Abstract
Aging is, by far, the greatest risk factor for most neurodegenerative diseases. In non-diseased conditions, normal aging can also be associated with declines in cognitive function that significantly affect quality of life in the elderly. It was recently shown that inhibition of mTOR activity in mice by chronic rapamycin treatment extends lifespan, possibly by delaying aging {Harrison, 2009 #4}{Miller, 2011 #168}. To explore the effect of chronic rapamycin treatment on normal brain aging we determined cognitive and non-cognitive components of behavior throughout lifespan in male and female C57BL/6 mice that were fed control- or rapamycin-supplemented chow. Our studies show that rapamycin enhances cognitive function in young adult mice and blocks age-associated cognitive decline in older animals. In addition, mice fed with rapamycin-supplemented chow showed decreased anxiety and depressive-like behavior at all ages tested. Levels of three major monoamines (norepinephrine, dopamine and 5-hydroxytryptamine) and their metabolites (3,4-dihydroxyphenylacetic acid, homovanillic acid, and 5-hydroxyindolacetic acid) were significantly augmented in midbrain of rapamycin-treated mice compared to controls. Our results suggest that chronic, partial inhibition of mTOR by oral rapamycin enhances learning and memory in young adults, maintains memory in old C57BL/6J mice, and has concomitant anxiolytic and antidepressant-like effects, possibly by stimulating major monoamine pathways in brain.
Keywords: mammalian target of rapamycin, memory, depression, anxiety, brain aging, monoamines
The target of rapamycin (TOR) is a major cellular signalling node that controls cellular metabolism and organismal lifespan in invertebrates and mammals (Kapahi and Zid, 2004). Mammalian TOR (mTOR) controls cell growth, proliferation, and survival through two distinct multiprotein complexes, mTORC1 and mTORC2. mTORC1 functions as a nutrient/energy/redox sensor and controls protein homeostasis. mTORC2 activates Akt/PKB by phosphorylation of Ser473 (Martin and Hall, 2005). This event inhibits the activity of FoxO transcription factors, which have a central role in the control of metabolism, cell stress resistance and autophagy (Michalek and Rathmell, 2008, Salih and Brunet, 2008). In addition, mTOR is involved in the modulation of long-lasting synaptic plasticity (Hoeffer and Klann, 2009). Although acute inhibition of mTOR has generally been associated with defects in long-term plasticity required for memory (Tang et al., 2002), inhibition of mTOR can also block the opposite process, the long-term reduction in synaptic responsiveness or long-term depression (LTD) (Huber et al., 2001). Moreover, disruption of signalling mechanisms that inhibit mTOR results in high mTOR activity and significant plasticity and memory deficits (Hoeffer and Klann, 2009). These observations suggest that mTOR does not act as a synaptic ‘on-off’ switch but may serve as a rheostat that modulates long-lasting synaptic change. The mechanisms by which mTOR inhibits memory have not been explored. Partial inhibition of mTOR function in vivo in rodent experimental models became possible only recently, when a method for effective chronic oral delivery of this drug was developed and used to establish that chronic systemic inhibition of mTOR in mice extends lifespan (Harrison et al., 2009). In agreement with these studies, we previously showed that treatment of mice modelling Alzheimer’s disease (AD) fed with the same rapamycin-supplemented diet that extends lifespan blocked AD-like impairments in spatial learning and memory (Spilman et al., 2010). To explore the effect of chronic rapamycin treatment on normal brain aging we determined cognitive and non-cognitive components of behavior in C57BL/6J animals that were fed control- or rapamycin-supplemented chow at different ages throughout their lifespan, and for periods ranging from 8 to 40 weeks. Our results demonstrate that rapamycin treatment enhances cognitive function in young C57BL/6J mice and blocks age-associated cognitive decline in older animals. In addition, our data suggests that rapamycin has anxiolytic and antidepressant-like effects at all ages tested. Levels of three major monoamines (norepinephrine, dopamine and 5-hydroxytryptamine) were significantly augmented in midbrain of rapamycin-treated mice, suggesting that the effects of rapamycin on cognitive and non-cognitive components of behavior may be explained by the stimulation of major monoamine pathways in brain.
1.2. EXPERIMENTAL PROCEDURES
1.2.1. Mice
Mice used in these studies were C57BL/6J obtained from the Jackson Laboratories (JAX, Bar Harbor, ME) or were non-transgenic mice arising from crosses of C57BL/6J breeders from JAX and heterozygous transgenic hAPP(J20) mice fully congenic in the C57BL/6J background as indicated in the figure legends and in the Results section. The twenty-five month-old C57BL/6 mouse group was purchased from Charles River. Numbers of animals per experimental group are indicated in the legends to the Figures.
1.2.2. Rapamycin treatment
Mice were fed chow containing either microencapsulated rapamycin or a control diet as described by Harrison et al., (2009). Rapamycin was used at 14 mg per kg of food (verified by HPLC). On the assumption that the average C57BL/6J mouse weighs 30 g and consumes 5 g of food/day, this rapamycin concentration in the chow results in an average dose of 2.24 mg rapamycin per kg body weight/day (Harrison et al., 2009). All mice were given ad libitum access to rapamycin or control food and water for the duration of the experiment. Body weights and food intake were measured weekly. Duration of rapamycin treatment for different groups are indicated in the Results section.
1.2.3. Passive avoidance
An inhibitory avoidance task was used to test long-term memory of an aversive event. Animals were habituated to the testing apparatus on day 1 by being placed in a lit chamber of a GEMINI active and passive avoidance system, a trough-shaped alley where two compartments (one lighted and one dark) are separated by a guillotine-like door. During the first 30 seconds (sec) of habituation the guillotine door was down. After 30 sec the door was opened, allowing mice access to a similar but darkened chamber. At any point, mice that failed ot enter the darkened chamber were taken out of the study. Twenty-four hours later mice were placed in the light side of the passive avoidance apparatus with the guillotine door down. After 30 sec the door was opened allowing the mouse to cross to the darkened chamber. When the mouse crossed into the darkened chamber the door closed and an electric foot shock (2mA for 2 sec) was delivered through the grid floor. Mice were left in the chamber for 30 sec before being returned to their home cage. Twenty-four hours later mice were again placed in the light side of the passive avoidance apparatus with the guillotine door down. After 30 sec the door opened allowing the mouse to cross to the darkened chamber. The amount of time that it took for the mouse to cross from the light to the dark chamber (latency) was recorded. Each mouse had a maximum of 300 sec to cross. No crossing was scored as 300 sec.
1.2.4. Morris water maze (MWM)
The MWM (Morris, 1984, Galvan et al., 2006, Galvan et al., 2008, Zhang et al., 2009) was used to test spatial memory. All groups were assessed for swimming ability before testing. None of the animals showed deficiencies in swimming abilities, directional swimming or climbing onto a cued platform during pre-training and had no sensorimotor deficits as determined with a battery of neurobehavioral tasks performed prior to testing. Briefly, mice were given a series of 3 trials per day, 20-30 minutes (min) apart in a tank filled with water opacified by the addition of non-toxic paint at a temperature of 24.0±1.0°C. Animals were trained to find a 12×12-cm submerged (1 cm below water surface) platform placed in one quadrant of the pool. The animals were released at different locations in each 60 sec trial. If mice did not find the platform in 60 sec, they were gently guided to it. After remaining on the platform for 20 sec, the animals were removed and placed in a dry cage under a warm heating lamp. The water tank was surrounded by opaque dark panels marked with geometric designs at approximately 30 cm from the edge of the pool that served as distal cues. The animals were trained for 4 days. At the end of training, a 30-sec probe trial was administered in which the platform was removed from the pool. The number of times that each animal crossed the previous platform location was determined as a measure of platform location retention. During the course of testing, animals were monitored daily, and their weights were recorded weekly. Performance in all tasks was recorded by a computer-based video tracking system (Water2100, HVS Image, U.K). Data were compiled offline using HVS Image and compiled using Microsoft Excel before statistical analyses.
1.2.5. Tail suspension test
Mice were suspended from a wooden pole 45 cm above a procedure table using adhesive tape placed approximately 2.5 cm from the base of the tail. Periods of immobility and active movement were recorded for 5 min. Mice were considered immobile when they hung motionless.
1.2.6. Elevated plus maze
The elevated plus maze was used to assess emotionality and reactivity (Rodgers, 1997). The plus maze consists of two enclosed arms and two open arms. Mice are placed in the center of the maze and allowed free access to all arms for 5 min. The animals can spend their time either in a closed safe area (closed arms) or in an open area (open arms). While the open arms are, by design, anxiogenic, the animal is free to move into the closed arms. Mouse movements were recorded by a computer-based video tracking system (Maze2100, HVS Image). Time spent in the open and closed arms, distance moved and number of open and closed arm entries were determined. Data were compiled offline using HVS Image and then compiled with Microsoft Excel before statistical analyses.
1.2.7. Western blotting
Mice were euthanized by isoflurane overdose followed by cervical dislocation. Hemibrains were flash frozen. One hemibrain was homogenized in liquid N2 and used for whole-brain western blotting (5-6 per group); the other hemibrain was used for hippocampal dissections (5-6 per group). Half brains were microdissected to isolate the hippocampus by peeling away the cortex from the underlying hippocampus and releasing the hippocampus from the surrounding tissue, in particular the fimbria, by using fine surgical tweezers (Fine Science Tools) and then lifting toward the midline. The hippocampus separates easily but does include some adjacent white matter, which is then carefully tweezed off. We also obtained hippocampal tissue from at least 12 × 10 ⎧m unfixed frozen sections mounted on glass slides. All but the hippocampal area was removed using a scalpel under a SZ60 Olympus dissecting microscope. The hippocampal tissue itself was removed by beading 10 ⎧l of RIPA lysis buffer (25mM Tris-HCl, pH 7.6; 150mM NaCl; 1% NP40; 1% sodium deoxycholate; 0.1% sodium dodecyl sulfate) on it and then pipetting it up. For Western blot analyses, proteins from soluble fractions of brain LN2 homogenates and from hippocampal dissections were resolved by SDS/PAGE (Invitrogen, Temecula, CA) under reducing conditions and transferred to a PVDF membrane, which was incubated in a 5% solution of non-fat milk or in 5% BSA for 1 hour at 20°C. After overnight incubation at 4°C with primary antibodies, the blots were washed in TBS-Tween 20 (TBS-T) (0.02% Tween 20, 100 mM Tris pH 7.5; 150 nM NaCl) for 20 minutes and incubated at room temperature with appropiate secondary antibodies. The blots were then washed 3 times for 20 min each in TBS-T and then incubated for 5 min with Super Signal (Pierce, Rockford, IL), washed again and exposed to film or imaged with a Typhoon 9200 variable mode imager (GE Healthcare, NJ). Antibodies used were anti-phospho-p70 (Cell Signaling, #9206) and anti-ß-actin (Sigma, A3853), anti phospho-Ser473 Akt (Cell Signaling, #9271) and anti-norepinephrine transporter (NET, MAb Technologies, NET05-1).
1.2.8. Determination of monoamines
Animals were sacrificed by isoflurane inhalation overdose followed by cervical dislocation. Brains were removed, snap frozen on dry ice, and stored at −80°C until dissection. Brains were slightly thawed on a cold block and the midbrain was dissected out and refrozen on dry ice. The midbrain was stored again at −80°C until it was processed for analysis. Midbrain samples were processed for HPLC analysis by homogenizing in ice-cold 0.1 M perchloric acid containing 10 ng/mL DHBA as an internal standard. Samples were incubated on ice for 2 min and subsequently centrifuged at 12,000 rpm at 4°C for 2 minutes. The supernatant was transferred to a 0.45 mm microcentrifuge filter tube (Millipore, Billerica, MA) and centrifuged at 12,000 rpm at 4 °C for 1 min. Monoamines were separated using a HPLC system that consisted of a dual-piston pump (Solvent Delivery Module, Model 580), a refrigerated autosampler (Model 540), and a Coulochem II dual-potentiostat electrochemical detector (Model 500; all by ESA Biosciences Inc., Chelmsford, MA). System control and data collection were performed using a PC-based data station (Model 500). Separation of monamines was executed on a HR-80 reverse-phase C18 column (4.6 × 80 mm). Analytes were detected on a dual-electrode analytical cell (Model 5011A) with the first electrode set at −50 mV and the second set at +280 mV. A guard cell (Model 5020) was positioned between the pump and the autosampler and set at +350 mV to oxidize contaminants in the mobile phase. The mobile phase contained 75 mM sodium phosphate monobasic, 4 mM heptanesulfonic acid, 25 μM EDTA, 0.01% triethylamine, and 6% acetonitrile (v/v), pH 3.1 (adjusted with phosphoric acid. The pump was set at 1.0 mL/min. All analyses were performed at 27°C and in duplicate. A separate cohort was bred for these experiments. Since the effects of rapamycin treatment on anxiety and motivation were observed in both genders (Figure 2), females were used to minimize housing costs.
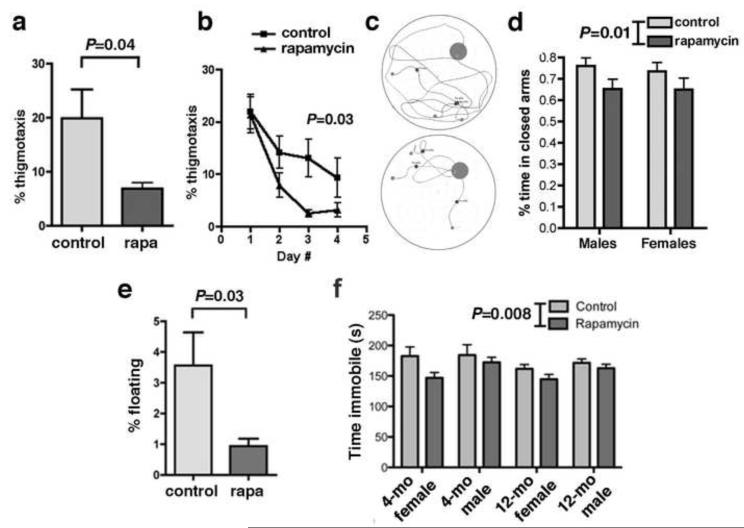
Figure 2. Non-cognitive components of behavior in control- and rapamycin-treated C57Bl/6J mice. a-d. Decreased anxiety in rapamycin-fed mice.
a. Percent time spent engaged in thigmotactic swimming was significantly reduced in male rapamycin-fed mice. b. Percent time spent in thigmotactic swimming on the first day of training was indistinguishable between the two experimental groups; percent time of thigmotaxis decreased significantly as training progressed for both groups [significant effect of day number on thigmotaxis, F(3,54)=14.12; P<0.0001, two-way ANOVA]. Rapamycin-fed mice spent less times swimming close to the tank wall at all times during training (P as indicated, Tukey’s post-hoc test applied to a significant effect of day number on thigmotaxis as above, n=10 for each group). c. Swim traces for day 4 trials (3 representative swim traces are shown for each experimental group; Top panel, control-fed; Lower panel, rapamycin-fed mice). Mice were fed with rapamycin-supplemented chow for 16 weeks. d. Elevated plus maze. Fourteen month-old mice fed with rapamycin for 40 weeks spent less time in the closed arms of the elevated plus maze, indicating decreased anxiety (F(1,45)=7.06; P as indicated, two-way ANOVA). n=11-14 for each group. e-f. Decreased depressive-like behavior in rapamycin-fed mice. e. Floating. Percent time spent floating was significantly lower [F(2,75)=3.3, P=0.04, two-way ANOVA] for rapamycin-fed 8 month-old male mice as compared to control-fed animals (P as indicated, unpaired Student’s t test, n=10 for each group). Mice were fed with rapamycin-supplemented chow for 16 weeks. f. Tail suspension test. Both young (4 month-old) and adult (12 month-old) mice fed with rapamycin for 16 and 40 weeks respectively spent less time immobile when suspended from their tails, indicating that rapamycin treatment reduces depressive-like behavior [F(1,83)=7.26; P as indicated, two-way ANOVA). n=10-16 for each group. Data are means ± SEM.
1.2.9. Statistical analyses
Statistical analyses were performed using GraphPad Prism (GraphPad, San Diego, CA) and Sigma Stat (Aspire Software International, Ashburn, VA). In two-variable experiments, as when scoring performance of different treatments across several days of training, repeated measures two-way ANOVA followed by Bonferroni’s post hoc tests were used to evaluate significance of differences between group means. When analyzing one-variable experiments with more than 2 groups, as when scoring retention across genotypes in probe trials, significance of differences between means was evaluated using one-way ANOVA followed by Tukey’s post-hoc test. Significance of differences in means between two different groups or between the same group at two different testing times were determined with Student’s t test or using a t test with Welch’s correction when experimental samples did not show equal variances. Values of P < 0.05 were considered significant. Pearson correlation coefficients between variables were calculated with regression analyses performed using GraphPad Prism (GraphPad, San Diego, CA).
1.3. RESULTS
1.3.1. Chronic rapamycin treatment inhibits mTORC1 but not mTORC2 in brains of C57BL/6J mice
It had been previously shown (Cloughesy et al., 2008) that rapamycin crosses the blood-brain barrier. Similarly, C57BL/6J mice fed a diet containing 14 ppm encapsulated rapamycin had rapamycin brain concentrations of 8.65 ± 0.66 ng/mg wet weight, similar to the rapamycin levels found in plasma in the same animals (Randy Strong, personal communication). Food consumption was higher for rapamycin-fed females, but not for males, during the first 8 weeks of treatment (by an average of 1.25±0.12 g/mouse/week, P<0.0001; two-way ANOVA). This may be a result of the inhibition of the mTOR pathway, which is expected to mimic the unfed state. In spite of the differences in food consumption during the first 8 weeks of treatment, overall body weight of control- and rapamycin-fed groups was not significantly different (P=0.77 for females and P=0.4 for males, two-way ANOVA, with overall averages of 22.71±2.04 vs 22.31±1.54 g for females and 30.1±3.05 vs 28.75±2.38 g for males, control-fed and rapamycin-fed groups respectively). However, body weight increased progressively for both control- and rapamycin-fed groups (increases were 23% for females regardless of treatment, 17% for rapamycin-fed males and 26% for control-fed males respectively), possibly as a result of the change in base chow composition (the formulation of rapamycin-supplemented chow and control chow (Harrison et al., 2009) differs from that of the standard chow provided by our animal facility).
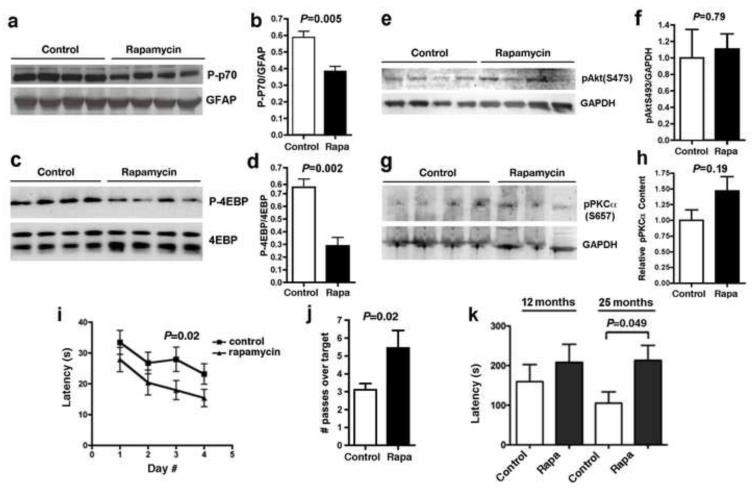
To determine whether rapamycin treatment inhibited mTOR in brain, we examined the phosphorylation status of the mTORC1 targets p70 S6 kinase (p70) in brains of control- and rapamycin-fed mice. Consistent with our prior studies (Spilman et al., 2010) and with the rapamycin concentrations achieved in brain tissues by chronic rapamycin feeding, phosphorylation of p70 by mTORC1 was significantly decreased in brains of rapamycin-fed animals (Figure 1 a-b). In agreement with this observation, the phosphorylation of a different mTORC1 target, eiF4E-binding protein (4EBP) was decreased in hippocampi of rapamycin-treated mice (Figure 1c-d). In contrast, phosphorylation of Akt/PKB at Ser473, a target of mTORC2, was unaffected both in whole brain lysates (Figure 1 e-f) as well as in hippocampi (Figure 1g-h). Taken together, these data indicate that long-term oral treatment with rapamycin significantly inhibits mTORC1 but does not reduce mTORC2 activity in brain.
Figure 1. Rapamycin enhances cognitive function in C57BL/6J mice. a-h. mTORC1 but not mTORC2 activity are decreased in rapamycin-treated brains.
a, c, e, g. Representative immunoblots of whole brain (a and e) or hippocampal lysates (c and g) from control- and rapamycin-treated mice; b, d, f, h. Quantitative densitometric analyses. Phosphorylated (activated) p70 and phosphorylated 4EBP (b and d), but not S473 phosphorylated PKB/Akt nor phosphorylated PKCalpha (f and h) were decreased in brains of rapamycin-fed mice (P as indicated, unpaired Student’s t test, n=6-10 for each group). Mice were fed with rapamycin-supplemented chow for 16 weeks. i-j. Rapamycin-fed mice C57BL/6J show improved spatial learning and memory. i. Spatial training. Mean latencies to reach a hidden platform were significantly reduced for rapamycin-fed male mice compared to control-fed animals (Significant effect of treatment on performance, F(3,54)=6.40, P as indicated, two-way ANOVA). j. Probe trial. Retention of the former platform site was enhanced in rapamycin-fed male mice with respect to the control-fed group (P as indicated, one-way ANOVA). n=10 for each group. Mice were fed with rapamycin-supplemented chow for 16 weeks. k. Old rapamycin-fed mice show improved memory of an aversive stimulus. Latencies to enter a dark compartment were increased as a result of enhanced memory of an aversive event (2-second foot shock) in rapamycin-fed mice of mixed gender at 25 months of age (P as indicated, upaired Student’s t test). n=9-14 for each group. Data are means ± SEM.
1.3.2. Chronic rapamycin treatment enhances spatial learning and memory in 8 month-old C57BL/6J mice
Our laboratory previously demonstrated that rapamycin treatment prevents cognitive decline in a mouse model of Alzheimer’s disease (Spilman et al., 2010). The present study aimed to determine whether the effects of chronic rapamycin treatment would extend to normal brain aging. Thus, to assess the effects of chronic rapamycin supplementation on cognitive function in wild-type animals, we used the Morris water maze (MWM) test (Morris, 1984, Galvan et al., 2006, Galvan et al., 2008), a widely used tool in the assessment of hippocampal-dependent learning and memory in rodents. Only males were used in MWM experiments since female rodents tend to form a weaker representation of the environment than males (Gresack and Frick, 2003, Hawley et al.). Eight month-old wild-type male C57BL/6J mice that had been fed rapamycin starting at 4 months of age (thus treated for 16 weeks) had significantly better learning compared to control-fed animals (significant effect of treatment on performance, F(3,54)=6.40; P<0.02; two-way ANOVA, Figure 1e). There was no significant interaction (P=0.92) between treatment and training day (two-way ANOVA), thus rapamycin had the same effect at all times during training. Consistent with improved learning, rapamycin-fed mice showed enhanced memory of the former location of the escape platform as compared to control-fed mice [P<0.04 as a result of Welch’s t test, Figure 1f]. Although performance of control- and rapamycin-treated mice at day 1 during training in the Morris water maze task was effectively indistinguishable (P=0.32 as a result of unpaired Student’s t test applied to the comparison of performance mean values for control- and rapamycin-treated mice), a trend to decreased latencies in the rapamycin-treated group on day 1 of training could indicate lower basal anxiety levels (Figure 2 a-d), or increased motivation (Figure 2 e-f) in rapamycin-treated groups as compared to control-treated animals. To determine whether the difference in performance between control and rapamycin-treated groups could be explained by differences in anxiety or in motivation during water maze training, we performed linear regression analyses relating thigmotaxis (as a measure of anxiety) or floating (as a measure of depressive-like behavior) with performance for individual animals at each swim during spatial training. Changes percent time spent in thigmotaxis explained approximately half of the variance in performance for both control- (r2=0.44) and rapamycin-treated mice (r2=0.57). Since the contribution of thigmotaxic to performance did not change as a result of rapamycin treatment, differences in basal anxiety could not explain the differences observed in performance between control- and rapamycin-treated groups during spatial training (learning) in the water maze. Changes in motivation were not correlated to performance in control- (r2=0.06) nor in rapamycin-treated mice (r2=0.02).
1.3.3. Chronic rapamycin treatment restores memory of an aversive event in 25 month- old C57BL/6J mice
To determine whether rapamycin treatment would affect memory in older (12 and 25 month-old) mice we used the passive avoidance task, which determines avoidance of an aversive stimulus (a 2-sec mild foot shock) as a result of the suppression of an innate preference in rodents (moving from a bright to a dark compartment), contingent on memory of the aversive event (Cho et al.). The relatively small number of male mice available to us at 12 and 25 months of age precluded a water maze study. No significant differences in latency to enter the dark compartment 24 hours after training (a measure of retention of the aversive stimulus) was observed among 12 month-old mice of mixed gender that had been fed with rapamycin-supplemented chow for 40 weeks (Figure 1g). In contrast, latency to enter the dark compartment was significantly increased in 25 month-old rapamycin-fed mice that were treated with rapamycin for the same length of time, indicating that rapamycin improved memory of the aversive event in older animals. No significant differences in latencies were found among experimental groups on the day of training, before the shock was administered. Thus, the differences in retention could not be attributed to differences in motivation to move into the dark compartment. Taken together, our results suggest that long-term rapamycin treatment can enhance spatial learning and memory in young animals and improve recall of an aversive event in older mice. Of note, memory was maintained in aged mice that were fed with rapamycin-supplemented chow starting at 18 months of age (Figure 1e), suggesting that chronic rapamycin treatment preserves cognitive function during aging, even when treatment is started late in life.
1.3.4. Chronic rapamycin treatment reduces anxiety in C57BL/6J mice
Making no attempt to swim (floating) and swimming in close proximity to the pool wall (‘wall hugging’ or thigmotaxis) during training in the MWM are frequent performance deficits to which certain strains of mice are prone (Tecott and Nestler, 2004). These patterned behaviours are believed to be manifestations of emotional responses, such as anxiety and helplessness in stressful situations. Immobility, defined as the absence of active, escape-oriented behaviors such as swimming in the water tank, is considered indicative of depressive-like behavior in experimental paradigms such as the forced swim test (Crawley, 2000, Pechnick et al., 2004). Since measures of performance in the water maze and in many other behavioral tests can be influenced by anxiety we determined the fraction of time spent in thigmotactic swimming (defined as the fraction of swim paths restricted to distances ~10 cm from the tank wall) during training for young (8 month-old) male mice that were fed rapamycin for 16 weeks. Even though the average percent time of thigmotaxis in control-fed animals was low [marginally higher than what would be expected from random swimming within the 10 cm-wide external annulus of the tank (mean=20 s, 30% of trial time, Figure 2a)] consumption of a rapamycin-supplemented diet significantly reduced the average time swimming close to the tank walls (mean=7 s, Figure 2a). This decrease in thigmotaxis was not related to lower basal anxiety levels (i.e. the water maze environment was intrinsically less anxiogenic to rapamycin-treated mice), since the percent time spent in thigmotactic swimming on the first day of training was indistinguishable between the two experimental groups (Figure 2b). Percent time engaged in thigmotactic swimming decreased with training day in both groups (significant effect of day number on performance, F(3,54)=14.12; P<0.0001, two-way ANOVA). As training progressed, however, rapamycin-fed animals, which learned faster (Figure 1c), spent significantly less time swimming in close proximity to the tank walls (Figure 2b). This observation is consistent with improved learning and was associated, as expected, in more direct paths towards the escape platform (Figure 2c).
To determine whether anxiety was affected by rapamycin treatment in older mice we used a behavioral paradigm that is not associated with learning, the elevated plus maze, which assesses rodent’s reactivity using their innate aversion of open spaces and provides a direct measure of anxiety (Crawley, 2000). In agreement with the effect of rapamycin on percent time spent in thigmotactic swimming in the water maze, 14 month-old mice that were treated with rapamycin for 40 weeks spent less time in the closed arms of the maze (significant effect of treatment on time spent in the closed arms, F(1,45)=7.06; P<0.01, two-way ANOVA, Figure 2d).
1.3.5. Reduced depressive-like behavior in C57BL/6J mice chronically fed with rapamycin
In addition to anxiety, motivation is an important aspect of affect that may impinge on cognitive outcomes. To determine whether rapamycin treatment modulates depressive-like behavior we determined percent time spent floating during the training (learning) phase of the water maze task. As shown in Figure 2e, the percent time spent by mice making no attempt to escape was significantly decreased in 8 month-old mice fed with rapamycin-supplemented chow for 16 weeks, suggesting that chronic rapamycin treatment decreased depressive-like behavior. To determine whether effects of rapamycin on motivation would also be observed in younger (4 month-old) or older (12 month-old) animals, we used the tail suspension test (TST), a task that provides a robust measure of depressive-like behavior (Cryan et al., 2005). Since TOR is essential for development (Polak and Hall, 2009), to avoid potential confounds related to inhibition of mTORC1 before brain development was completed we started feeding control- or rapamycin-supplemented chow to experimental groups at 8 weeks of age. Rapamycin feeding for 16 weeks decreased the time spent immobile (making no attempt to escape) in the TST at both ages tested (4 and 12 months of age) and in both genders [significant effect of treatment on time spent immobile, F(1,83)=7.26; P<0.0085, two-way ANOVA, Figure 2f]. Thus, these results suggest that chronic rapamycin treatment has antidepressant-like effects in C57BL/6J mice, and indicate that 8 weeks of rapamycin treatment are sufficient for this effect.
1.3.6. Increased monoamine levels in midbrain of rapamycin-fed mice
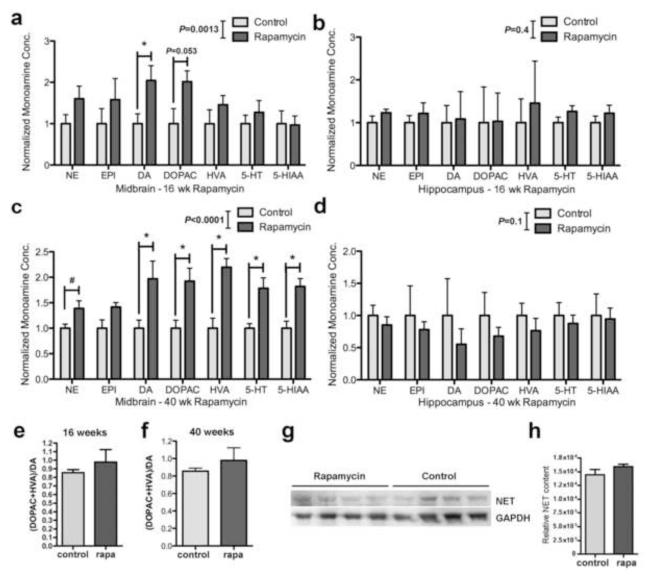
Since we observed anxiolytic and antidepressant-like effects of rapamycin in cohorts ranging from 4 to 14 months of age and in animals of both genders, to investigate the mechanisms by which rapamycin may have anxiogenic and antidepressant-like actions we generated cohorts of female mice at an intermediate age (12 months) that were treated with rapamycin for 16 or 40 weeks and determined levels of major monoamines [norepinephrine (NE), epinephrine (EPI), dopamine (DA) and 5-hydroxytryptamine (5-HT)] and their metabolites [3,4-dihydroxyphenylacetic acid (DOPAC), homovanillic acid (HVA), and 5-hydroxyindolacetic acid (5-HIAA)] in midbrain and hippocampus. While levels of major monoamines were highly variable and overall not significantly changed in hippocampus at either length of rapamycin treatment (Figure 3 b and d) monoamine levels were consistently and significantly increased in midbrain of rapamycin-treated animals both after 16 weeks (Figure 3 a, F(1,55)=11.57; P=0.0013, two-way ANOVA) and after 40 weeks (Figure 3 c) of rapamycin treatment (F(1,56)=65.8; P<0.0001, two-way ANOVA). Even though both 16 and 40 weeks of rapamycin treatment resulted in significantly increased monoamine levels in midbrain, levels of individual monoamines increased with increasing length of rapamycin treatment (Figure 3 a and c). Bonferroni’s post-hoc tests showed that DA, DOPAC, HVA, 5-HT AND 5-HIAA were all significantly increased by 40 weeks of rapamycin treatment (P=0.007, P=0.01, P<0.001, P=0.02 and P=0.01 respectively, Figure 3a) while only DA showed significant increases at 16 weeks of rapamycin treatment (P=0.04). Although differences in mean NE levels at 40 weeks were not significant in this analysis, pairwise determinations of significance of differences between mean levels for each monoamine using Student’s t test suggested an increase in NE levels (P=0.018, Figure 3c) at 40 weeks of rapamycin treatment, and a marginal, not statistically significant trend to increase in DOPAC (P=0.053) at 16 weeks of rapamycin treatment (Figure 3a). No significant differences in midbrain levels of EPI were found among experimental groups at 16 nor 40 weeks of rapamycin treatment. Thus, rapamycin treatment increases midbrain levels of major monoamines but does not significantly affect EPI.
Figure 3. Rapamycin treatment increases monoamines levels in midbrain of rapamycin-treated mice.
a and c. Monoamine levels in midbrain. a. Monoamine levels were significantly increased in midbrain of mice fed with rapamycin for 16 weeks (F(1,55)=11.57; P=0.0013, two-way ANOVA) and c. for 40 weeks (F(1,56)=65.8; P<0.0001, two-way ANOVA). DA was significantly increased in midbrain at 16 weeks of rapamycin treatment (P=0.04) while DA, DOPAC, HVA, 5-HT AND 5-HIAA were significantly increased in midbrain at 40 weeks (P=0.007, P=0.01, P<0.001, P=0.02 and P=0.01 respectively). NE levels showed a significant increase when analyzed independently (P=0.018, unpaired Student’s t test). n=5 for each group. c and d. Monoamine levels in hippocampus. Monoamine levels were not significantly different between experimental groups at 16 weeks (b) nor at 40 weeks (d) of rapamycin treatment. e and f. No differences in DA metabolism were found among experimental groups at 16 (e) nor at 40 (f) weeks of rapamycin treatment. g-h. Cortical NET levels are not affected by rapamycin treatment. e. Representative immunoblots of cortical lysates from control- and rapamycin-treated mice probed with antibodies specific for the indicated proteins. f. Quantitative densitometric analyses indicate that cortical NET levels are not affected by rapamycin treatment (P>0.05, unpaired Student’s t test). n=5 for each group. Data are means ± SEM.
To determine whether the observed increases in DA resulted from decreases in DA metabolism, we calculated midbrain DA/DOPAC+HVA ratios for animals subjected to 16 or 40 weeks of control or rapamycin treatment. As shown in Figure 3e and f, DA metabolism was unaffected in midbrain by rapamycin after 16 or 40 weeks of treatment, suggesting that rapamycin treatment does not affect DA degradation.
It was recently suggested that decreased levels of mTORC2 by genetic ablation of the mTORC2 regulatory protein rictor in neurons leads to reduced levels of prefrontal DA, elevated NE, enhanced expression of the NE transporter (NET), resulting in hypodopaminergia and schizophrenia-like behaviors in mice (Siuta et al.). Even though rapamycin is considered to be a specific mTORC1 inhibitor, it was recently shown that long-term in vitro rapamycin treatment may result in the inhibition of mTORC2 as well (Sarbassov et al., 2006). Although we observed no differences in the phosphorylation of mTORC2 targets in whole brain and in hippocampi of mice treated chronically with rapamycin (Figure 1 e-h), to rule out potential confounding effects of cortical hypodopaminergia in rapamycin-treated mice arising from enhanced expression of the NET transporter (Siuta et al.), we determined NET levels in cortex of control- and rapamycin-fed animals. Rapamycin treatment did not affect cortical NET levels (Figure 3 g and h), suggesting that the increase in NE in brains of rapamycin-fed mice is not related to effects of rapamycin on NET.
1.4. DISCUSSION AND CONCLUSIONS
Normal aging is associated with specific changes in brain structure and connectivity, and frequently with declines in cognitive function that significantly affect quality of life. Signaling pathways that regulate the aging process have in common a role in the control of cellular metabolism in response to availability of nutrients or growth factors (Kapahi et al.). mTOR controls cellular metabolism and organismal lifespan in invertebrates and mammals (Kapahi and Zid, 2004). It was recently shown that inhibition of mTOR activity in mice by chronic rapamycin treatment extends lifespan, possibly by delaying aging (Harrison et al., 2009). To explore the effect of rapamycin on normal brain aging we determined cognitive and non-cognitive components of behavior throughout lifespan in C57BL/6 animals that were fed control- or rapamycin-supplemented chow. Our studies show that feeding encapsulated rapamycin in chow at 14 ppm for at least 16 weeks is sufficient to improve cognitive outcomes in both adult (8 month-old) and in old (25 month-old) C57BL/6 mice compared to control-fed littermates (Figure 1). Even though the acute inhibition of TOR has generally been associated with defects in long-term plasticity (Tang et al., 2002), inhibition of TOR can also block LTD (Huber et al., 2001). Moreover, high TOR activity in neuronal progenitors or in neuronal/glial precursors during development results in significant plasticity and memory deficits (Hoeffer and Klann, 2009) and aberrant excitability, suggesting that adequate plasticity requires that TOR activity be within a range that allows for appropriate regulation of protein synthesis at synaptic sites. Our results suggest that an approximate 30% reduction in TOR activity in brain [Figure 1, (Spilman et al., 2010)] for 16 weeks or longer improves performance of C57BL/6 mice in tasks that involve long-term plasticity and are dependent on hippocampus or on hippocampus and prefrontal cortex. Considered together with prior knowledge on mTOR function (Huber et al., 2001, Tang et al., 2002, Sarbassov et al., 2005, Hoeffer and Klann, 2009), our results suggest that, while acute and complete inhibition of TOR abolishes plasticity required for long-term memory (Tang et al., 2002, Hoeffer and Klann, 2009), chronically decreased TOR activity (in our studies, approximately 30%) can improve learning and retention of a spatial task in young mice and improve memory of an aversive event in old animals (Figure 1). Consistent with these observations, caloric restriction, which decreases mTOR activity (Dogan et al.) and extends lifespan, improves cognitive function in some, but not all, rodent (Mattson et al., 2002, Martin et al., 2007, Adams et al., 2008, Komatsu et al., 2008), primate (Witte et al., 2009, Dal-Pan et al.) or invertebrate (Burger et al.) experimental models. Thus, it is conceivable that long-term, partial reduction of mTOR activity by rapamycin administered in the chow mimics the effect of calorie restriction, enhancing cognitive outcomes in young mice and maintaining intact cognitive performance in older animals. Of note, cognitive effects of rapamycin feeding were observed when mice were fed for 16 weeks or as long as 40 weeks, suggesting that the effect of chronic rapamycin treatment is sustained for protracted lengths of time.
It has been suggested that there was little selective pressure to evolve effective mechanisms of neuroprotection in old age (Finch and Austad, 2012). It is therefore conceivable that brain mTOR activity levels that are adequate during the reproductive years may become detrimental as mammals age (Blagosklonny, 2010). In agreement with this hypothesis, we (Spilman et al., 2010) and others (Caccamo et al 2010) previously showed that chronic (> 16 weeks) inhibition of mTOR activity by rapamycin in brain preserves cognitive function in mice modeling AD, possibly by increasing autophagy.
It had been previously reported that treatment with a rapamycin analog everolimus may decrease anxiety and depressive-like behaviour (Lang et al., 2009) although other studies did not confirm this effect on transplant patients treated with sirolimus or tacrolimus (Martinez-Sanchis et al.). The studies presented here show that C57BL/6 mice chronically fed encapsulated rapamycin exhibit reduced anxiety and decreased depressive-like behavior at all ages tested and in animals of both genders (Figure 2). These results suggest that a partial decrease in TOR activity may impinge on the activity of limbic structures like the amygdala, which mediate emotion output such as fear. It is conceivable that at least one mechanism mediating the effect of long-term oral rapamycin on memory involves the downregulation of synaptic plasticity in limbic networks. This potential mechanism, however, cannot explain the observed effects of chronic rapamycin treatment on spatial learning and memory (Figure 1).
In agreement with the observed changes in behavioral outcomes, however, rapamycin-treated animals showed significantly increased levels of three major monoamines (NE, DA and 5-HT) and their metabolites (DOPAC, HVA, and 5-HIAA) specifically in midbrain (Figure 3 a and c). Even though monoamine levels were not increased in hippocampal tissue (Figure 3 b and d), the observed increase of monoamine content in midbrain is consistent with effects on key target areas such as the septo-hippocampal complex through the mesolimbic pathway. The mesocorticolimbic dopamine system is widely implicated in reward and in reinforcement processes (Morgane et al., 2005). The midbrain contains major dopaminergic projecting neurons in the substantia nigra pars compacta (PC) and ventral tegmental area (VTA). Thus, increases in monoamine content in midbrain is consistent with effects on key target areas such as the striatum (involved in reward/motivation) through the mesostriatal pathway, as well as on the limbic cortices, the septo-hippocampal complex, the amygdala and the nucleus accumbens (involved in emotion, long-term memory and olfaction) through the mesolimbic pathway. Pathways arising from dopaminergic midbrain nuclei also innervate the prefrontal and insular cortices, several thalamic and hypothalamic nuclei and the monoaminergic nuclei of the brain stem, superior colliculus, reticular formation and periaqueductal grey, all having important roles in regulating arousal as well as affective and cognitive processes. In addition, the PC is important in spatial learning and some studies have suggested its involvement in a response-based memory system that utilizes a dorsostriatal pathway and may function independently of the hippocampus (Da Cunha et al., 2003). In addition, the VTA is strongly involved in the reward circuitry of the brain, with important roles in cognition and motivation (Margolis et al., 2006). Dopaminergic input from VTA contributes to processing of emotion output from the amygdala, thus playing a role in avoidance and fear conditioning (Figure 1 k). An increase in dopamine levels in midbrain, therefore, is expected to have a broad impact on both affect and cognitive domains. Thus, our results suggest that increased levels of DA in midbrain may underlie the memory-enhancing, anxiolytic and antidepressant-like effects observed in rapamycin-fed C57BL/6J mice.
DA is a precursor to NE and then to EPI in these neurotransmitters’ biosynthetic pathway (Mains and Patterson, 1973). The observed increase in NE in midbrain of rapamycin-treated animals may therefore be a direct consequence of increased DA levels (Figure 3a). A simple direct precursor relationship, however, is unlikely since EPI was not increased. In midbrain, noradrenergic neurons originate in the lateral tegmental field and exert effects on large areas of the brain, increasing alertness and arousal as well as influencing the reward system through activation of adrenergic receptors in amygdala, cingulated gyrus, hippocampus, hypothalamus, neocortex, striatum and thalamus. Midbrain NE has been associated with the regulation of anxiety (Simon et al., 2009, Watt et al., 2009) and motivation (Heimovics et al.). Moreover, increasing NE and 5-HT levels by inhibition of NE-DA uptake is a common intervention used to treat anxiety in depressive disorders (Tollefson et al., 1991, Papakostas et al., 2008). Uptake of NE-DA in prefrontal cortex is mediated by NET, and it was recently demonstrated (Siuta et al.) that animals with impaired mTORC2 function show increased prefrontal NET, resulting in increased levels of prefrontal NE concomitant with decreased DA. Our results show that neither mTORC2 activity (Figure 1 e-h) nor NET levels (Figure 3 g-h) are affected by rapamycin treatment, suggesting that increased noradrenergic input to cortex is intact in rapamycin-treated mice. Thus, increased NE levels in midbrain may further contribute to the observed memory-enhancing, anxiolytic and antidepressant-like effects of rapamycin treatment in C57BL/6J mice. 5-HT (serotonin) regulates mood, appetite and sleep as well as memory and learning (Hariri and Holmes, 2006, Elliott et al.). The modulation of 5-HT levels at synapses is a major site of action for antidepressants (Sharp and Cowen). In midbrain, the 5-HT in the raphe nuclei (RN) controls the activity of ascending serotonergic systems and the release of 5-HT in forebrain (Adell et al., 2002). 5-HT-containing neurons in the dorsal RN provide part of the serotonergic innervation to widely distributed areas in the brain (Monti). Dysfunctions in forebrain serotonergic innervation from the RN are thought to underlie the neural mechanisms of depression (Stamford et al., 2000). NOR, gamma-amino butyric acid and glutamate regulate the extracellular concentration of 5-HT in raphe nuclei. Thus, it is possible that high NOR stimulates the synthesis and release of 5-HT in midbrain. The pivotal role of 5-HT in the regulation of mood and motivation as well as its role in the regulation of learning and memory provides an additional explanation for the observed improvements in cognitive and non-cognitive components of behavior in rapamycin-treated mice.
The question as to how TOR function is mechanistically linked to the regulation of midbrain monoamine levels, however, remains unanswered. Rapamycin has been shown to inhibit evoked dopamine release in striatal slices through the activation of macroautophagy [Hernandez et al (2012) Neuron 74:277], demonstrating that macroautophagy is necessary for the maintenance of adequate numbers of synaptic vesicles available for release at synaptic sites. Of note, Hernandez et al. noted that rapamycin induced decreased numbers of synaptic vesicles also in non-dopaminergic terminals in striatum, suggesting that the effect of macroautophagy in the maintenance of an adequately sized vesicle pool at nerve terminals may be general and not specific to dopaminergic cells. Decreases in the size of readily releasable vesicle pools mobilizes a reserve pool of neurotransmitter-containing synaptic vesicles [Venton et al. (2006) J Neurosci 26:3206]. Thus, it is conceivable that the increases in monoamine content that we have observed in midbrain may reflect the activation of synthetic pathways to replenish a decreasing pool of synaptic vesicles as a result of a chronic increase in macroautophagy induced by rapamycin treatment. Consistent with this hypothesis, we previously demonstrated that chronic rapamycin treatment increases autophagy in hippocampus of mice modeling AD (Spilman et al., 2010). Since the specific midbrain/brainstem nuclei contain the major dopamine (substantia nigra) and serotonin (raphe nuclei) releasing clusters in the brain, this effect may be more pronounced, and thus readily detectable, in midbrain and brainstem.
In summary, the results of the present study demonstrate that chronic feeding with encapsulated rapamycin enhances memory in young C57BL/6 mice and delays cognitive decline associated with aging. Moreover, our results show that long-term feeding with encapsulated rapamycin has concomitant anxiolytic and antidepressant-like effects. Our findings are consistent with prior studies showing that long-term rapamycin treatment rescued learning and memory in mice modelling AD (Caccamo et al., Spilman et al., 2010). Our results suggest chronic, partial inhibition of mTOR may have widespread effects in the regulation of arousal and attention as well as of affective and cognitive processes, possibly by stimulating major monoamine pathways in brain. Rapamycin and rapamycin analogs, already used in the clinic, may have potential for therapeutic intervention in cognitive and affective dysfunctions associated with aging.
Chronic rapamycin decreased mTORC1 but not mTORC2 activity in mouse brains.
-
-
Chronic rapamycin enhanced learning and memory in young adult C57BL/6J mice.
-
-
Chronic rapamycin improved memory in aged mice.
-
-
Chronic rapamycin decreased anxiety and depressive-like behavior at all ages.
-
-
Monoamines were increased by rapamycin in midbrain but not in hippocampus.
ACKNOWLEDGEMENTS
We thank Ms. Katrine Krueger for excellent administrative assistance. We are also grateful to Dr. Elisabeth Fernandez and to Ms. Xiang Bai for their help with monoamine measurements, and to Ms. Vanessa Soto and Mr. John Ramos and to the South Texas Center for Biology in Medicine Animal Facility staff for excellent animal care. This work was supported in part by the San Antonio Nathan Shock Center of Excellence in the Basic Biology of Aging (AR and RS), RC2AG036613 NIH Recovery Act Grand Opportunities “GO” grant to AR, and by NIRG 04-1054 from the Alzheimer’s Association, AG-NS-0726-10 New Scholar Award in Aging from the Ellison Medical Foundation, and a University Research Council Award from UTHSCSA to VG.
ROLE OF THE FUNDING SOURCE
This work was supported by NIRG 04-1054 from the Alzheimer’s Association, AG-NS-0726-10 New Scholar Award in Aging from the Ellison Medical Foundation, and a University Research Council Award from UTHSCSA to VG, the San Antonio Nathan Shock Center of Excellence in the Basic Biology of Aging to AR and RS, the RC2AG036613 NIH Recovery Act Grand Opportunities “GO” grant to AR, and T32AG21890 to SAH.
GLOSSARY
- TOR
Target of rapamycin
- NE
norepinephrine
- EPI
epinephrine
- DA
dopamine
- 5-HT
5-hydroxytryptamine
- DOPAC
3,4-dihydroxyphenylacetic acid
- HVA
homovanillic acid
- 5-HIAA
5-hydroxyindolacetic acid
- LTD
long-term depression
- AD
Alzheimer’s disease
- NET
norepinephrine transporter
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE STATEMENT
The authors have no conflicts of interest to disclose.
REFERENCES
- Adams MM, Shi L, Linville MC, Forbes ME, Long AB, Bennett C, Newton IG, Carter CS, Sonntag WE, Riddle DR, Brunso-Bechtold JK. (Caloric restriction and age affect synaptic proteins in hippocampal CA3 and spatial learning ability. Exp Neurol. 2008;211:141–149. doi: 10.1016/j.expneurol.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adell A, Celada P, Abellan MT, Artigas F. Origin and functional role of the extracellular serotonin in the midbrain raphe nuclei. Brain Res Brain Res Rev. 2002;39:154–180. doi: 10.1016/s0165-0173(02)00182-0. [DOI] [PubMed] [Google Scholar]
- Blagosklonny MV. Revisiting the antagonistic pleiotropy theory of aging: TOR-driven program and quasi-program. Cell Cycle. 2010;9:3151. doi: 10.4161/cc.9.16.13120. [DOI] [PubMed] [Google Scholar]
- Burger JM, Buechel SD, Kawecki TJ. Dietary restriction affects lifespan but not cognitive aging in Drosophila melanogaster. Aging Cell. 2010;9:327–335. doi: 10.1111/j.1474-9726.2010.00560.x. [DOI] [PubMed] [Google Scholar]
- Caccamo A, Majumder S, Richardson A, Strong R, Oddo S. Molecular interplay between mammalian target of rapamycin (mTOR), amyloid-beta, and Tau: effects on cognitive impairments. J Biol Chem. 2010;285:13107–13120. doi: 10.1074/jbc.M110.100420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho SH, Sun B, Zhou Y, Kauppinen TM, Halabisky B, Wes P, Ransohoff RM, Gan L. CX3CR1 protein signaling modulates microglial activation and protects against plaque-independent cognitive deficits in a mouse model of Alzheimer disease. J Biol Chem. 2011;286:32713–32722. doi: 10.1074/jbc.M111.254268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloughesy TF, Yoshimoto K, Nghiemphu P, Brown K, Dang J, Zhu S, Hsueh T, Chen Y, Wang W, Youngkin D, Liau L, Martin N, Becker D, Bergsneider M, Lai A, Green R, Oglesby T, Koleto M, Trent J, Horvath S, Mischel PS, Mellinghoff IK, Sawyers CL. Antitumor activity of rapamycin in a Phase I trial for patients with recurrent PTEN-deficient glioblastoma. PLoS Med. 2008;5:e8. doi: 10.1371/journal.pmed.0050008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley JN. What’s wrong with my mouse? Wyley-Liss; New York: 2000. [Google Scholar]
- Cryan JF, Mombereau C, Vassout A. The tail suspension test as a model for assessing antidepressant activity: review of pharmacological and genetic studies in mice. Neurosci Biobehav Rev. 2005;29:571–625. doi: 10.1016/j.neubiorev.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Da Cunha C, Wietzikoski S, Wietzikoski EC, Miyoshi E, Ferro MM, Anselmo-Franci JA, Canteras NS. Evidence for the substantia nigra pars compacta as an essential component of a memory system independent of the hippocampal memory system. Neurobiol Learn Mem. 2003;79:236–242. doi: 10.1016/s1074-7427(03)00008-x. [DOI] [PubMed] [Google Scholar]
- Dal-Pan A, Pifferi F, Marchal J, Picq JL, Aujard F. Cognitive performances are selectively enhanced during chronic caloric restriction or resveratrol supplementation in a primate. PLoS One. 2011;6:e16581. doi: 10.1371/journal.pone.0016581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dogan S, Johannsen AC, Grande JP, Cleary MP. Effects of intermittent and chronic calorie restriction on mammalian target of rapamycin (mTOR) and IGF-I signaling pathways in mammary fat pad tissues and mammary tumors. Nutr Cancer. 2011;63:389–401. doi: 10.1080/01635581.2011.535968. [DOI] [PubMed] [Google Scholar]
- Elliott R, Zahn R, Deakin JF, Anderson IM. Affective cognition and its disruption in mood disorders. Neuropsychopharmacology. 2011;36:153–182. doi: 10.1038/npp.2010.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch CE, Austad SN. Primate aging in the mammalian scheme: the puzzle of extreme variation in brain aging. Age (Dordr) 2012 doi: 10.1007/s11357-011-9355-9. Jan 5 epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan V, Gorostiza OF, Banwait S, Ataie M, Logvinova AV, Sitaraman S, Carlson E, Sagi SA, Chevallier N, Jin K, Greenberg DA, Bredesen DE. Reversal of Alzheimer’s-like pathology and behavior in human APP transgenic mice by mutation of Asp664. Proc Natl Acad Sci U S A. 2006;103:7130–7135. doi: 10.1073/pnas.0509695103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan V, Zhang J, Gorostiza OF, Banwait S, Huang W, Ataie M, Tang H, Bredesen DE. Long-term prevention of Alzheimer’s disease-like behavioral deficits in PDAPP mice carrying a mutation in Asp664. Behav Brain Res. 2008;191:246–255. doi: 10.1016/j.bbr.2008.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresack JE, Frick KM. Male mice exhibit better spatial working and reference memory than females in a water-escape radial arm maze task. Brain Res. 2003;982:98–107. doi: 10.1016/s0006-8993(03)03000-2. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Holmes A. Genetics of emotional regulation: the role of the serotonin transporter in neural function. Trends Cogn Sci. 2006;10:182–191. doi: 10.1016/j.tics.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, Pahor M, Javors MA, Fernandez E, Miller RA. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley WR, Grissom EM, Barratt HE, Conrad TS, Dohanich GP. The effects of biological sex and gonadal hormones on learning strategy in adult rats. Physiol Behav. 2012;105:1014–1020. doi: 10.1016/j.physbeh.2011.11.021. [DOI] [PubMed] [Google Scholar]
- Heimovics SA, Salvante KG, Sockman KW, Riters LV. Individual differences in the motivation to communicate relate to levels of midbrain and striatal catecholamine markers in male European starlings. Horm Behav. 2011;60:529–539. doi: 10.1016/j.yhbeh.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeffer CA, Klann E. mTOR signaling: at the crossroads of plasticity, memory and disease. Trends Neurosci. 2009;33:67–75. doi: 10.1016/j.tins.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber KM, Roder JC, Bear MF. Chemical induction of mGluR5- and protein synthesis--dependent long-term depression in hippocampal area CA1. J Neurophysiol. 2001;86:321–325. doi: 10.1152/jn.2001.86.1.321. [DOI] [PubMed] [Google Scholar]
- Kapahi P, Chen D, Rogers AN, Katewa SD, Li PW, Thomas EL, Kockel L. With TOR, less is more: a key role for the conserved nutrient-sensing TOR pathway in aging. Cell Metab. 2010;11:453–465. doi: 10.1016/j.cmet.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapahi P, Zid B. TOR pathway: linking nutrient sensing to life span. Sci Aging Knowledge Environ. 2004;2004:PE34. doi: 10.1126/sageke.2004.36.pe34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu T, Chiba T, Yamaza H, Yamashita K, Shimada A, Hoshiyama Y, Henmi T, Ohtani H, Higami Y, de Cabo R, Ingram DK, Shimokawa I. Manipulation of caloric content but not diet composition, attenuates the deficit in learning and memory of senescence-accelerated mouse strain P8. Exp Gerontol. 2008;43:339–346. doi: 10.1016/j.exger.2008.01.008. [DOI] [PubMed] [Google Scholar]
- Lang UE, Heger J, Willbring M, Domula M, Matschke K, Tugtekin SM. Immunosuppression using the mammalian target of rapamycin (mTOR) inhibitor everolimus: pilot study shows significant cognitive and affective improvement. Transplant Proc. 2009;41:4285–4288. doi: 10.1016/j.transproceed.2009.08.050. [DOI] [PubMed] [Google Scholar]
- Mains RE, Patterson PH. Primary cultures of dissociated sympathetic neurons. II. Initial studies on catecholamine metabolism. J Cell Biol. 1973;59:346–360. doi: 10.1083/jcb.59.2.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis EB, Lock H, Hjelmstad GO, Fields HL. The ventral tegmental area revisited: is there an electrophysiological marker for dopaminergic neurons? J Physiol. 2006;577:907–924. doi: 10.1113/jphysiol.2006.117069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin B, Pearson M, Kebejian L, Golden E, Keselman A, Bender M, Carlson O, Egan J, Ladenheim B, Cadet JL, Becker KG, Wood W, Duffy K, Vinayakumar P, Maudsley S, Mattson MP. Sex-dependent metabolic, neuroendocrine, and cognitive responses to dietary energy restriction and excess. Endocrinology. 2007;148:4318–4333. doi: 10.1210/en.2007-0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin DE, Hall MN. The expanding TOR signaling network. Curr Opin Cell Biol. 2005;17:158–166. doi: 10.1016/j.ceb.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Martinez-Sanchis S, Bernal MC, Montagud JV, Candela G, Crespo J, Sancho A, Pallardo LM. Effects of immunosuppressive drugs on the cognitive functioning of renal transplant recipients: a pilot study. J Clin Exp Neuropsychol. 2011;33:1016–1024. doi: 10.1080/13803395.2011.595396. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Duan W, Chan SL, Cheng A, Haughey N, Gary DS, Guo Z, Lee J, Furukawa K. Neuroprotective and neurorestorative signal transduction mechanisms in brain aging: modification by genes, diet and behavior. Neurobiol Aging. 2002;23:695–705. doi: 10.1016/s0197-4580(02)00025-8. [DOI] [PubMed] [Google Scholar]
- Michalek RD, Rathmell JC. Methed-up FOXOs can’t in-Akt-ivate. Mol Cell. 2008;32:160–162. doi: 10.1016/j.molcel.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti JM. The role of dorsal raphe nucleus serotonergic and non-serotonergic neurons, and of their receptors, in regulating waking and rapid eye movement (REM) sleep. Sleep Med Rev. 2010;14:319–327. doi: 10.1016/j.smrv.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Morgane PJ, Galler JR, Mokler DJ. A review of systems and networks of the limbic forebrain/limbic midbrain. Prog Neurobiol. 2005;75:143–160. doi: 10.1016/j.pneurobio.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Papakostas GI, Trivedi MH, Alpert JE, Seifert CA, Krishen A, Goodale EP, Tucker VL. Efficacy of bupropion and the selective serotonin reuptake inhibitors in the treatment of anxiety symptoms in major depressive disorder: a meta-analysis of individual patient data from 10 double-blind, randomized clinical trials. J Psychiatr Res. 2008;42:134–140. doi: 10.1016/j.jpsychires.2007.05.012. [DOI] [PubMed] [Google Scholar]
- Pechnick RN, Chesnokova VM, Kariagina A, Price S, Bresee CJ, Poland RE. Reduced immobility in the forced swim test in mice with a targeted deletion of the leukemia inhibitory factor (LIF) gene. Neuropsychopharmacology. 2004;29:770–776. doi: 10.1038/sj.npp.1300402. [DOI] [PubMed] [Google Scholar]
- Polak P, Hall MN. mTOR and the control of whole body metabolism. Curr Opin Cell Biol. 2009;21:209–218. doi: 10.1016/j.ceb.2009.01.024. [DOI] [PubMed] [Google Scholar]
- Rodgers RJ. Animal models of ‘anxiety’: where next? Behav Pharmacol. 1997;8:477–496. doi: 10.1097/00008877-199711000-00003. [DOI] [PubMed] [Google Scholar]
- Salih DA, Brunet A. FoxO transcription factors in the maintenance of cellular homeostasis during aging. Curr Opin Cell Biol. 2008;20:126–136. doi: 10.1016/j.ceb.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarbassov DD, Ali SM, Sabatini DM. Growing roles for the mTOR pathway. Curr Opin Cell Biol. 2005;17:596–603. doi: 10.1016/j.ceb.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Sarbassov DD, Ali SM, Sengupta S, Sheen JH, Hsu PP, Bagley AF, Markhard AL, Sabatini DM. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell. 2006;22:159–168. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- Sharp T, Cowen PJ. 5-HT and depression: is the glass half-full? Curr Opin Pharmacol. 2011;11:45–51. doi: 10.1016/j.coph.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Simon NM, Kaufman RE, Hoge EA, Worthington JJ, Herlands NN, Owens ME, Pollack MH. Open-label support for duloxetine for the treatment of panic disorder. CNS Neurosci Ther. 2009;15:19–23. doi: 10.1111/j.1755-5949.2008.00076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siuta MA, Robertson SD, Kocalis H, Saunders C, Gresch PJ, Khatri V, Shiota C, Kennedy JP, Lindsley CW, Daws LC, Polley DB, Veenstra-Vanderweele J, Stanwood GD, Magnuson MA, Niswender KD, Galli A. Dysregulation of the norepinephrine transporter sustains cortical hypodopaminergia and schizophrenia-like behaviors in neuronal rictor null mice. PLoS Biol. 2010;8:e1000393. doi: 10.1371/journal.pbio.1000393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spilman P, Podlutskaya N, Hart MJ, Debnath J, Gorostiza O, Bredesen D, Richardson A, Strong R, Galvan V. Inhibition of mTOR by rapamycin abolishes cognitive deficits and reduces amyloid-beta levels in a mouse model of Alzheimer’s disease. PLoS One. 2010;5:e9979. doi: 10.1371/journal.pone.0009979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamford JA, Davidson C, McLaughlin DP, Hopwood SE. Control of dorsal raphe 5-HT function by multiple 5-HT(1) autoreceptors: parallel purposes or pointless plurality? Trends Neurosci. 2000;23:459–465. doi: 10.1016/s0166-2236(00)01631-3. [DOI] [PubMed] [Google Scholar]
- Tang SJ, Reis G, Kang H, Gingras AC, Sonenberg N, Schuman EM. A rapamycin-sensitive signaling pathway contributes to long-term synaptic plasticity in the hippocampus. Proc Natl Acad Sci U S A. 2002;99:467–472. doi: 10.1073/pnas.012605299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tecott LH, Nestler EJ. Neurobehavioral assessment in the information age. Nat Neurosci. 2004;7:462–466. doi: 10.1038/nn1225. [DOI] [PubMed] [Google Scholar]
- Tollefson GD, Lancaster SP, Montague-Clouse J. The association of buspirone and its metabolite 1-pyrimidinylpiperazine in the remission of comorbid anxiety with depressive features and alcohol dependency. Psychopharmacol Bull. 1991;27:163–170. [PubMed] [Google Scholar]
- Watt MJ, Burke AR, Renner KJ, Forster GL. Adolescent male rats exposed to social defeat exhibit altered anxiety behavior and limbic monoamines as adults. Behav Neurosci. 2009;123:564–576. doi: 10.1037/a0015752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte AV, Fobker M, Gellner R, Knecht S, Floel A. Caloric restriction improves memory in elderly humans. Proc Natl Acad Sci U S A. 2009;106:1255–1260. doi: 10.1073/pnas.0808587106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Gorostiza OF, Tang H, Bredesen DE, Galvan V. Reversal of learning deficits in hAPP transgenic mice carrying a mutation at Asp664: A role for early experience. Behav Brain Res. 2009 doi: 10.1016/j.bbr.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]