Abstract
In nature, individuals vary tremendously in condition and this may be an important source of variation in mutation rate. Condition is likely to affect cell state and thereby impact the amount of DNA damage sustained and/or the way it is repaired. Here, we focus on DNA repair. If low-condition individuals are less capable of devoting the same level of resources to accurate repair, they may suffer higher mutation rates. However, repair decisions are also governed by various aspects of cell physiology, which may render the prediction that “higher-condition individuals use better repair mechanisms” too simplistic. We use a larval diet manipulation in Drosophila melanogaster to create high- and low-condition individuals and then contrast their relative usage of three repair pathways [homologous recombination (HR), single-strand annealing (SSA), and nonhomologous end joining (NHEJ)] that differ in their mechanistic requirements and their mutational consequences. We find that low-condition flies are more likely than high-condition flies to use the most conservative of these three repair pathways, suggesting that physiological constraints on repair pathway usage may be more important than energetic costs. We also show that the repair differences between high- and low-condition flies resemble those between young and old flies, suggesting the underlying mechanisms may be similar. Finally, we observe that the effect of larval diet on adult repair increases as flies age, indicating that developmental differences early in life can have long-lasting consequences.
Keywords: DNA repair, mutations, condition, aging
DESPITE being necessary for adaptation, the majority of spontaneous mutations are deleterious (Morgan 1903; Keightley and Lynch 2003). Their accumulation imposes a reduction in population mean fitness, known as mutation load (Haldane 1937), that challenges the genetic health (Kondrashov 1995; Crow 1997) of natural populations and can lead to their accelerated extinction (Lande 1994; Lynch et al. 1995). Mutation load is also implicated as a driving force behind evolutionary phenomena (Lynch et al. 1999) such as the evolution of sex (Kondrashov 1988; Agrawal 2001; Keightley and Otto 2006) and senescence (Partridge and Barton 1993), and the maintenance of genetic variation (Barton and Turelli 1989; Bulmer 1989).
Mutation rate (U) determines the magnitude of the mutation load (Haldane 1937); thus, it is important to understand the selective forces and biological mechanisms underlying its evolution. Theory suggests that selection should favor very low mutation rates in sexual taxa (Leigh 1970; Sniegowski et al. 2000), yet genome-wide rates of nonneutral mutation (U) are quite high in multicellular organisms (Baer et al. 2007). Traditionally, equilibrium mutation rate is thought to be determined by trade-offs between the “costs of DNA fidelity,” and the deleterious consequences of new mutations (Kimura 1967; Baer et al. 2007; but see Lynch 2008). Boundaries on the cellular resources devoted to maintaining DNA replication and repair can arise through a number of different paths. Increased fidelity may involve more energetic expenditure, as suggested by in vitro studies in bacteriophage T4 (Bessman et al. 1974; Clayton et al. 1979). In addition, vigilant DNA replication and repair might require more time (Kirkwood et al. 1986) and lead to slower cell growth (Preston et al. 2006b).
The idea that costs prevent trait values from reaching their optima is a recurring theme in evolutionary biology. Trait variation is thought to arise from differences in condition, as individuals with higher levels of resource acquisition are expected to be able to allocate more to all traits (Van Noordwijk and De Jong 1986; Houle 1991; Rowe and Houle 1996; David et al. 2000; Tomkins et al. 2004). Most types of DNA damage can be repaired via multiple alternative repair pathways. Some types of repair are more conservative (i.e., less likely to result in a mutation) but may be more costly to employ. As U is mediated by an individual’s ability to repair DNA damage, condition-dependent DNA repair can lead to mutation rate variation, especially if low-condition individuals suffer higher U as a result of lower investment into DNA maintenance.
Among the different forms of DNA damage, DNA double-strand breaks (DSBs) are thought to be the most toxic lesions (Kanaar et al. 1998; Jackson 2002). The frequency of spontaneous DSBs in early passage human fibroblast cells has been estimated at ∼10 per day per cell (Lieber 2010) and occur through oxidative damage, ionizing radiation, and replication fork collapse during DNA replication. Cells likely devote significant resources to the repair of DSBs, as defects in their repair lead to elevated cancer risk and phenotypes resembling accelerated aging (Engels et al. 2007). There is a fair degree of flexibility in DSB repair processes (Ohnishi et al. 2009). Importantly, the different DSB repair pathways may be associated with different costs and they confer varying levels of risk for inducing mutations at the repair site. However, DSB repair pathway choice also appears to be regulated by numerous physiological factors including cell cycle phase (Langerak and Russell 2011), genomic context (Moore and Haber 1996), developmental timing, and age (Preston et al. 2006a).
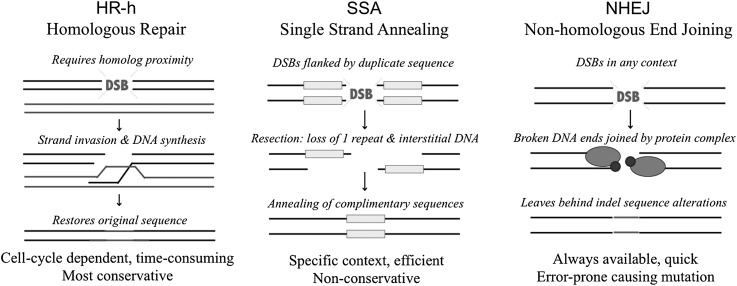
We focus on three major DSB repair pathways: homologous recombination with the homolog as a repair template (HR-h), single-strand annealing (SSA), and nonhomologous end-joining (NHEJ) (Figure 1). HR-h is the most conservative among these three pathways as it involves conversion from an undamaged homologous template, which restores the original sequence around the DSB. While HR-h repair is highly accurate, usage of this pathway is cell cycle dependent (Aylon et al. 2004; Saleh-Gohari and Helleday 2004). In addition, involvement of DNA synthesis means that utilization of HR-h is much more time consuming (Mao et al. 2008). SSA can only occur for DSBs flanked by duplicated sequence because it employs a simple annealing mechanism following single-strand pairing between the complementary duplicate sequences. It is thought to be highly efficient because it is the most commonly used repair pathway in this genomic context (Preston et al. 2002; 2006a). However, use of SSA always leads to loss of one of the repeats as well as any interstitial sequence. By removing a complete repeat, this type of sequence change might have severe fitness consequences in some cases but may often be neutral or only weakly deleterious. The third repair pathway, NHEJ, requires no sequence homology and is typically the least conservative (Mao et al. 2008). It uses simple ligation to join together the broken DNA strands following modification of the DSB ends, which usually introduces an indel. Differences in condition may cause individuals to differ in their available energy to devote to DNA repair as well as in cell cycle dynamics that may constrain the usage of different pathways. Though the deleterious effects of DSBs could be minimized by preferentially using the more conservative among these three pathways (e.g., HR-h > SSA > NHEJ), this could be countered by energetic trade-offs or physiological constraints.
Figure 1 .
Comparison of the alternative DSB repair pathways (HR-h, SSA, and NHEJ) measured by the repair reporter construct system. Horizontal lines above represent single strands of DNA, with each pair of lines forming a duplex DNA molecule (for HR-h, the additional duplex without the DSB represents a homologous chromosome serving as a repair template).
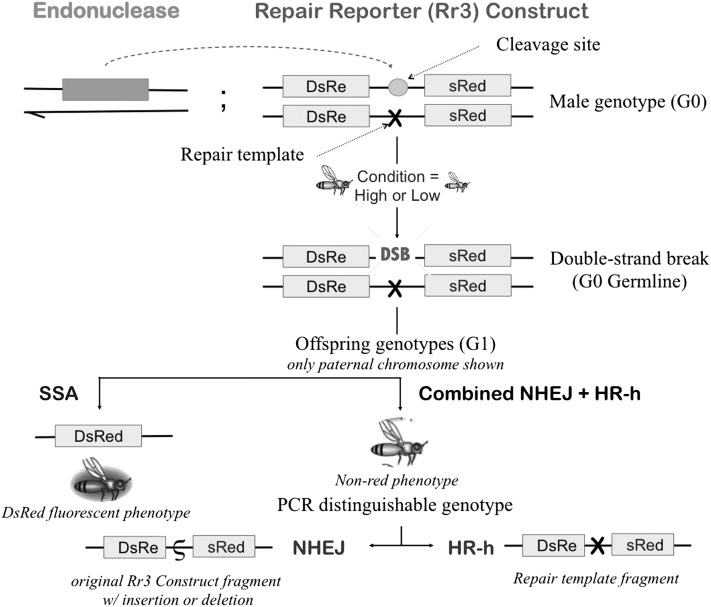
To test for condition dependence in DSB repair, male Drosophila melanogaster of high or low condition were created through manipulation of larval diet. We then used a repair reporter construct system (Preston et al. 2006a) that allows us to induce DSBs in the germ-line cells of adult male D. melanogaster and compare the relative frequencies of HR-h, SSA, and NHEJ across the two condition groups (Figure 2). These males bear gene constructs that serve as the site of DSB creation as well as a homologous repair template. They also carry an endonuclease construct and continuous expression of this gene results in DSBs being created at the same recognition site in individual germ-line cells. Successful repair by each of these three different pathways leaves behind a unique repair product whose genetic signature can be traced in the progeny of the males.
Figure 2 .
Assay used to measure DSB repair pathway use. Males being tested for DSB repair carry an endonuclease and a cleavage recognition (DSB induction) site construct on their first and second chromosomes, respectively. The genotype of the females mated to the males do not contain either of these constructs but do bear visible markers to allow classification of offspring as having inherited the Rr3 construct and/or the endonuclease from their father. Three separate offspring types can be scored, allowing measurement of the relative usage of HR-h, SSA, and NHEJ for each individual male.
An important caveat of the repair reporter construct system used here is that it is unable to quantify the absolute frequencies of all possible DSB repair events as some forms of repair yield undetectable genetic signatures. First, the homologous recombination pathway can also utilize the sister chromatid as its repair template (HR-s). Second, there is some chance that NHEJ repair could be perfectly precise. Both these cases (HR-s or precise NHEJ) would result in restoration of the DSB cut site. These repair products would remain available for recutting by the endonuclease. In principle, cutting and restoration via HR-s or precise NHEJ could occur repeatedly. The process would eventually be terminated when use of HR-h, SSA, or imprecise NHEJ repairs the DSB without restoring the cut site. Although we cannot measure the rate at which HR-s and precise NHEJ repair occur, this system can be used to measure the relative frequencies of HR-h, SSA, and imprecise NHEJ repair when the other pathways are effectively unavailable (Preston et al. 2006a,b). By monitoring the relative HR-h, SSA, and imprecise NHEJ frequencies at weekly intervals for each male, for up to 5 weeks of its lifespan, we are able to assess how experimentally induced differences in condition affect the relative usage of alternative repair pathways and how these differences change with age.
Materials and Methods
Drosophila stocks and crosses
Stocks carrying the gene constructs (Rr3, Rr3EJI, and UIE) of the repair reporter system were obtained from the W. R. Engels lab (University of Wisconsin, Madison, WI). Details of these constructs can be found in Preston et al. (2006a). We introgressed each of the repair reporter constructs onto the genetic background of our standard large outbred population (Dah). Originally collected in 1970 from Dahomey (now Benin), West Africa, this population has been maintained at large population size in various labs since then, and in our lab for 4 years at the time of introgression. All stocks were cultured using standard Drosophila protocol at 25° on a 12L:12D cycle, with 70% relative humidity (Ashburner et al. 2005).
Male D. melanogaster of high or low condition were produced through manipulation of larval diet. High-condition individuals were raised on 7.5 ml of standard sugar-yeast media while low-condition individuals were raised on media in which sugar and yeast concentrations were reduced by 75%. Density was constant between the two treatment conditions [∼50 larvae per 8-dr (32 ml) vial]. Adult males were collected within 16 hr of eclosion on the same day and were therefore of the same age ±8 h; these males were used in one of three assays: weight, siring success, or DSB repair. Males were held in vials containing standard media with live yeast for 2 days prior to being used for the assays. For the weight assay, males were dehydrated in a drying oven overnight and individually measured for dry mass on a fine balance scale. Males for use in the repair reporter assay were mated individually to four virgin females (2–4 days of age). Males for use in the competitive mating ability assay were mated in groups of two males to four virgin females (2–4 days of age) with three competitor males (2 days of age). All females and competitor males shared the same outbred genetic backgrounds but were marked with visible phenotypic mutations (repair: al1bw1, competitive mating ability: se1) to allow us to distinguish between different progeny types. Mating occurred over a 3-day period in both assays, after which the males being tested for competitive mating ability were transferred to holding vials and the females and competitor males were discarded. Repair and competitive mating ability assays were then repeated weekly, through up to 5 weeks of the males’ lifetimes, with a fresh set of females and competitor males being used each week.
Fitness measurements and competitive mating ability assay
We quantified the condition differences between our high- and low-condition treatments through several fitness measurements. First, we compared mean dry mass of high- and low-condition males using a t-test analysis. Second, we looked for differences in survivorship and fertility of the two condition groups. Survival data (dead or alive) were collected weekly for the males in both the repair reporter and competitive mating ability assays, while fertility data (presence or absence of viable adult offspring) were collected only from the males in the repair reporter assays. Survivorship and fertility curves were estimated using the Kaplan–Meier estimators (“survfit” function in Survival package of R) (R Development Core Team, 2012). Differences in the average survivorship and fertility of high- vs. low-condition males were tested using Cox proportional hazards regression analysis. This analysis included a censoring indicator since the experiment concluded at week 5 for all males, and individuals still surviving at that time were not tracked until their natural death or sterility. Log-likelihood ratio tests were employed to find the best fitted model for age-specific survivorship and fertility rates. Significance of the model parameters were then compared among groups by constraining one parameter to be the same for both groups and comparing log-likelihood values of constrained models to the unconstrained model for both groups.
Finally, we compared the relative siring successes of high- and low-condition males through a competitive mating ability assay conducted in parallel with the repair assays. Offspring emerging from the competitive mating ability assay were scored weekly for a total of 5 weeks. Paternity of the offspring was distinguishable through eye color phenotype, and the proportion of noncompetitor sired progeny was calculated at each time point. Only the replicates in which both males being tested for competitive mating ability remained alive at the end of the 5-week experimental duration were included in the statistical analysis to avoid the confounding effects of mortality. To test for condition dependence in siring success, we analyzed the complete lifetime proportion data using a generalized linear model (“glm” function in R) (R Development Core Team, 2012) specifying a quasibinomial error structure.
Repair reporter assay
Use of the repair reporter assay allows us to induce DSBs in the germ-line cells of adult male D. melanogaster and compare the relative frequencies of HR-h, SSA, and NHEJ across our two condition groups (Figure 2). Males bear two gene constructs—P{Rr3}48C and P{Rr3EJI}48C—that serve as the site of DSB creation and the homologous repair template, respectively. They also bear P{UIE}5B, which is a construct carrying a transgenic endonuclease gene under a Ubiquitin promoter; continuous expression of this gene results in DSBs being created at the recognition site (P{Rr3}48C) in individual germ-line cells. Successful repair by each of the three pathways leaves behind a unique repair product whose genetic signature can be traced in the progeny of the males. Monitoring of relative HR-h, SSA, and NHEJ frequencies was done at weekly intervals for each male for up to 5 weeks of its lifespan. (Throughout, NHEJ implicitly refers to imprecise NHEJ, as we cannot detect precise NHEJ events.) Each time point consisted of germ-line cells sampled over 3 days within that week.
Exact details of the assay can be found in Preston et al. (2006a,b); a brief summary is given in Figure 2. The frequency of SSA was estimated from the proportion of male offspring that are DsRed+ (as male progeny do not inherit the X-linked endonuclease gene construct and cannot experience somatic repair to generate fluorescent protein). The combined frequency of HR-h and NHEJ was estimated as the proportion of female offspring that are DsRed−. These proportions were calculated for the high- and low-condition treatment groups at all five time points. In addition, at weeks 1 and 4, the combined frequency of HR-h and NHEJ was partitioned into its two components through PCR scoring of a subset (5–10 randomly chosen or as many as available) of each male’s DsRed-daughters. Two sets of primers were used in the PCR reactions: a control set amplifying the region containing the mutated Rr3 induction site and another that was specific to the homologous repair template sequence. PCRs positive for the repair template fragment (EJI+) were classified as HR-h and those expressing only the control fragment were classified as NHEJ. HR-h events were also subclassified as either long (HR-lg) or short (HR-sh) tract conversions (Preston et al. 2006b). Due to a 16-bp deletion lying 156 bp to the right of the endonuclease cleavage site in the repair reporter construct, conversion tracts extending further then 156 bp in that direction result in a visibly shorter PCR product.
As mentioned above, frequencies of the three measured DSB repair pathways were estimated from the progeny (proportion of different offspring genotypes) of the high- and low-condition males. Since males used to estimate DSB repair pathway use were mated individually to several females, the progeny from each individual male can be considered as independent trials. However, the individual offspring produced by a single male are not independent from one another. One important reason for this is that a single repair event, especially those occurring in the earlier stages of germ cell development, can be represented in multiple offspring of an individual male (Preston et al. 2006a). To make statistically valid inferences about the effects of condition, each male should be used as the level of replication rather than each individual offspring. In the analyses described below, we treat the datum from each male (represented as the proportion of offspring of a given type) as an independent observation. Each datum is a proportion and is statistically modeled as such rather than treating proportions as continuous real numbers.
We first used complete lifetime data to test for differences in usage of SSA; using a generalized linear model (“glm” function in R) (R Core Development Team, 2012), the proportion of SSA-derived offspring from each male was fitted as a function of the fixed factor condition, using a quasibinomial error structure. We also analyzed the data for each week separately to confirm that the treatment groups were different at all time points. Similar models were fit for the proportion of HR-h and NHEJ and for the proportion of HR-lg and HR-sh at weeks 1 and 4. Finally, we examined whether repair pathway use varied with age and whether the effect of age was the same for high- and low-condition individuals. This was done by fitting the proportion of SSA-derived offspring from each male using a generalized mixed model function (“lmer” function in R) (R Development Core Team, 2012), with condition, age, and a condition × age interaction as fixed factors, and male identity as a random factor and assuming a binomial error structure. Inclusion of male identity in the model structure accounts for the nonindependence of sets of offspring taken from a single male at different ages.
Results
High- and low-condition males differ in fitness
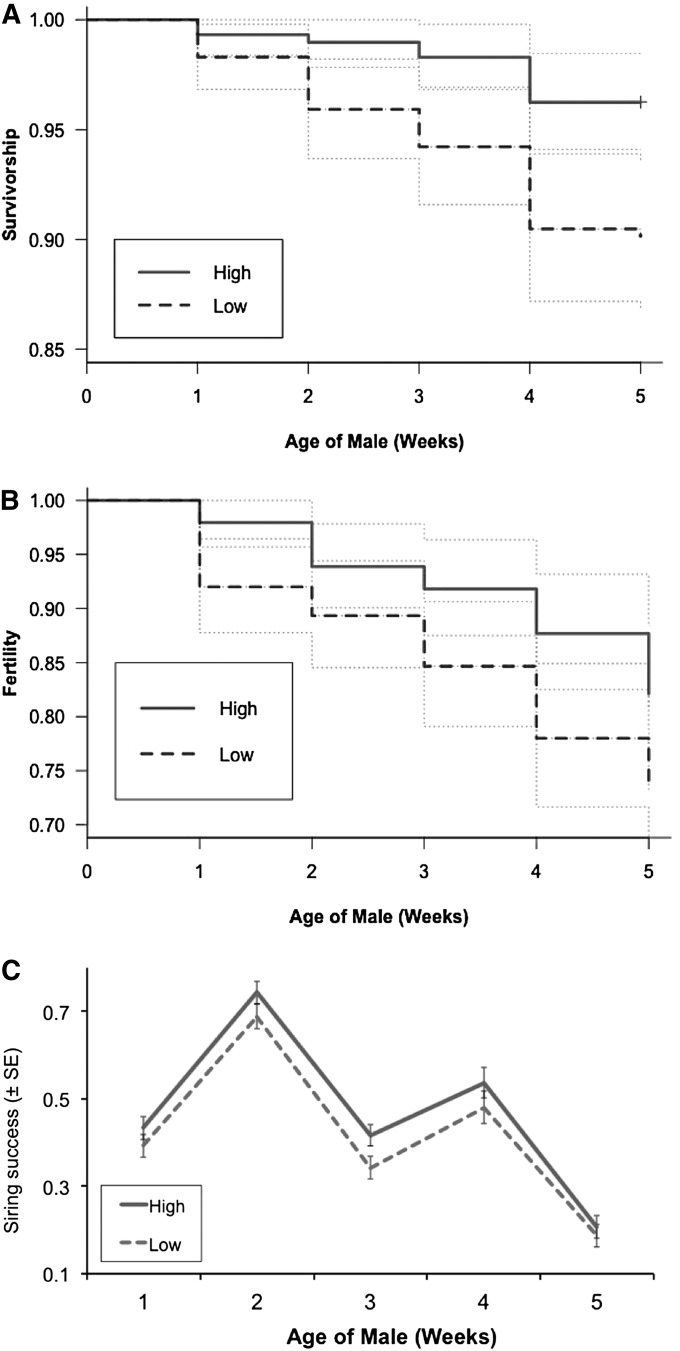
The diet manipulation was effective in producing flies that differed in condition as measured by mass: on average, males emerging from the high-condition treatment were ∼29% larger (t = 24.96, d.f. = 145, P < 10−16) than low-condition males. As expected, condition affected the fitness of the flies (Figure 3). Mean time to mortality was ∼7% longer for high-condition individuals and they exhibited higher survivorship (χ2 = 8.97, d.f. = 1, P = 0.003) throughout the 5 weeks of the experiment (Figure 3A). Similar results were observed for fertility (Figure 3B). High-condition individuals remained fertile ∼16% longer than low-condition individuals (χ2 = 3.71, d.f. = 1, P = 0.05). Moreover, high-condition males consistently sired more offspring in competitive mating assays than their low-condition counterparts through all 5 weeks of the experiment (Figure 3C) and had significantly higher lifetime siring success (t = −2.19, d.f. = 145, P = 0.0304).
Figure 3 .
Differences in fitness-related traits between high- and low-condition males: (A) survival, (B) fertility, and (C) siring success against a competitor. High-condition males are better across all three fitness measurements. Note that in C, males are tested against a new set of competitors each week and siring success is measured as the percentage of offspring sired by the test males. Because of random fluctuations in rearing conditions of competitors among weeks, the quality of competitors likely varies across weeks, contributing to the nonmonotonic change in siring success through time. However, high-condition males consistently perform better against competitors than do low-condition males.
High- and low-condition males differ in repair pathway use
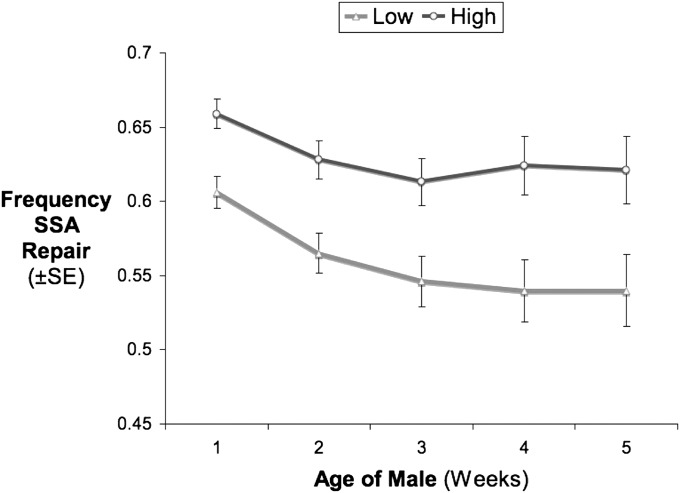
Usage estimates of the three repair pathways are summarized in Table 1 and are comparable with results from previous studies (Preston et al. 2006a,b). (Table 1 shows offspring counts pooled across all males for simplicity, but the statistical analyses account for the nonindependence of offspring from the same male.) SSA was the most frequently used repair pathway in both condition groups, but high-condition males use this pathway ∼11% more than low-condition males considering lifetime reproduction (t = −4.43, d.f. = 282, P = 1.33e-05) (Figure 4). Consistent with the results of Preston et al. (2006b), we also observed a decrease in SSA use with age (χ2 = 49.9, d.f. = 1, P < 1.65e-12). Interestingly, we found that the rate of change in SSA repair with age was almost double for low-condition males than for high-condition males (χ2 = 6.07, d.f. = 1, P = 0.014). SSA use decreased by ∼6% by week 5 in the high-condition group, whereas it decreased by ∼11% in the low-condition group (Figure 4).
Table 1 . Estimates of the relative usage of DSB repair pathways in the germ-line cells of males.
| No. PCRs scored |
Estimates |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Week | No. males | DsRed+/all Endo− | DsRed−/all Endo+ | NHEJ | HR-sh | HR-lg | SSA | NHEJ | HR-h |
| (A) High-condition group | |||||||||
| 1 | 146 | 3087/4687 | 1499/4521 | 326 | 99 | 140 | 0.659 | 0.202 | 0.138 |
| 2 | 138 | 2311/3682 | 1300/3623 | — | — | — | 0.628 | — | — |
| 3 | 133 | 1933/3155 | 1235/3354 | — | — | — | 0.613 | — | — |
| 4 | 127 | 1970/3155 | 1170/3235 | 69 | 111 | 169 | 0.624 | 0.098 | 0.270 |
| 5 | 118 | 1563/2516 | 1102/2860 | — | — | — | 0.621 | — | — |
| Life | — | 0.632 | 0.150 | 0.204 | |||||
| (B) Low-condition group | |||||||||
| 1 | 137 | 2654/4380 | 1499/4521 | 302 | 115 | 133 | 0.606 | 0.207 | 0.160 |
| 2 | 135 | 2032/3596 | 1300/3623 | — | — | — | 0.565 | — | — |
| 3 | 122 | 1588/2907 | 1235/3354 | — | — | — | 0.546 | — | — |
| 4 | 115 | 1590/2945 | 1345/2949 | 53 | 132 | 183 | 0.540 | 0.099 | 0.379 |
| 5 | 107 | 1213/2247 | 1223/2592 | — | — | — | 0.540 | — | — |
| Life | — | 0.565 | 0.153 | 0.270 | |||||
Figure 4 .
Relative usage of SSA in the germ-line cells of male D. melanogaster through the first 5 weeks of their life. SSA use differs between the high- vs. low-condition groups and declines with age in a condition-dependent manner.
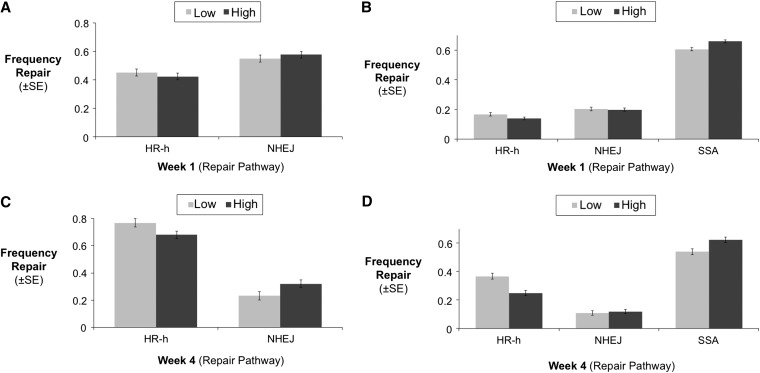
DSBs not repaired through SSA can be repaired through either HR-h or NHEJ. However, young (week 1, Figure 5A) and old flies (week 4, Figure 5C) differ in their usage of these pathways (χ2 = 168.4, d.f. = 1, P = 2.2e-16). When DSBs were not repaired through SSA, the two other pathways were used at similar rates when flies were young, with NHEJ being used slightly more frequently than HR-h (Figure 5A). However, in older flies (week 4), HR-h was used substantially more than NHEJ (Figure 5C). Analyzing the data from young and old flies separately, there is no effect of condition on the relative usage of HR-h to NHEJ (for non-SSA repairs) in young flies (t = 0.82, d.f. = 273, P = 0.414) but there is a condition effect in old flies (t = 2.10, d.f. = 224, P = 0.037). This result provides another example of how high- and low-condition flies age differently with respect to repair pathway usage.
Figure 5 .
Relative usage of HR-h and NHEJ in the germ-line cells of male D. melanogaster at A and B, week 1 and C and D, week 4. (A and C) Usage of HR-h and NHEJ for DSB that were not repaired by SSA [e.g., frequency of HR-h = HR-h/(HR-h + NHEJ)]. While no condition dependence is observed in the use of HR-h relative to NHEJ in young flies, there is a substantial effect of condition in week 4. (B and D) Usage of each pathway as a percentage of the total [e.g., frequency of HR-h = (HR-h/(HR-h + NHEJ)) × (percentage non-SSA)]. Declines in SSA with age appear to be primarily compensated by increases in HR-h.
Not only did high- and low-condition flies differ in how often they used the most conservative of the three pathways, HR-h, but they also differed in how it was used. Condition had a significant effect on the proportion of long (HR-lg) and short (HR-sh) tract HR-h events (χ2 = 20.122, d.f. = 2, P = 4.272e-05), with low-condition flies having significantly fewer long-tract conversions (Table 1). While a previous study (Preston et al. 2006b) documented a 60% increase in the proportion of HR-lg events with age, we did not observe a significant age effect (χ2 = 1.214, d.f. = 1, P = 0.27), though there was a modest increase in the point estimate of the frequency of HR-lg tract conversions.
Above, we evaluated the usage of HR-h relative to NHEJ to focus on changes in pathway use that were statistically independent of the changes in SSA we previously discussed. However, if we consider all three pathways together (measuring each pathway as a percentage of the total), we see that both SSA and NHEJ decline with age, while usage of the most conservative pathway, HR-h, increases (Figure 5, B and D). Overall, the condition treatment groups differ most with respect to SSA and HR-h usage, and these differences are greatest at older ages (Figure 5D).
Discussion
Possible causes of condition-dependent DNA repair
We found that low-condition flies use the most conservative pathway (HR-h) more than high-condition flies. This pattern is counter to the result expected under the assumption that condition dependence in repair arises solely due to a presumed trade-off between accuracy and the energetic costs of different pathways. However, this perspective omits consideration of any physiological constraints on the usage of alternative pathways.
The cell cycle phase at which DSBs occur and the duration of each phase may be critical factors determining which repair pathway is used. Recent studies have shown that the frequency of HR-h can vary across cell cycle phases (Aylon et al. 2004; Saleh-Gohari and Helleday 2004). In yeast, this appears to be driven by the cell cycle regulated activity of the Clb-CDK protein, which has low activity from mitosis to late G1 but becomes highly active during S and G2 (Nasmyth 1996). Specifically, Clb-CDK seems to be involved in resection of DSB ends to produce ssDNA tails (Aylon et al. 2004). As HR-h requires extensive end resection to invade the homologous repair template, it is less frequently used outside of S and G2. While SSA has not been measured in the same way for cell cycle dependency, it may also be cell cycle regulated, as it too requires the formation of extensive ssDNA (Fishman-Lobell et al. 1992). NHEJ on the other hand, is operational throughout the cell cycle (Moore and Haber 1996). Moreover, DSB repair pathway use may be related to cell cycle duration. In vitro measurements in human cells have shown that while NHEJ takes only ∼30 min to complete, HR repair can take upwards of 7 hr (Mao et al. 2008). This is likely because conservative HR-h repair involves seeking out a repair template as well as extensive DNA synthesis. These observations lead to two alternative hypotheses, one nonadaptive and the other adaptive, for the observed effect of condition on repair pathway use.
Cell cycle length is an important determinant of overall growth rate and its overall duration likely trades off directly with the rate of cell growth. While we are aware of no studies directly examining whether condition affects cell cycle dynamics, numerous studies examining life-history traits (i.e., development time, body size, longevity) have shown that individuals in higher condition have increased growth-related fitness (Jenkins et al. 2001; Hunt et al. 2004; Reitzel 2004; Perry and Rowe 2010). Therefore, high-condition individuals, whose cells likely have sufficient resources to get ready for mitosis faster, might spend proportionally less time in the growth phases of the cell cycle (e.g., G2) so that fewer of their DSBs will occur during the phase when they have the greatest probability of being repaired by HR-h. In contrast, low-condition flies may spend proportionally longer amounts of time in the growth phase prior to mitosis, providing greater opportunity to use the conservative HR-h pathway.
Alternatively, individuals may adaptively deploy alternative repair pathways to maximize cell cycle rate. In germ-line cells, an increased rate of cell cycling enhances gamete production. As the majority of mutations will tend to be neutral or only very weakly selected, high-condition males may not utilize the conservative DSB repair pathways at the expense of reducing their rate of gamete production. Low-condition flies, on the other hand, may already be limited to slower cell cycles due to energetic constraints, thus allowing for use of the slower but more accurate pathways.
An alternative to hypotheses based on cell cycling emphasizes the importance of changes in repair pathway usage during the progression through spermatogenesis. Chan et al. (2011) showed that HR-h is more frequently used in early rather than late stages of spermatogenesis. If progression through the early stages of spermatogenesis is slow in low-condition flies relative to high-condition flies, then there will be more opportunity for DSBs to occur at the stages when HR-h is more likely to be used.
DNA repair is affected similarly by increased age and reduced condition
The age-dependent changes in DSB repair pathway use we found are consistent with the pattern previously established (Preston et al. 2006b; Engels et al. 2007). While use of the two error-prone pathways, NHEJ and SSA, both decline with increasing age, the most conservative of the three pathways, HR-h, increases. This pattern is similar to the condition-dependent differences we observed, suggesting there may be a shared mechanism driving repair pathway change. As the duration of the cell cycle is known to increase with age in the Drosophila male germline (Wallenfang et al. 2006), cell cycle dynamics may be driving both the age and condition effects.
However, Chan et al. (2011) offered an alternative, developmentally based explanation for the aging pattern observed by Preston et al. (2006b). Compared to sperm from young flies, sperm from old flies will have spent more time in the early stages of spermatogenesis (as germline stem cells and spermatogonia) (Wallenfang et al. 2006; Wong and Jones 2012). Because HR-h appears to be more commonly used in the early stages of spermatogenesis (Chan et al. 2011), sperm from old flies are more likely to have experienced a DSB at a spermatogenic stage where repair by HR-h is more frequent. This idea could also explain the condition effect if the early stages of spermatogenesis are disproportionally protracted in low-condition flies.
Though the age and condition effects are similar and these similarities might be driven by the same mechanisms, it is worth noting that the effects of age and condition are not entirely the same. NHEJ use is reduced with age but does not appear to be affected by condition (Figure 5, B and D). Furthermore, while age causes a slight increase in the proportion of HR-long tract conversions, low-condition flies have a lower proportion of HR-lg events overall. Such discrepancies might occur if cell cycle rates change with both age and condition, but differ in which phases of the cell cycle are lengthened.
Effect of condition-dependent DNA repair on mutation rate
Due to the different repair products of the three pathways examined here (HR-h = no mutation, SSA = deletion of repeat, NHEJ = indel), the number and nature of new mutations will differ between the offspring of high- and low-condition individuals, i.e., condition-dependent repair usage can cause the mutation rate to be condition dependent. Theoretical work has shown that condition dependence of mutation rates can have important evolutionary consequences. In stressful environments, condition dependence can accelerate adaptation if poor condition, brought on by poor environmental quality, results in elevated U (Echols 1981). Condition dependence can also alter the mutation load (Agrawal 2002) and could lead to a divergence in genetic architecture between sexual and asexual taxa (Shaw and Baer 2011). However, these theoretical ideas are based on the assumption that low-condition individuals experience elevated mutation rates.
The effect of condition on DNA repair reported above would seem to imply the opposite, i.e., low-condition flies have a reduced mutation rate. However, we need to consider two issues: (i) Based on these results alone, how much lower would the rate be? (ii) Are factors other than those considered here likely to be important in determining the net effect of condition on mutation rate?
To determine an upper limit on how the condition-based differences in DSB repair would affect mutation rate, we can assume that both nonconservative repair pathways (SSA and NHEJ) always result in a deleterious mutation, but HR-h never does. Even under this scenario, the absolute difference predicted for U is small. High-condition flies will pass on ∼3% more new deleterious mutations than low-condition flies in the first week of reproductive life (86 vs. 83% combined SSA, NHEJ use, Table 1 estimates), although this difference increases to 10% by week 4 (73 vs. 63% combined SSA, NHEJ use, Table 1 estimates). Additionally, there is reason to believe that each NHEJ-based mutation occurring in a low-condition individual is more likely to be deleterious. This is because the fidelity of the error-prone pathway, NHEJ, is known to be plastic (Seluanov et al. 2004) and appears to induce larger deletions in senescent cells compared with young cells. If low-condition results in similar decreases in the fidelity of NHEJ repair, then even though low-condition flies use NHEJ less frequently, each individual NHEJ-based mutation is more likely to result in fitness consequences.
Though our results provide evidence for a link between condition and DNA repair pathway use, this study is not intended to provide a complete picture of how condition affects total mutation rate. First, repair of the DSBs in germ-line cells is not limited to the three repair pathways measured by the Rr3 system. As mentioned previously, the repair reporter construct disables monitoring of any HR events in which the sister chromatid is used as a repair template (HR-s) or any precise NHEJ events, as both these repair events will restore the DSB cleavage site, allowing for recutting by the endonuclease. Since HR-s is known to be the preferred and most error-free repair pathway during S phase (Saleh-Gohari and Helleday 2004), it is possible that high-condition individuals could be using this pathway more than low-condition individuals in this nongrowth-related phase of the cell cycle. Similarly, it is possible NHEJ repair in high-condition individuals could be more precise such that use of this pathway is less likely to result in a new mutation. Because HR-s and precise NHEJ are rendered ineffective in this experimental context, we must use caution in extrapolating these results to how condition affects repair of natural DSBs. Though our data shows that pathway usage differs between high- and low-condition flies under the constraints of the Rr3 system, the nature of these differences could depend on what range of pathways are available.
Second, DSBs represent only a fraction of the total DNA damage encountered by an organism, with each category having its own repertoire of repair pathways. If condition dependence in repair pathways is ubiquitous across the genome, then the potential exists for the repair response to different types of damage to go in the opposite direction (i.e., high condition using the more conservative pathways). This seems to be the case in a previous experiment looking at postfertilization repair by female flies of MMS-induced alkylation lesions (excision repair, etc.) (Agrawal and Wang 2008).
Finally, it is important to note that the condition-dependent changes in DSB repair we observe reflect changes in the relative usage of the repair pathways and not an overall increase or decrease in repair events. The amount of DNA damage can also differ due to condition. For instance, condition may impact cellular metabolism such that the production of reactive oxidative species (ROS) differs between high- and low-condition individuals. Because ROS lead to DNA damage, this could lead to an increased demand for repair.
In a recent study, Sharp and Agrawal (2012) found that focal chromosomes that were maintained in unfit genetic backgrounds during a mutation accumulation experiment declined in fitness more quickly than those maintained in wild-type genetic backgrounds. That result suggests that low-condition individuals do experience elevated mutation rates. However, that study does not provide any insight into whether this is due to differences in rates of damage or repair response. Our current study suggests that a condition-based difference in repair of DSBs is unlikely to be the explanation.
Conclusion
Although differences in DNA repair capacities have been invoked as an explanation for mutation rate differences among taxonomic groups (Britten 1986; Eisen and Hanawalt 1999), its role in generating mutation rate variation within a species has received less attention. We have shown that the relative ratios of three DSB repair pathways in the germ-line cells of D. melanogaster change in response to differences in individual condition. While we suspect that condition dependence in DNA repair pathway usage is likely to be a common phenomenon, the magnitude and direction of such effects may depend on the type of damage and the organism in which it occurs. Predicting the nature of condition dependence is difficult because it depends, not only on energetic costs, but also cell cycle dynamics and other essential functions in DNA metabolism. Future studies should also examine how condition affects overall differences in DNA damage, as this may play a larger role in generating mutation rate variation.
Acknowledgments
We thank the W. R. Engels lab (University of Wisconsin) for providing us with the repair reporter construct lines. L. Yuan and R. Badame assisted with molecular work. Funding for this project was supported by the Natural Sciences and Engineering Research Council of Canada (A.D.W. and A.F.A.) and an Ontario Graduate Studies fellowship (A.D.W.).
Footnotes
Communicating editor: J. Sekelsky
Literature Cited
- Agrawal A. F., 2001. Sexual selection and the maintenance of sexual reproduction. Nature 411: 692–695 [DOI] [PubMed] [Google Scholar]
- Agrawal A. F., 2002. Genetic loads under fitness-dependent mutation rates. J. Evol. Biol. 15: 1004–1010 [Google Scholar]
- Agrawal A. F., Wang A. D., 2008. Increased transmission of mutations by low-condition females: evidence for condition-dependent DNA repair. PLoS Biol. 6: e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner M., Golic K. G., Hawley R. S., 2005. Drosophila: A Laboratory Handbook. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- Aylon Y., Liefshitz B., Kupiec M., 2004. The CDK regulates repair of double-strand breaks by homologous recombination during the cell cycle. EMBO J. 23: 4868–4875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer C. F., Miyamoto M. M., Denver D. R., 2007. Mutation rate variation in multicellular eukaryotes: causes and consequences. Nat. Rev. Genet. 8: 619–631 [DOI] [PubMed] [Google Scholar]
- Barton N. H., Turelli M., 1989. Evolutionary quantitative genetics: How little do we know? Annu. Rev. Genet. 23: 337–370 [DOI] [PubMed] [Google Scholar]
- Bessman M. J., Muzyczka N., Goodman M. F., Schnaar R. L., 1974. Studies on the biochemical basis of spontaneous mutation. II. The incorporation of a base and its analogue into DNA by wild-type, mutator and antimutator DNA polymerases. J. Mol. Biol. 88: 409–421 [DOI] [PubMed] [Google Scholar]
- Britten R. J., 1986. Rates of DNA sequence evolution differ between taxonomic groups. Science 231: 1393–1398 [DOI] [PubMed] [Google Scholar]
- Bulmer M., 1989. Estimating the variability of substitution rates. Genetics 123: 615–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan Y. S., Naujoks D. A., Huen D. S., Russell S., 2011. Insect population control by homing endonuclease-based gene drive: an evaluation in Drosophila melanogaster. Genetics 188: 33–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton L. K., Goodman M. F., Branscomb E. W., Galas D. J., 1979. Error induction and correction by mutant and wild type T4 DNA polymerases. Kinetic error discrimination mechanisms. J. Biol. Chem. 254: 1902–1912 [PubMed] [Google Scholar]
- Crow J. F., 1997. The high spontaneous mutation rate: Is it a health risk? Proc. Natl. Acad. Sci. USA 94: 8380–8386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- David P., Bjorksten T., Fowler K., Pomiankowski A., 2000. Condition-dependent signalling of genetic variation in stalk-eyed flies. Nature 406: 186–188 [DOI] [PubMed] [Google Scholar]
- Echols H., 1981. SOS functions, cancer and inducible evolution. Cell 25: 1–2 [DOI] [PubMed] [Google Scholar]
- Eisen J. A., Hanawalt P. C., 1999. A phylogenomic study of DNA repair genes, proteins, and processes. Mutat. Res. 435: 171–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engels W. R., Johnson-Schlitz D., Flores C., White L., Preston C. R., 2007. A third link connecting aging with double strand break repair. Cell Cycle 6: 131–135 [DOI] [PubMed] [Google Scholar]
- Fishman-Lobell J., Rudin N., Haber J. E., 1992. Two alternative pathways of double-strand break repair that are kinetically separable and independently modulated. Mol. Cell. Biol. 12: 1292–1303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldane J. B. S., 1937. The effect of variation of fitness. Am. Nat. 71: 337–349 [Google Scholar]
- Houle D., 1991. Genetic covariance of fitness correlates: what genetic correlations are made of and why it matters. Evolution 45: 630–648 [DOI] [PubMed] [Google Scholar]
- Hunt J., Brooks R., Jennions M. D., Smith M. J., Bentsen C. L., et al. , 2004. High-quality male field crickets invest heavily in sexual display but die young. Nature 432: 1024–1027 [DOI] [PubMed] [Google Scholar]
- Jackson S. P., 2002. Sensing and repairing DNA double-strand breaks. Carcinogenesis 23: 687–696 [DOI] [PubMed] [Google Scholar]
- Jenkins K., Hawley D., Farabaugh C., Cristol D., 2001. Ptilochronology reveals differences in condition of captive White-throated Sparrows. Condor 103: 579–586 [Google Scholar]
- Kanaar R., Hoeijmakers J. H., van Gent D. C., 1998. Molecular mechanisms of DNA double strand break repair. Trends Cell Biol. 8: 483–489 [DOI] [PubMed] [Google Scholar]
- Keightley P. D., Lynch M., 2003. Toward a realistic model of mutations affecting fitness. Evolution 57: 683–689 [DOI] [PubMed] [Google Scholar]
- Keightley P. D., Otto S. P., 2006. Interference among deleterious mutations favours sex and recombination in finite populations. Nature 443: 89–92 [DOI] [PubMed] [Google Scholar]
- Kimura M., 1967. On evolutionary adjustment of spontaneous mutation rates. Genet. Res. 9: 23–34 [Google Scholar]
- Kirkwood T. B. L., Rosenberger R. F., Galas D. J., 1986. Accuracy in Molecular Processes: Its Control and Relevance to Living Systems. Chapman & Hall, London [Google Scholar]
- Kondrashov A. S., 1988. Deleterious mutations and the evolution of sexual reproduction. Nature 336: 435–440 [DOI] [PubMed] [Google Scholar]
- Kondrashov A. S., 1995. Contamination of the genome by very slightly deleterious mutations: Why have we not died 100 times over? J. Theor. Biol. 175: 583–594 [DOI] [PubMed] [Google Scholar]
- Lande R., 1994. Risk of population extinction from fixation of new deleterious mutations. Evolution 48: 1460–1469 [DOI] [PubMed] [Google Scholar]
- Langerak P., Russell P., 2011. Regulatory networks integrating cell cycle control with DNA damage checkpoints and double-strand break repair. Proc. Biol. Sci. 366: 3562–3571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leigh E., Jr, 1970. Natural selection and mutability. Am. Nat. 104: 301–305 [Google Scholar]
- Lieber M. R., 2010. The mechanism of double-strand DNA break repair by the nonhomologous DNA end-joining pathway. Annu. Rev. Biochem. 79: 181–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch M., 2008. The cellular, developmental and population-genetic determinants of mutation-rate evolution. Genetics 180: 933–943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch M., Conery J., Burger R., 1995. Mutation accumulation and the extinction of small populations. Am. Nat. 146: 489–518 [Google Scholar]
- Lynch M., Blanchard J., Houle D., Kibota T., Schultz S., et al. , 1999. Perspective: spontaneous deleterious mutation. Evolution 53: 645–663 [DOI] [PubMed] [Google Scholar]
- Mao Z., Bozzella M., Seluanov A., Gorbunova V., 2008. Comparison of nonhomologous end joining and homologous recombination in human cells. DNA Repair (Amst.) 7: 1765–1771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore J. K., Haber J. E., 1996. Cell cycle and genetic requirements of two pathways of nonhomologous end-joining repair of double-strand breaks in Saccharomyces cerevisiae. Mol. Cell. Biol. 16: 2164–2173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan T. H., 1903. Evolution and Adaptation. Macmillan, New York [Google Scholar]
- Nasmyth K., 1996. Viewpoint: putting the cell cycle in order. Science 274: 1643–1645 [DOI] [PubMed] [Google Scholar]
- Ohnishi T., Mori E., Takahashi A., 2009. DNA double-strand breaks: their production, recognition, and repair in eukaryotes. Mutat. Res. 669: 8–12 [DOI] [PubMed] [Google Scholar]
- Partridge L., Barton N. H., 1993. Optimality, mutation and the evolution of aging. Nature 362: 305–311 [DOI] [PubMed] [Google Scholar]
- Perry J. C., Rowe L., 2010. Condition-dependent ejaculate size and composition in a ladybird beetle. P Roy Soc Lond B Bio 277: 3639–3647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston C. R., Engels W., Flores C., 2002. Efficient repair of DNA breaks in Drosophila: evidence for single-strand annealing and competition with other repair pathways. Genetics 161: 711–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston C. R., Flores C. C., Engels W. R., 2006a Differential usage of alternative pathways of double-strand break repair in Drosophila. Genetics 172: 1055–1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston C. R., Flores C. C., Engels W. R., 2006b Age-dependent usage of double-strand-break repair pathways. Curr. Biol. 16: 2009–2015 [DOI] [PubMed] [Google Scholar]
- R Development Core Team, 2012. R: A language and environment for statistical computing R Foundation for Statistical Computing, Vienna. [Google Scholar]
- Reitzel A. M., 2004. Growth, development and condition of Dendraster excentricus (Eschscholtz) larvae reared on natural and laboratory diets. J. Plankton Res. 26: 901–908 [Google Scholar]
- Rowe L., Houle D., 1996. The lek paradox and the capture of genetic variance by condition dependent traits. Proc. Biol. Sci. 263: 1415–1421 [Google Scholar]
- Saleh-Gohari N., Helleday T., 2004. Conservative homologous recombination preferentially repairs DNA double-strand breaks in the S phase of the cell cycle in human cells. Nucleic Acids Res. 32: 3683–3688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seluanov A., Mittelman D., Pereira-Smith O. M., Wilson J. H., Gorbunova V., 2004. DNA end joining becomes less efficient and more error-prone during cellular senescence. Proc. Natl. Acad. Sci. USA 101: 7624–7629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp N. P., Agrawal A. F., 2012. Evidence for elevated mutation rates in low-quality genotypes. Proc. Natl. Acad. Sci. USA 109: 6142–6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw F. H., Baer C. F., 2011. Fitness-dependent mutation rates in finite populations. J. Evol. Biol. 24: 1677–1684 [DOI] [PubMed] [Google Scholar]
- Sniegowski P. D., Gerrish P. J., Johnson T., Shaver A., 2000. The evolution of mutation rates: separating causes from consequences. Bioessays 22: 1057–1066 [DOI] [PubMed] [Google Scholar]
- Tomkins J. L., Radwan J., Kotiaho J. S., Tregenza T., 2004. Genic capture and resolving the lek paradox. Trends Ecol. Evol. 19: 323–328 [DOI] [PubMed] [Google Scholar]
- Van Noordwijk A., de Jong G., 1986. Acquisition and allocation of resources: their influence on variation in life-history tactics. Am. Nat. 128: 137–142 [Google Scholar]
- Wallenfang M. R., Nayak R., DiNardo S., 2006. Dynamics of the male germline stem cell population during aging of Drosophila melanogaster. Aging Cell 5: 297–304 [DOI] [PubMed] [Google Scholar]
- Wong C., Jones D. L., 2012. Efficiency of spermatogonial dedifferentiation during aging. PLoS ONE 7: e33635. [DOI] [PMC free article] [PubMed] [Google Scholar]