of systemic lupus erythematosus. Arthritis Rheum. 2010;62(9):2776–2786. doi: 10.1002/art.27560. [DOI] [PubMed] [Google Scholar]
- Schrum LW, Marriott I, Butler BR, Thomas EK, Hudson MC, Bost KL. Functional CD40 expression induced following bacterial infection of mouse and human osteoblasts. Infect Immun. 2003;71(3):1209–1216. doi: 10.1128/IAI.71.3.1209-1216.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selmani Z, Naji A, Zidi I, Favier B, Gaiffe E, Obert L, Borg C, Saas P, Tiberghien P, Rouas-Freiss N, Carosella ED, Deschaseaux F. Human leukocyte antigen-G5 secretion byhuman mesenchymal stem cells is required to suppress T lymphocyteand natural killer function and to induce CD4+CD25highFOXP3+regulatory T cells. Stem Cells. 2008;26(1):212–222. doi: 10.1634/stemcells.2007-0554. [DOI] [PubMed] [Google Scholar]
- Sheng H, Wang Y, Jin Y, Zhang Q, Zhang Y, Wang L, et al. A critical role of IFNgamma in priming MSC-mediated suppression of T cell proliferation through up-regulation of B7-H1. Cell Res. 2008;18(8):846–857. doi: 10.1038/cr.2008.80. [DOI] [PubMed] [Google Scholar]
- Shi S, Gronthos S. Perivascular niche of postnatal mesenchymal stem cells in human bone marrow and dental pulp. J Bone Miner Res. 2003;18(4):696–704. doi: 10.1359/jbmr.2003.18.4.696. [DOI] [PubMed] [Google Scholar]
- Sodian R, Lueders C, Kraemer L, Kuebler W, Shakibaei M, Reichart B, Daebritz S, Hetzer R. Tissue engineering of autologous human heart valves using cryopreserved vascular umbilical cord cells. Ann Thorac Surg. 2006;81(6):2207–16. doi: 10.1016/j.athoracsur.2005.12.073. [DOI] [PubMed] [Google Scholar]
- Sotiropoulou PA, Perez SA, Gritzapis AD, Baxevanis CN, Papamichail M. Interactions between human mesenchymal stem cells and natural killer cells. Stem Cells. 2006;24(1):74–85. doi: 10.1634/stemcells.2004-0359. [DOI] [PubMed] [Google Scholar]
- Spaggiari GM, Abdelrazik H, Becchetti F, Moretta L. MSCs inhibit monocyte-derived DC maturation and function by selectively interfering with the generation of immature DCs: central role of MSC-derived prostaglandin E2. Blood. 2009;113:6576–6583. doi: 10.1182/blood-2009-02-203943. [DOI] [PubMed] [Google Scholar]
- Spaggiari GM, Capobianco A, Becchetti S, Mingari MC, Moretta L. Mesenchymal stem cell-natural killer cell interactions: evidence that activated NK cells are capable of killing BMMSCs, whereas MSCs can inhibit IL-2-induced NK-cell proliferation. Blood. 2006;107(4):1484–1490. doi: 10.1182/blood-2005-07-2775. [DOI] [PubMed] [Google Scholar]
- Spaggiari GM, Capobianco A, Abdelrazik H, Becchetti F, Mingari MC, Moretta L. Mesenchymal stem cells inhibit natural killer-cell proliferation, cytotoxicity, and cytokine production: role of indoleamine 2,3-dioxygenase and prostaglandin E2. Blood. 2008;111(3):1327–1333. doi: 10.1182/blood-2007-02-074997. [DOI] [PubMed] [Google Scholar]
- Stagg J, Pommey S, Eliopoulos N, Galipeau J. Interferon-gamma-stimulated marrow stromal cells: a new type of nonhematopoietic antigen-presenting cell. Blood. 2006;107(6):2570–2577. doi: 10.1182/blood-2005-07-2793. [DOI] [PubMed] [Google Scholar]
- Sun L, Akiyama K, Zhang H, Yamaza T, Hou Y, Zhao S, et al. Mesenchymal stem cell transplantation reverses multiorgan dysfunction in systemic lupus erythematosus mice and humans. Stem Cells. 2009;27(6):1421–1432. doi: 10.1002/stem.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzawa M, Takada I, Yanagisawa J, Ohtake F, Ogawa S, Yamauchi T, et al. Cytokines suppress adipogenesis and PPAR-gamma function through the TAK1/TAB1/NIK cascade. Nat Cell Biol. 2003;5(3):224–230. doi: 10.1038/ncb942. [DOI] [PubMed] [Google Scholar]
- Suzawa M, Takada I, Yanagisawa J, Ohtake F, Ogawa S, Yamauchi T, et al. Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol. 2005;57(3):874–882. doi: 10.1002/ana.20501. [DOI] [PubMed] [Google Scholar]
- Tabera S, Pérez-Simón JA, Díez-Campelo M, Sánchez-Abarca LI, Blanco B, López A, et al. The effect of mesenchymal stem cells on the viability, proliferation and differentiation of B-lymphocytes. Haematologica. 2008;93(9):1301–1309. doi: 10.3324/haematol.12857. [DOI] [PubMed] [Google Scholar]
- Tang KC, Trzaska KA, Smirnov SV, Kotenko SV, Schwander SK, Ellner JJ, et al. Down-regulation of MHC II in mesenchymal stem cells at high IFN-gamma can be partly explained by cytoplasmic retention of CIITA. J Immunol. 2008;180(3):1826–1833. doi: 10.4049/jimmunol.180.3.1826. [DOI] [PubMed] [Google Scholar]
- Tasso R, Fais F, Reverberi D, Tortelli F, Cancedda R. The recruitment of two consecutive and different waves of host stem/progenitor cells during the development of tissue-engineered bone in a murine model. Biomaterials. 2010;31:2121–2129. doi: 10.1016/j.biomaterials.2009.11.064. [DOI] [PubMed] [Google Scholar]
- Traggiai E, Volpi S, Schena F, Gattorno M, Ferlito F, Moretta L, et al. Bone marrow-derived mesenchymal stem cells induce both polyclonal expansion and differentiation of B cells isolated from healthy donors and systemic lupus erythematosus patients. Stem Cells. 2008;26(2):562–569. doi: 10.1634/stemcells.2007-0528. [DOI] [PubMed] [Google Scholar]
- Wu Y, Chen L, Scott PG, Tredget EE. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells. 2007;25(10):2648–2659. doi: 10.1634/stemcells.2007-0226. [DOI] [PubMed] [Google Scholar]
- Yamaza T, Miura Y, Bi Y, Liu Y, Akiyama K, Sonoyama W, et al. Pharmacologic stem cell based intervention as a new approach to osteoporosis treatment in rodents. PLoS ONE. 2008;3(7):e2615. doi: 10.1371/journal.pone.0002615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SH, Park MJ, Yoon IH, Kim SY, Hong SH, Shin JY, et al. Soluble mediators from mesenchymal stem cells suppress T cell proliferation by inducing IL-10. Exp Mol Med. 2009;41(5):315–324. doi: 10.3858/emm.2009.41.5.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zandonella C. Tissue engineering: The beat goes on. Nature. 2003;421(6926):884–886. doi: 10.1038/421884a. [DOI] [PubMed] [Google Scholar]
- Zhang B, Liu R, Shi D, Liu X, Chen Y, Dou X, et al. Mesenchymal stem cells induce mature dendritic cells into a novel Jagged-2-dependent regulatory dendritic cell population. Blood. 2009;113(1):46–57. doi: 10.1182/blood-2008-04-154138. [DOI] [PubMed] [Google Scholar]
- Zhang W, Ge W, Li C, You S, Liao L, Han Q, et al. Effects of mesenchymal stem cells on differentiation, maturation, and function of human monocyte-derived dendritic cells. Stem Cells Dev. 2004;13(3):263–271. doi: 10.1089/154732804323099190. [DOI] [PubMed] [Google Scholar]
- Zhao S, Wehner R, Bornhauser M, Wassmuth R, Bachmann M, Schmitz M. Immunomodulatory properties of mesenchymal stromal cells and their therapeutic consequences for immune-mediated disorders. Stem Cells Dev. 2010;19(5):607–614. doi: 10.1089/scd.2009.0345. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Liu Y, Zhang CM, Zhang HY, Li WH, Shi S, Le AD, Wang SL. Stem cells from deciduous tooth repair mandibular defect in swine. J Dent Res. 2009;88(3):249–254. doi: 10.1177/0022034509333804. [DOI] [PMC free article] [PubMed] [Google Scholar]