Abstract
Understanding sensory systems that perceive environmental inputs and neural circuits that select appropriate motor outputs is essential for studying how organisms modulate behavior and make decisions necessary for survival. Drosophila melanogaster oviposition is one such important behavior, in which females evaluate their environment and choose to lay eggs on substrates they may find aversive in other contexts. We employed neurogenetic techniques to characterize neurons that influence the choice between repulsive positional and attractive egg-laying responses toward the bitter-tasting compound lobeline. Surprisingly, we found that neurons expressing Gr66a, a gustatory receptor normally involved in avoidance behaviors, receive input for both attractive and aversive preferences. We hypothesized that these opposing responses may result from activation of distinct Gr66a-expressing neurons. Using tissue-specific rescue experiments, we found that Gr66a-expressing neurons on the legs mediate positional aversion. In contrast, pharyngeal taste cells mediate the egg-laying attraction to lobeline, as determined by analysis of mosaic flies in which subsets of Gr66a neurons were silenced. Finally, inactivating mushroom body neurons disrupted both aversive and attractive responses, suggesting that this brain structure is a candidate integration center for decision-making during Drosophila oviposition. We thus define sensory and central neurons critical to the process by which flies decide where to lay an egg. Furthermore, our findings provide insights into the complex nature of gustatory perception in Drosophila. We show that tissue-specific activation of bitter-sensing Gr66a neurons provides one mechanism by which the gustatory system differentially encodes aversive and attractive responses, allowing the female fly to modulate her behavior in a context-dependent manner.
Keywords: Drosophila melanogaster oviposition program, simple decision-making, gustatory sensory system, gustatory receptor 66a (Gr66a), mushroom body
PROPER perception of the environment is essential for an organism to modulate its behavior and make choices necessary to both survival of individuals and propagation of the species. In Drosophila melanogaster, the selection of appropriate oviposition sites that will benefit survival of the progeny is one such behavior (Richmond and Gerking 1979; Jaenike 1982; Chess and Ringo 1985; van Delden and Kamping 1990; Ruiz-Dubreuil et al. 1994; Amlou et al. 1998; Mery and Kawecki 2002). Recent studies have demonstrated that during egg-laying site selection female fruit flies actively explore the different options available before choosing where to lay their eggs (Yang et al. 2008; Miller et al. 2011; Schwartz et al. 2012). Interestingly, females do not always remain on the substrate where they have deposited their eggs (Joseph et al. 2009) and will often choose to lay eggs on substrates they normally find aversive for foraging and feeding (Fuyama 1976; Moreteau et al. 1994; Eisses 1997; Matsuo et al. 2007; Lee et al. 2009; Sellier et al. 2011; Weiss et al. 2011). Since a fly cannot be in two places at once, a choice must be made between these competing preference pathways. Thus, with regard to oviposition behavior, a decision is defined as the selection between one of two mutually exclusive responses: (1) avoid the substrate and hold eggs, or (2) choose the substrate in order to lay eggs. Taken together, these findings suggest that during oviposition, female Drosophila employ an evaluation process that meets the criteria of simple decision-making (Kristan 2008; Kable and Glimcher 2009).
Although previous studies have identified compounds that can induce avoidance responses (Fuyama 1976; Lee et al. 2009; Sellier et al. 2011; Weiss et al. 2011) and attractive egg-laying preference in Drosophila (Moreteau et al. 1994; Eisses 1997; Matsuo et al. 2007; Yang et al. 2008; Miller et al. 2011), the analysis has been performed independently, i.e., aversion and attraction have been measured in separate assays. To study choice behavior, it is important for both responses to be measured concurrently within the same assay (Joseph et al. 2009) and to identify a stimulus that can simultaneously generate two competing responses. Lobeline has been shown to induce avoidance-related responses (Marella et al. 2006; Sellier et al. 2011; Weiss et al. 2011) and egg-laying attraction (Yang et al. 2008) in independent behavioral assays. Lobeline is an alkaloid naturally produced by the diverse genus of Lobelia plants (Krochmal et al. 1972), which serves as a feeding repellent for several insect species (Wink and Schneider 1990; Detzel and Wink 1993). Furthermore, bitter-sensing Gr66a-expressing sensory neurons in the Drosophila gustatory system have been shown to detect lobeline (Lee et al. 2010). Thus, when employed with a two-choice assay that concurrently measures positional and egg-laying preferences (Joseph et al. 2009), lobeline is an ideal substrate to study the choice that female flies make when deciding between these two competing responses.
Unlike olfactory neurons, which typically express a single odorant receptor/co-receptor pair that defines their identity (Hallem et al. 2004; Larsson et al. 2004), gustatory neurons co-express multiple gustatory receptors; this includes the Gr66a-expressing neurons that detect bitter compounds such as lobeline (Thorne et al. 2004; Wang et al. 2004; Jiao et al. 2008; Lee et al. 2009; Weiss et al. 2011). Gustatory neurons are present in sensilla located in multiple tissues of the fly, including the labellum, pharynx, legs, wings, and abdomen (Stocker and Schorderet 1981; Taylor 1989; Stocker 1994; Gendre et al. 2004; Thorne and Amrein 2008; Mitri et al. 2009; Shimono et al. 2009; Masek and Scott 2010). The Gr66a-expressing neurons that detect bitter compounds are present in most of these tissues (Dunipace et al. 2001; Mitri et al. 2009; Shimono et al. 2009; Weiss et al. 2011), and axons from these gustatory neurons project from taste bristles to the subesophageal ganglion (SOG) for first-order processing (Thorne et al. 2004; Wang et al. 2004; Miyazaki and Ito 2010).
Important questions remain unanswered about the gustatory circuits involved in the decision-making processes regulating the Drosophila oviposition program. Which sensory neurons detect the relevant environmental cues? What determines whether the response is aversion or attraction? Are there central brain regions involved in choosing the response that is most appropriate? To begin addressing these questions, we selectively inactivated either specific sensory neurons or central brain regions and analyzed responses to lobeline using a two-choice preference assay, which allows the quantification of egg-laying and positional preference concurrently (Joseph et al. 2009). Surprisingly, we found that sensory neurons expressing the same gustatory receptor, Gr66a, receive input for both the aversive positional and attractive egg-laying responses. Furthermore, the analysis of mosaic flies revealed that different groups of Gr66a-expressing neurons are responsible for attraction and repulsion. Finally, we show that the mushroom body, which has been implicated in sensory integration (Xi et al. 2008), switches between motivational states (Krashes et al. 2009; Serway et al. 2009) and Drosophila decision-making behaviors (Zhang et al. 2007; Brembs 2009; Wu and Guo 2011), plays a crucial role in both positional aversion and egg-laying attraction to lobeline.
In summary, we propose that tissue-specific activation of Gr66a-expressing gustatory neurons allows a female fly to execute distinct behaviors in response to a single sensory input, and that the tissue-specific inputs are possibly integrated and evaluated in the mushroom body prior to behavioral output selection. Our findings therefore provide novel insights into the complex nature of sensory perception and behavioral modulation in the decision-making process employed by D. melanogaster during oviposition.
Materials and Methods
Fly stocks and growth
Flies were reared on standard cornmeal/molasses/yeast/agar media under constant light at 25° and 70% humidity. General behavioral characterization of positional aversion and egg-laying attraction responses to lobeline was typically performed in w1118 Berlin background, unless otherwise specified. GAL4 lines from our P-element insertion library were also in the w1118 Berlin background. The pox-neuro lines were backcrossed at least four generations to w1118 Berlin, excluding the second chromosome, which carries the unmarked poxnΔM22-B5 deficiency. Flies used in single female clonal analysis experiments were in a w1118 background as well (Gordon and Scott 2009).
UAS-Shibirets flies contain two insertions of the transgene in a w1118 Canton-S background. To ensure there was no variation in behavior due to mixed backgrounds, we assayed w1118 Berlin controls with all UAS-Shibirets and GAL80 trials. w1118 Berlin controls, mixed background w1118 Berlin/UAS-Shibirets flies, and w1118 Berlin/GAL4 females exhibited similar behaviors in all tests at both 23° and 30°. Furthermore, our observations in Supporting Information, Figure S1B show that responses to 0.50 mM lobeline are nearly identical in females with different genetic backgrounds, demonstrating that the presence of Canton-S background likely has minimal effects on positional and egg-laying preferences.
Two-choice assay of egg laying and positional responses
The experimental assay to simultaneously measure egg-laying and positional responses to lobeline was performed as previously described (Joseph et al. 2009), with some modifications. Briefly, the base of plastic 6-ounce round bottom bottle (E & K Scientific, Santa Clara, CA) was cut off using a razor blade, and a 60-mm Petri dish lid was inserted into the removed portion of the bottle to facilitate scoring of female positional preference. Molten standard cornmeal/molasses/yeast/agar media was mixed with the appropriate volume of either aqueous (−)-lobeline hydrochloride (Sigma-Aldrich, St. Louis, MO) or water. Thirty-five millimeter Petri dish lids (Becton Dickinson Labware, Franklin Lakes, NJ) were divided in half using a razor blade, and either lobeline- or water-containing food was poured into each half to construct the two-choice plates. Groups of 12–15 females, typically 1–2 days old, were collected and allowed to mate with three males for 2–3 days before being tested. Flies were gently knocked into bottles without CO2 anesthesia to reduce behavioral perturbations; the bottle was capped with the two-choice plate and then inverted for observation. Females were allowed to acclimate to the bottle apparatus for 1–2 hr, after which positional preferences were recorded. Bottles were then placed in dark conditions to reduce environmental distractions. For temperature-sensitive assays using UAS-Shibirets, experimental procedures were conducted as described above, except that flies tested at the nonpermissive temperature were put in a heated incubator with a transparent case, allowing for visualization of positional behavior at 30°.
To obtain positional preference indexes (PI), the number of flies on each half of the plate was scored at 10-min intervals for 80 min. Values were totaled and a PI value was calculated: PI = (total flies on experimental food − total flies on control food)/(total flies on experimental food + total flies on control food). To obtain oviposition preference indexes (OI), the number of eggs on each half of the plate was counted after females laid eggs overnight: OI = (no. of eggs laid on experimental food − no. of eggs laid on control food)/total no. of eggs laid).
Extended 24-hr time interval assays
For behavioral assays that measured positional and egg-laying preferences for lobeline at times greater than 1–2 hr after initial bottle entry, experimental procedures were conducted as described above, with the following modifications: (i) positional preferences were assayed at 3, 7, 11, 15, 19, and 23 hr after grouped females were first introduced to 0.50 mM lobeline; (ii) two-choice dishes were collected and total eggs were counted immediately after the scoring of positional preferences; and (iii) females were left in lighted conditions before and throughout testing.
Two-choice feeding assay
To determine feeding preferences for food containing 0.50 mM lobeline, the experimental assay and calculation of the feeding index (FI) was identical as previously described (Joseph et al. 2009), with the following minor modifications: (i) we used 0.05% as the final dye concentrations of Erioglaucine (FD&C Blue no. 1) or Fast Green FCF dye (Green no. 3) (Sigma-Aldrich); (ii) females sampled lobeline-containing dye substrates for a longer time period (6 hr) to ensure a sufficient number of eggs were laid to check that egg-laying preference was not altered by the presence of Blue no. 1 or Green no. 3. Positional preferences were also scored, and females exhibited normal OI and PI values in the presence of dye.
Surgeries
To impair olfaction, females were anesthetized with CO2 and the third antennal segment was removed with sharp forceps. To impair gustation on the legs, sharpened forceps were used to make a cut at the junction between the first and second tarsal segments on either the anterior, medial, or posterior pairs of legs (see Figure 3C for position of the cut). After surgeries, females were allowed to recover and mate for 2–3 days before being tested.
Figure 3 .
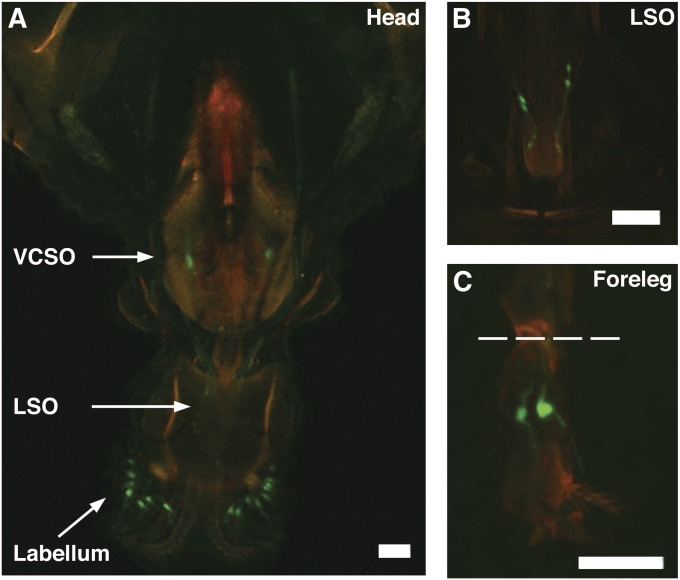
Gr66aGAL4 expresses in gustatory neurons present in the Drosophila proboscis and legs. (A) Gr66aGAL4 is expressed in sensory neurons in the labellum, lateral sensory organ (LSO), and ventral cibarial sensory organ (VCSO) of the Drosophila proboscis. Image in A was taken from the posterior side of the head. (B) Image of the LSO taken from the anterior side of the head, such that Gr66aGAL4 expression can be better visualized. (C) Gr66aGAL4 is expressed in the first tarsi of the anterior forelegs in female Drosophila. Dashed line represents the location where cuts in tarsal ablation experiments were performed (Figure 4B). In A–C, Gr66aGAL4 was visualized with UAS-CD8-GFP (green channel); cuticle autofluoresence was used to define boundaries of the head and the leg (red channel). Bars, 40 μm.
Imaging and immunohistochemistry
Representative imaging of the Gr66aGAL4 expression pattern (Figure 3) and clonal analysis experiments (Figure 6) were performed by directly visualizing the fluorescence of GAL4/UAS-CD8-GFP, UAS-T2-GFP or GAL4/UAS-CD8-GFP using a Leica confocal microscope (Leica Microsystems, Bannockburn, IL). The green channel detects GFP expression induced by Gr66aGAL4, while the red channel was utilized to detect autofluorescence of the Drosophila cuticle. Immunostaining of 5-120GAL4/+; UAS-CD8-GFP/+ and 5-120GAL4/MB{GAL80}; UAS-CD8-GFP/+ fly brains (Figure 7) was performed with antibodies against GFP and the nc82 antibody.
Figure 6 .
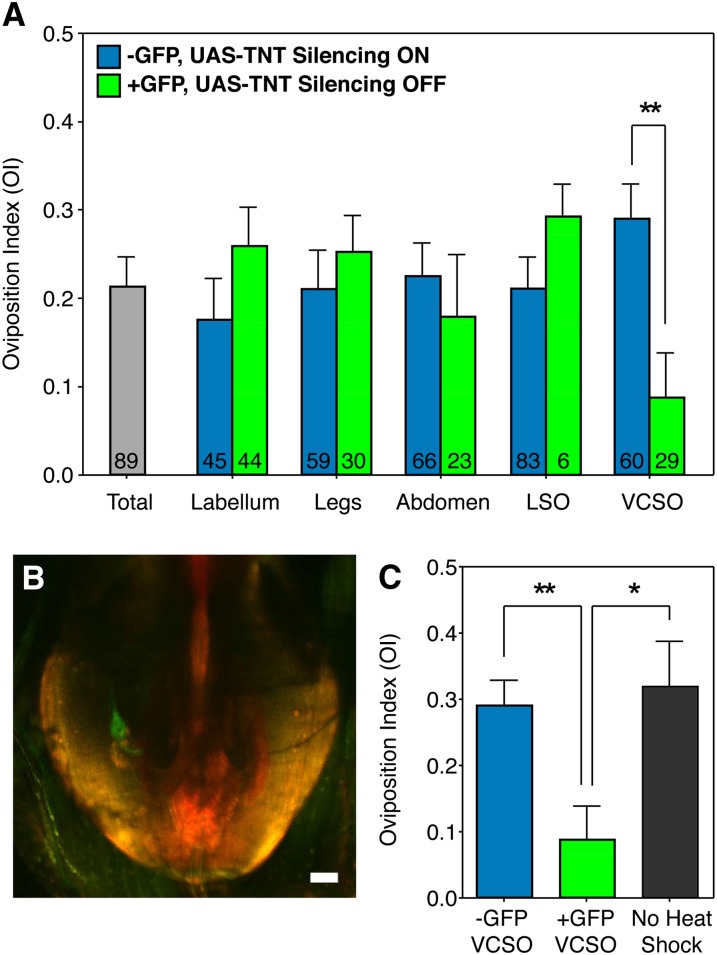
Gr66a neurons in the pharynx receive sensory input for egg-laying attraction to lobeline. (A) Average oviposition indexes of females grouped as either lacking or possessing GFP-labeled, and thus TeTx silenced clones (blue and green bars, respectively) in the following Gr66a-expressing tissue regions: labellum, legs, abdomen, lateral sensory organ (LSO) and ventral cibarial sensory organ (VCSO). A significant disruption in egg-laying preference to 0.50 mM lobeline was only observed when comparing females that were grouped as GFP− or GFP+ for silencing of neurons in the VCSO (**P < 0.01; unpaired two-tailed t-test; number of flies for for GFP− vs. GFP+ mean OI values for each tissue grouping are listed within respective blue and green bars). (B) Representative image of a single GFP-labeled, UAS-TeTx silenced clone within the VCSO. Genotype of the representative female is: tubulin-FRT-GAL80-FRT/+; Gr66aGAL4/UAS-TeTx; heat shock-FLP/UAS-CD8-GFP. Imaging of UAS-CD8-GFP is shown with the green channel; cuticle autofluorescence was recorded with the red channel. Bar, 20 μm. (C) Comparison between females with GFP+, UAS-TeTx silenced clones in the VCSO (green bar), females of the same genotype that did not undergo heat shock (gray bar), and GFP−, UAS-TeTx females that underwent heat shock but did not possess silenced neurons within the VCSO (blue bar) (*P < 0.05; **P < 0.01; one-way ANOVA, Bonferroni post-test; n ≥ 19).
Figure 7 .
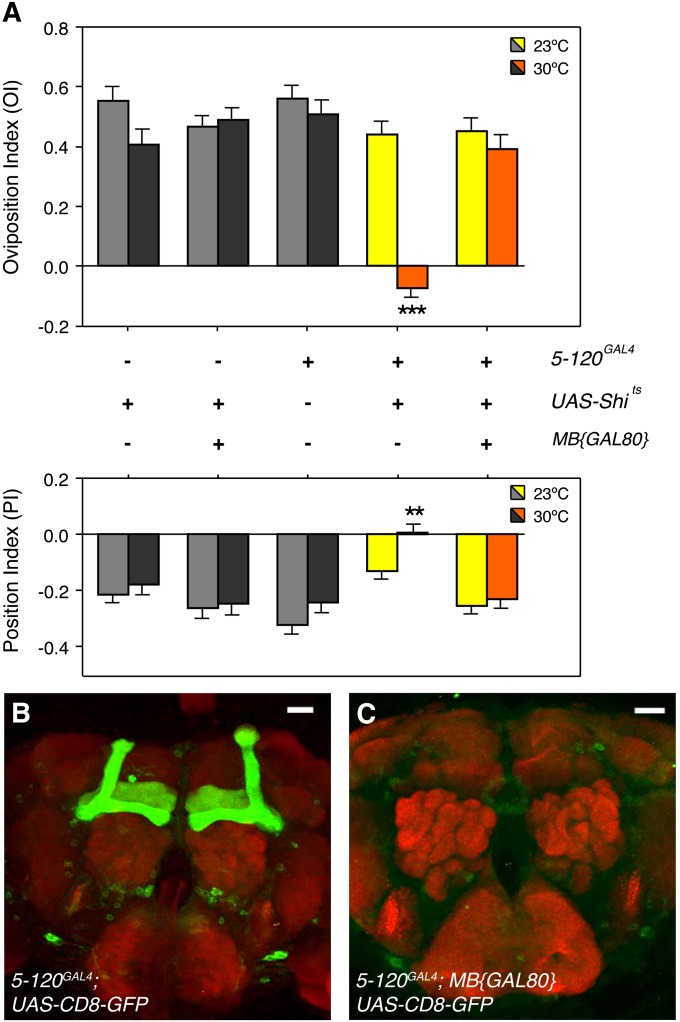
Silencing the mushroom body simultaneously disrupts positional and egg-laying responses to lobeline. (A) At the nonpermissive temperature (30°), 5-120GAL4 females expressing UAS-Shits in the mushroom body lose both positional aversion and egg-laying attraction to 0.50 mM lobeline, when compared to relevant controls (gray bars). In contrast, 5-120GAL4 females expressing both UAS-Shits and mushroom body-GAL80 (MB{GAL80}) exhibit normal behavioral responses to 0.50 mM lobeline (**P < 0.01; ***P < 0.001; one-way ANOVA, Bonferroni post-test for comparison between columns within the 23° or 30° groups; two-way ANOVA, Bonferroni post-test for comparison between temperatures within same genotypes; n ≥ 13). (B) Confocal image stacks of 5-120GAL4/+; UAS-CD8-GFP/+ females reveal strong GAL4 expression in the mushroom body, as well as some other neurons within the brain. (C) Inclusion of MB{GAL80} in 5-120GAL4/MB{GAL80}; UAS-CD8-GFP/+ females suppresses GAL4 expression specifically in the mushroom body, while maintaining expression in other extraneous neurons. In B and C, GAL4 was visualized in immunostained brains using antibodies against GFP (green channel) and the nc82 antibody that recognizes synapses (red channel). Bar, 20 μm.
Single fly clonal analysis and dissections
To generate transgenic females that possessed silenced clones restricted to a limited number of cells within the Gr66a expression pattern, we crossed tubulin-FRT-GAL80-FRT; UAS-TeTx; heat shock-FLP (see Gordon and Scott 2009 for strain construction) to Gr66aGAL4; UAS-CD8-GFP flies. Resulting tubulin-FRT-GAL80-FRT/+; UAS-TeTx/Gr66aGAL4; heat shock-FLP/UAS-CD8-GFP progeny were then heat shocked for 1 hr 15 min at the pupal stage to generate clones. Briefly, heat-shock activation of hs-FLP randomly causes FRT sites to recombine GAL80 away from its promoter, thereby halting GAL80 repressor production. As a result, the UAS/GAL4 system is derepressed (i.e., activated), inducing UAS-TeTx neuronal silencing and UAS-CD8-GFP labeling in these Gr66a neurons that underwent a stochastic recombination event. Single females were then collected after eclosion and allowed to mate with three males for 2–3 days before being assayed for both positional aversion and egg-laying attraction responses to 0.50 mM lobeline. Experimental protocols and preference index calculations for single fly assays were identical to those described above for the two-choice assay of egg-laying and positional responses, except that bottles only contained individual females. After behavioral analysis, individual flies were immediately collected and dissected to ascertain which Gr66a-expressing tissue regions contained UAS-CD8-GFP labeled, and thus UAS-TeTx silenced clones. The head, anterior legs, and abdomen were separated from the thorax using a razor blade, and then whole mounted on a microscope slide with two bridging cover slips, to prevent compression of dissected samples. Of note, the abdomen was placed ventral surface facing up, to facilitate imaging of Gr66a-expressing cells. Tissue samples were then imaged using a confocal microscope. After obtaining z-stacks of each dissected specimen, individual flies were assigned as either GFP+ or GFP− for each particular tissue region.
After obtaining expression data for 89 single clonal females, and 19 individual control flies of the same genotype that did not undergo heat shock, we divided the assayed females into two groups for each different tissue region: (1) flies possessing GFP+, and hence UAS-TeTx silenced clones in a particular tissue region within the Gr66aGAL4 expression pattern, and (2) flies that were GFP−, and thus lacked UAS-TeTx activity. We then performed unpaired t-tests comparing the mean OI values of each group to see whether there was a significant decrease in egg-laying attraction in the GFP positive females when compared to the GFP− females. Specifically, if Gr66a neurons in a particular tissue region were responsible for the egg-laying attraction, then the mean OI value of GFP+, UAS-TeTx silenced flies should differ significantly from GFP− siblings, since GFP+ grouping should be enriched with flies exhibiting disrupted egg-laying preference. Meanwhile the GFP− grouping should primarily contain individuals with wild-type egg-laying preference, and therefore exhibit a mean OI value very similar to the no heat-shock controls. This OI comparison analysis was performed on the following tissue region groupings of the same 89 clonal females: labellum, legs, abdomen, labial sensory organ (LSO), and ventral cibarial sensory organ (VCSO).
Statistics
Statistical analyses are as described in figure legends and the main text, and unless otherwise specified, the data are presented as means ± SEM, with associated raw P-values. All analyses were performed using GraphPad Prism, version 4.0 (GraphPad Software, San Diego, CA).
Results
Detection of lobeline by Gr66a-expressing neurons induces opposing egg-laying and positional preferences
In a previous study, we showed that egg-laying preference and positional aversion toward acetic acid could be effectively used as a model for choice behavior in female Drosophila (Joseph et al. 2009). With regard to acetic acid, the attraction was mediated by the gustatory system, while aversion required intact olfaction. We next asked whether a single compound could elicit opposing oviposition and positional preferences when detected by the same sensory modality. Lobeline, a bitter-tasting compound, has been shown to be an egg-laying attractant (Yang et al. 2008) as well as a general repellant (Marella et al. 2006; Lee et al. 2009; Sellier et al. 2011; Weiss et al. 2011). We confirmed that in our two-choice assay, in which we monitor both egg-laying and positional preference for regular food or food supplemented with lobeline, female flies preferentially laid eggs on media containing 0.50 mM lobeline, as reflected by positive oviposition index (OI) values (Figure 1, A and B). Females also avoided the same lobeline-containing medium when not laying eggs, as reflected by negative positional index (PI) values (Figure 1, A and B). We confirmed that females perceived lobeline as repulsive in feeding assays (Figure S1A). Both behavioral responses were dose dependent: at high doses of lobeline (0.75–1.00 mM), females exhibited very high positional aversion and substantially decreased egg-laying preference (Figure 1A).
Figure 1 .
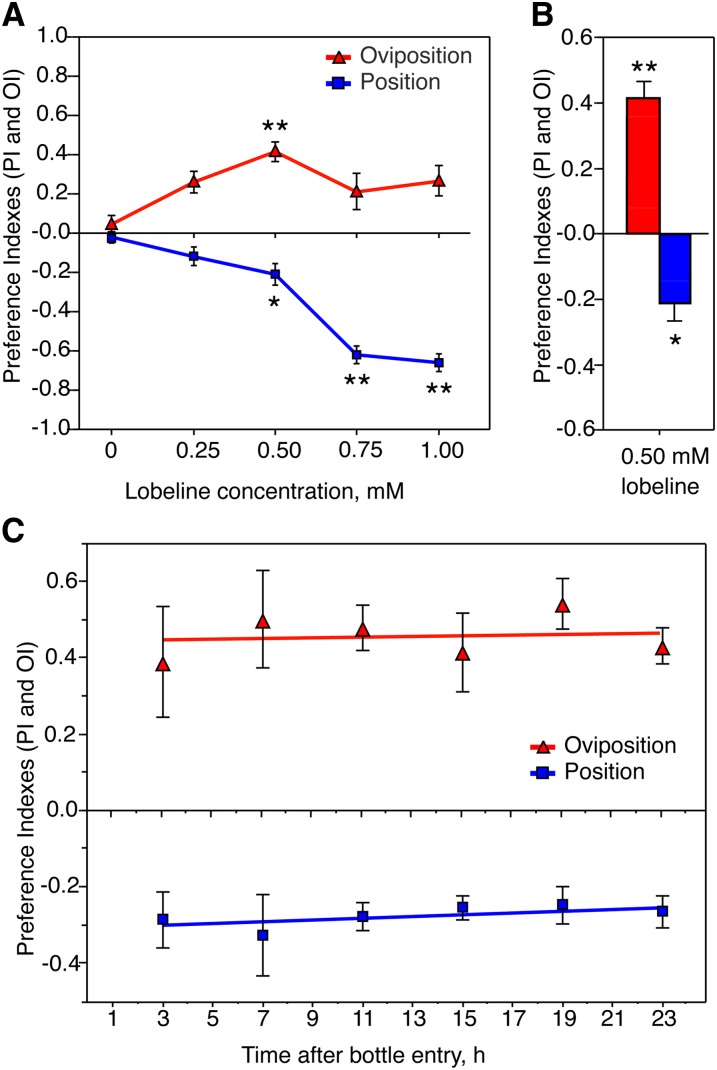
Bitter-tasting lobeline concurrently induces aversive positional and attractive egg-laying responses in Drosophila females. (A) Dose–response curve for positional and egg-laying responses to increasing concentrations of lobeline. Values for the positional preference index (PI) and oviposition preference index (OI) were collected from the same groups of flies (see Materials and Methods for calculation of PI and OI). Significant differences between no-lobeline control assays and 0.50 mM two-choice dishes were observed (*P < 0.05; **P < 0.01; one-way ANOVA, Bonferroni post-test; n ≥ 9). (B) Bar graph representation of average PI and OI values demonstrated with 0.50 mM lobeline; subsequent experiments were performed at the 0.50 mM dose. (C) PI and OI values of females assayed 3, 7, 11, 15, 19, 23 hr after being introduced to the two-choice assay with regular food and food supplemented with 0.50 mM lobeline. Both positional aversion and egg-laying attraction remained constant between different time intervals (P > 0.05; nonzero linear regression test; n ≥ 6). Linear regression plots for PI values (blue line) and OI values (red line) had slopes = 0.002 and 0.001, respectively. No significant differences were observed between average PI or OI values across different time intervals (P > 0.05; one-way ANOVA; n ≥ 6).
Data for positional preference were recorded 1–2 hr after the females had been introduced and acclimated to the two-choice chamber. In contrast, to ensure that females laid an adequate number of eggs to reliably calculate oviposition preference indexes, eggs were counted after the flies laid eggs overnight. Given the difference in time intervals in data collection, the possibility remained that positional preference may become attractive over extended periods of time. Similarly, the attractive egg-laying preference could simply be a result of females adapting and reducing their aversive response to bitter-tasting lobeline over time. To determine whether positional aversion and egg-laying attraction remained consistent throughout the entire egg-laying period, we concurrently recorded data for both OI and PI values within the same time period, observing both behaviors beginning at 3, 7, 11, 15, 19, or 23 hr after females were first introduced to the two-choice chambers. Both the positional repulsion and egg-laying attraction to 0.50 mM lobeline remained constant over a 24-hr period (Figure 1C).
Furthermore, to verify that these responses were not unique to our particular laboratory stock of wild-type flies, we tested additional wild-type strains in our behavioral assay, and observed similar responses (Figure S1D). We selected 0.50 mM lobeline for subsequent experiments, since it generated moderately strong preferences for aversive and attractive behaviors. Quinine (10 mM) produced similar behavioral responses in female flies (Figure S1B), suggesting that opposing positional aversion and egg-laying attraction are not specific to lobeline, but rather a more general response toward bitter compounds. In summary, our data show that lobeline induces attractive egg-laying and repulsive positional responses in our experimental model of choice-like behavior in Drosophila.
Given past studies, in which attractive and repulsive behavioral outputs were induced by activation of the gustatory and olfactory circuits, respectively (Suh et al. 2004; Fischler et al. 2007; Joseph et al. 2009; Ai et al. 2010), we asked whether lobeline would function in a similar manner. To determine whether olfactory input was necessary for lobeline responses, we tested females in which the primary olfactory organs, the third antennal segments (Hallem et al. 2004), had been surgically removed. In addition, we assayed mutants lacking the critical Or83b co-receptor, which is required for most olfactory signaling (Larsson et al. 2004). Flies with a compromised olfactory system exhibited normal responses to lobeline (Figure S2), indicating that neither egg-laying attraction nor positional aversion to lobeline was mediated by the olfactory system.
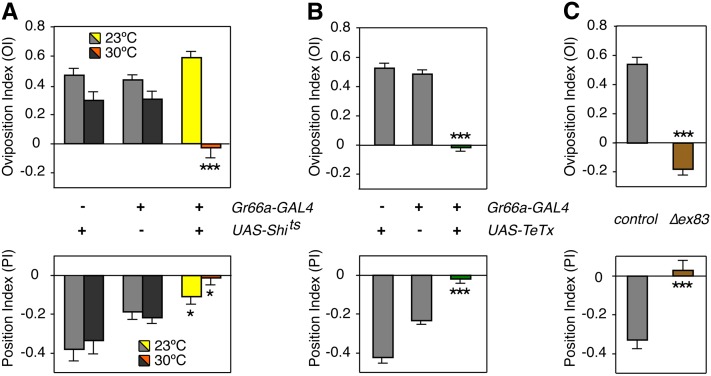
These data suggested that input for both positional aversion and egg-laying attraction to lobeline is received by the gustatory system, which is supported by previous studies where lobeline was aversive in other taste-based behavioral assays (Marella et al. 2006; Sellier et al. 2011; Weiss et al. 2011). Given the broad expression of Gr66a gustatory receptor in most bitter-sensing neurons (Mitri et al. 2009; Isono and Morita 2010; Weiss et al. 2011), we predicted that Gr66a-expressing sensory neurons would mediate at least the positional aversion response. To test this, we used a temperature-sensitive Shibire transgene (UAS-Shits) (Kitamoto 2001) to inhibit endocytosis and thus block neurotransmission in Gr66a-expressing neurons. Indeed, synaptic silencing of Gr66a-expressing neurons disrupted the positional aversion to lobeline in Gr66aGAL4/+; UAS-Shits/+ females at the nonpermissive temperature (Figure 2A, lower axis). A modest decrease in positional aversion to lobeline in Gr66aGAL4/+; UAS-Shits/+ females also occurred at the permissive temperature, likely due to residual activity of the strong UAS-Shits transgene employed in our experiments. Interestingly, silencing Gr66a-expressing neurons caused a loss of egg-laying attraction to lobeline as well (Figure 2A, upper axis). To independently verify that these results were not due to nonspecific secondary effects of the UAS-Shits transgene, we used tetanus toxin (UAS-TeTx) to abolish synaptic vesicle release (Sweeney et al. 1995). Gr66aGAL4/UAS-TeTx females also exhibited loss of both positional and egg-laying responses to lobeline (Figure 2B). Finally, to demonstrate the Gr66a receptor is necessary for the detection of lobeline and the resulting behavioral responses, we assayed ΔGr66aex83 mutant flies that lack the taste receptor but still have sensory neurons capable of signaling (Moon et al. 2006). Indeed, ΔGr66aex83 females did not exhibit either positional aversion or egg-laying attraction (Figure 2C). Taken together, our results show that signaling through gustatory neurons expressing the Gr66a receptor is required for the proper execution of positional aversion and egg-laying attraction for lobeline.
Figure 2 .
Silencing Gr66a neurons disrupts both aversive positional and attractive egg-laying responses. (A) Behavioral responses in the two-choice assay of females expressing UAS-Shits in Gr66a neurons. Gr66aGAL4/UAS-Shits flies exhibited a loss of positional aversion and egg-laying preference for 0.50 mM lobeline when shifted from permissive (23°) to nonpermissive (30°) temperatures. PI and OI preferences of experimental Gr66aGAL4/UAS-Shits flies (colored bars) were significantly different from UAS-Shits/+ and Gr66aGAL4/+ controls (gray bars) at 30°. (*P < 0.05; **P < 0.01; one-way ANOVA, Bonferroni post-test for comparison between columns within the 23° or 30° groups; two-way ANOVA, Bonferroni post-test for comparison between temperatures within same genotypes; n ≥ 9). In addition, Gr66aGAL4/UAS-Shits flies also exhibited a significant loss of aversion at 25°, likely due to leaky activity of the strong UAS-Shits transgene at room temperature. (B) When compared to Gr66aGAL4/+ and UAS-TeTx/+ controls, Gr66aGAL4/UAS-TeTx also possessed a significant loss of both positional aversion and egg-laying attraction to 0.50 mM lobeline (***P < 0.001; one-way ANOVA; n ≥ 28). Of note, positional aversion in the UAS-TeTx/+ control was greater than both Gr66aGAL4/+ (***P < 0.001) and the wild-type aversion responses observed for 0.50 mM lobeline in Figure 1. However the increased repulsion associated with UAS-TeTx construct alone did not affect Gr66aGAL4/UAS-TeTx experimental flies, since they demonstrated a complete lack of positional repulsion to lobeline. (C) ΔGr66aex83/ΔGr66aex83 flies exhibited a loss in positional aversion and egg-laying attraction to 0.50 mM lobeline when compared to w1118 Berlin controls (***P < 0.001; unpaired two-tailed t-test; n ≥ 10).
Positional aversion to lobeline is mediated by Gr66a-expressing neurons on the anterior legs
How do females produce two opposing behavioral outputs from a single gustatory input? Gustatory sensory neurons that express Gr66a are present in diverse regions of the fly, including bristles on the labellum, internal mouthparts lining the pharynx, tarsal segments of the legs, and abdominal tissues (Dunipace et al. 2001; Lee et al. 2009; Mitri et al. 2009; Shimono et al. 2009; Weiss et al. 2011). We hypothesized that activation of Gr66a receptors in distinct sensory organs may explain the opposing behavioral responses to lobeline.
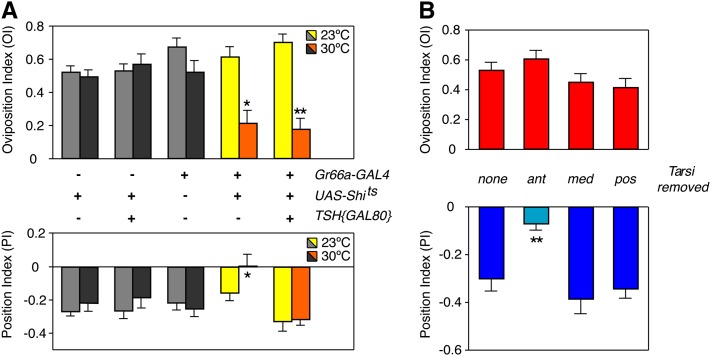
Gr66aGAL4 is expressed, in addition to other structures, in gustatory neurons that innervate sensory bristles on the anterior legs (Figure 3C). To begin dissecting which neurons in the Gr66a expression pattern mediate the behavioral responses to lobeline, we assayed Gr66aGAL4/+;UAS-Shits/+ females that also carried teashirt-GAL80 (TSH{GAL80}), a GAL4 repressor with expression in the thorax and legs (J. H. Simpson, unpublished data; Clyne and Miesenböck 2008). If synaptic activation of Gr66a neurons on the legs was necessary for the repulsive response to lobeline, then GAL80 inhibition of Gr66aGAL4/UAS-Shits expression in the thoracic leg segments should restore positional aversion in these females. Indeed, Gr66aGAL4/TSH{GAL80}; UAS-Shits/+ females exhibited normal positional aversion to lobeline (Figure 4A, lower axis). Furthermore, addition of TSH{GAL80} did not rescue the loss of egg-laying attraction, indicating that Gr66a receptors on the legs do not mediate oviposition preference (Figure 4A, upper axis).
Figure 4 .
Gr66a neurons on Drosophila legs receive sensory input for the positional aversion response. (A) Restoration of positional aversion to lobeline using thorax-specific TSH{GAL80} to suppress Gr66aGAL4/UAS-Shits silencing in leg sensory neurons. Females expressing TSH{GAL80}, Gr66aGAL4 and UAS-Shits exhibited normal positional aversion at the nonpermissive temperature (30°) when compared to flies with only Gr66aGAL4 and UAS-Shits, as well as the UAS-Shits/+, Gr66aGAL4/+, and TSH{GAL80}/+; UAS-Shits/+ controls. (*P < 0.05; one-way ANOVA, Bonferroni post-test; n ≥ 15). Egg-laying attraction remained disrupted at 30° in females expressing TSH{GAL80}, Gr66aGAL4 and UAS-Shits, when compared to relevant controls. (*P < 0.05; **P < 0.01; one-way ANOVA, Bonferroni post-test for comparison between columns within the 23° or 30° groups; two-way ANOVA, Bonferroni post-test for comparison between temperatures within same genotypes; n ≥ 15). (B) Behavioral responses to lobeline in females with the first tarsi removed on either the anterior (ant), medial (med), or posterior (pos) pairs of legs. A loss of positional aversion to 0.50 mM lobeline was only observed in flies lacking first tarsi gustatory bristles from the anterior legs (**P < 0.01; one-way ANOVA, Bonferroni post-test; n ≥ 10). Egg-laying responses were unaffected by tarsal ablation (P > 0.05; one-way ANOVA, n ≥ 10).
Given that TSH{GAL80} is expressed in all leg segments, the possibility remained that TSH{GAL80} rescued the aversive lobeline response by restoring signaling in thoracic neurons other than the characterized Gr66a cells on the forelegs. We therefore performed bilateral removal of the first tarsal segments on the forelegs, midlegs, and hindlegs. Females were allowed to recover from surgeries for 2 days and then assayed for positional aversion and egg-laying attraction to lobeline. Flies lacking the first tarsal segment of the forelegs lost positional aversion, while removal of tarsi of midlegs or hindlegs had no effect (Figure 4B). Furthermore, egg-laying attraction to lobeline was normal in all flies tested, confirming that taste bristles on the legs are dispensable for oviposition preference. Taken together, our findings demonstrate that Gr66a-expressing gustatory neurons on the first tarsi of the forelegs receive input for the positional aversion to lobeline.
Egg-laying attraction to lobeline is mediated by Gr66a-expressing neurons in the internal mouthparts of the pharynx
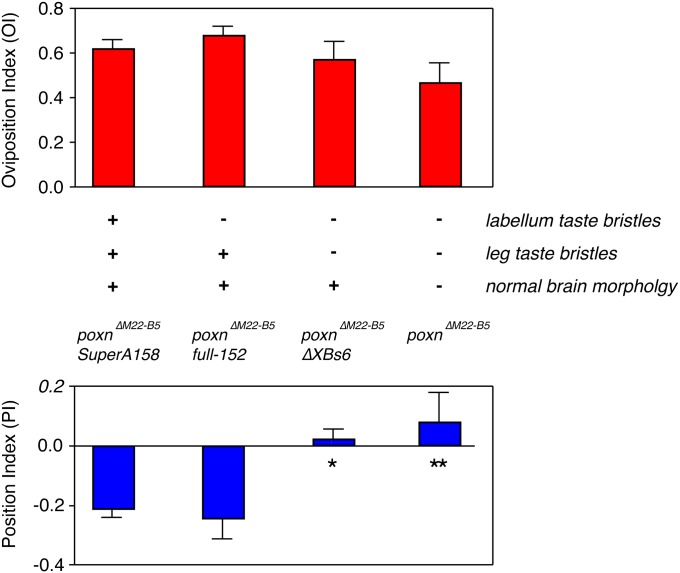
Pox-neuro (poxn) encodes a transcriptional regulator that is necessary for the development of polyinnervated chemosensory bristles in Drosophila; mutation of the poxn locus transforms most gustatory bristles into mono-innervated mechanosensory bristles that lack taste receptors (Awasaki and Kimura 1997). We assayed lobeline responses in the null poxnΔM22-B5 mutant and in transgenic strains in which the mutant defects are selectively rescued in different tissues (Boll and Noll 2002). As expected, null poxnΔM22-B5 mutants and ΔXBs6; poxnΔM22-B5 females lacking taste receptors on the legs exhibited a loss of positional aversion to lobeline (Figure 5, lower axis). Interestingly, we found that poxnΔM22-B5 females exhibited normal egg-laying attraction to lobeline, indicating that morphological changes and subsequent loss of Gr66a neurons in the labellum and abdomen had minimal effects on egg-laying preference (Figure 5, upper axis). Similarly, poxnΔM22-B5; full-152 females that only lack labellar taste receptors also showed normal preference (Figure 5, upper axis). Taken together, our data suggest that abdominal, labellar, and tarsal taste bristles are not needed for detecting lobeline with regard to oviposition preference.
Figure 5 .
pox-neuro mutants lacking taste bristles on legs only lose positional aversion to lobeline. pox-neuro (poxn) flies lacking taste bristles on their legs, namely the deficiency poxnΔM22-B5 homozygotes and ΔXBs6; poxnΔM22-B5 partial rescue, demonstrated a loss of positional aversion to 0.50 mM lobeline when compared to poxnΔM22-B5; full-152 and poxnΔM22-B5; SuperA158 rescue lines that have functional gustatory bristles on their tarsal segments (*P < 0.05; **P < 0.01; one-way ANOVA, Bonferroni post-test; n ≥ 6). Egg-laying attraction to 0.50 mM lobeline was normal in all lines tested, including poxnΔM22-B5 homozygotes (P > 0.05; one-way ANOVA; n ≥ 6), suggesting that the Gr66a gustatory neurons responsible for the egg-laying behavior are not transformed by the poxn developmental defect.
Thus, Gr66a-expressing neurons that mediate lobeline-induced egg-laying attraction are likely taste cells not affected by the poxn mutation. A few Gr66a-expressing neurons are present in the pharyngeal tissues (Dunipace et al. 2001; Lee et al. 2009; Mitri et al. 2009) and expression of taste-related proteins in these neurons appears to be poxn independent (Galindo and Smith 2001). We therefore hypothesized that Gr66a-expressing cells in the internal mouthpart organs, namely the VCSO and/or the LSO (Figure 3, A and B), may be responsible for receiving input for the egg-laying preference for lobeline. To test this, we generated mosaic females in which subsets of Gr66a-expressing neurons were silenced by expression of TeTx. In flies carrying tubulin-FRT-GAL80-FRT; Gr66aGAL4/UAS-TeTx; heat shock-FLP-recombinase/UAS-CD8-GFP, the gene encoding GAL80, a repressor of GAL4, can be excised upon heat shock (Gordon and Scott 2009). After producing individual females expressing UAS-TeTx in randomly generated GFP-labeled clones, we assayed both positional aversion and egg-laying attraction to lobeline in single mosaic females. Immediately following behavioral tests, heads, legs, and abdomens of individual experimental flies were dissected and imaged to determine which Gr66a cells were silenced, identified by GFP expression.
We obtained OI and PI values, and GFP expression data for ∼90 mosaic females and 20 control flies of the same genotype that did not undergo heat shock (and subsequent elimination of GAL80). We divided the assayed females into two groups: (1) those possessing GFP+, and hence UAS-TeTx silenced clones in a particular tissue region within the Gr66aGAL4 expression pattern, and (2) those that were GFP−, and thus lacked UAS-TeTx activity in the tissue. Flies carrying GFP+ and GFP− Gr66a neurons for the following tissues were compared: labellum, legs, abdomen, LSO, and VCSO.
We found that flies containing clones of TeTx-expressing cells in the VCSO had significantly decreased egg-laying preference when compared to flies that did not (Figure 6A). In contrast, egg-laying preference was not affected by clones within the labellum or legs, thus confirming our observed results with the poxnΔM22-B5; full-152 and poxnΔM22-B5; ΔXBs6 females, respectively (Figure 5). Furthermore, egg-laying attraction was not disrupted by silencing neurons in the abdomen or LSO (Figure 6A), thereby arguing that the VCSO plays a primary role in determining oviposition preference for lobeline. Interestingly, silencing a single cell within the VCSO was often sufficient to induce a decrease in egg-laying preference (Figure 6B). Furthermore, the egg-laying preference of females lacking silenced Gr66a neurons in the VCSO was nearly identical to that of controls that were not heat shocked (Figure 6C). Finally, similar analysis of PI values demonstrated a decrease in positional aversion in females with TeTx-expressing clones in Gr66a neurons on the legs (Figure S3), which supports our findings that the legs mediate positional aversion and validates the mosaic analysis. Thus, our results show that Gr66a-expressing gustatory neurons in the internal mouthparts lining the pharynx, specifically the VCSO, receive input for egg-laying attraction to lobeline.
The mushroom body is required for both positional and egg-laying responses
Gr66a sensory neurons project axons into the subesophageal ganglion (SOG) (Thorne et al. 2004; Wang et al. 2004; Miyazaki and Ito 2010). The SOG has been postulated to act as a relay center where signals from peripheral sensory neurons undergo primary processing. Although the selection of the specific behavioral output, positional aversion or egg-laying attraction, could theoretically occur within the SOG and then be transmitted in parallel to motor systems, previous work argues for the presence of additional processing centers in the circuits that connect the SOG to motor output neurons (Gordon and Scott 2009). Given its involvement in other decision-making processes (Zhang et al. 2007; Krashes et al. 2009; Serway et al. 2009; Wu and Guo 2011), we asked whether the mushroom body is involved in choice-related processing of gustatory signals.
To silence mushroom body neurons, we expressed Shits under the control of 5-120GAL4, which drives GAL4 expression broadly in all lobes of the mushroom body (Figure 7B) (Joseph et al. 2009; Kaun et al. 2011). Females of genotype 5-120GAL4/+; UAS-Shits/+ showed both a loss of positional aversion and egg-laying attraction to lobeline selectively at the nonpermissive temperature (Figure 7A). To confirm that these phenotypes resulted from specific silencing of the mushroom body, we utilized mushroom body-GAL80 (MB{GAL80}) to repress GAL4-mediated induction of UAS-Shits in mushroom body neurons. MB{GAL80} has been utilized reliably in numerous studies to repress GAL4/UAS induction specifically in neurons within the mushroom body, while maintaining GAL4 activity in other neurons (Krashes et al. 2007, 2009; Hekmat-Scafe et al. 2010; Shuai et al. 2011). Indeed, 5-120GAL4/MB{GAL80}; UAS-Shits/+ flies with restored neuronal signaling in the mushroom body exhibited normal responses to lobeline (Figure 7A). Imaging of the brains of flies carrying the UAS-GFP transgene confirmed that GAL4 expression was repressed only within neurons of the mushroom body in 5-120GAL4/MB{GAL80}; UAS-CD8-GFP/+ flies (Figure 7C). To provide further evidence for a role of the mushroom body in our choice behavior, we inhibited synaptic transmission using an additional, independently generated mushroom body-GAL4 line, 30YGAL4 (Aso et al. 2009). We observed a similar loss of both aversion and attraction to lobeline in 30YGAL4/+; UAS-Shits/+ females (Figure S4), corroborating that the mushroom body plays a role in mediating both behavioral responses. In summary, these results suggest that the mushroom body is a higher-order brain structure common to the neural circuits responsible for positional aversion and egg-laying attraction to bitter compounds, and may act as a site of intersection where signals from each pathway could be compared and integrated.
Discussion
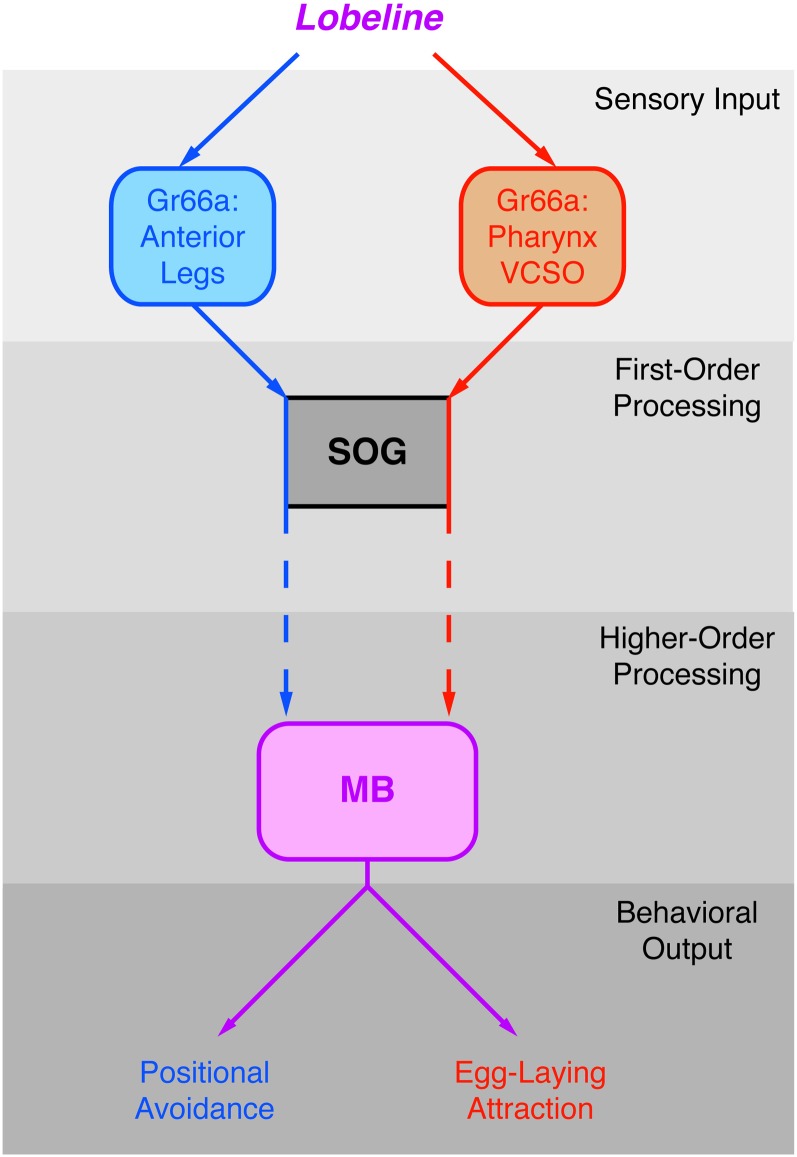
Characterizing both the neural systems that receive relevant sensory input and the central brain regions that select the appropriate motor output is critical to understanding how a female fly chooses between competing environmental preferences to lay eggs to optimize the survival and fitness of her progeny. We propose a model for how female flies decide to either avoid bitter-tasting compounds or approach them for egg-laying purposes (Figure 8).
Figure 8 .
A model for the neural circuits mediating positional and egg-laying responses to lobeline. Sensory input for lobeline is simultaneously received by Gr66a neurons in the legs that receive signals for the positional aversion pathway (blue lines) and by Gr66a neurons in the pharynx that receive signals for the egg-laying attraction pathway (red lines). Both types of sensory neurons project into distinct subregions of the SOG, where some separation of signals is likely maintained during first-order processing. Our data suggest that lobeline signals are relayed to the mushroom body, where they are integrated into a signal (purple lines) that is evaluated before an appropriate motor output is selected.
Using lobeline, a bitter-tasting compound, we observed that sensory input for both positional aversion and egg-laying attraction was received by gustatory neurons expressing the Gr66a gustatory receptor. Synaptic silencing of only thoracic Gr66a neurons and anatomical ablation experiments demonstrated that signaling in Gr66a-expressing neurons in the gustatory bristles of the first tarsal segment on anterior legs is necessary for the avoidance of lobeline, thereby arguing that these Gr66a-expressing foreleg neurons primarily receive input for positional aversion (Figure 8, blue lines). These results are supported by previous work showing that contact of bitter compounds to the legs can induce repulsive behavioral outputs such as inhibition of the proboscis extension reflex (Wang et al. 2004). Surprisingly, analysis of mosaic flies revealed that silencing gustatory neurons in the pharyngeal VCSO disrupted attraction to lobeline as an oviposition substrate (Figure 6), while disrupting signaling in abdominal or other Gr66a-expressing neurons had no effect (Figures 5 and 6). We obtained a relatively low number of mosaic females with silenced neurons in the other Gr66a-expressing pharyngeal organ, the LSO (n = 6), so the possibility remains that input from the LSO also contributes to egg-laying preference. Regardless, our findings show that gustatory signaling from pharyngeal organs appears to be the primary determinant of egg-laying preference for lobeline.
Sensory input for positional and egg-laying preferences occurs at Gr66a-expressing neurons in the anterior legs and the pharynx, respectively (Figure 8). Both foreleg and pharyngeal Gr66a-expressing neurons project to different regions of the SOG (Miyazaki and Ito 2010). This leaves open the possibility that Gr66a neurons in the pharynx and forelegs relay signals through independent pathways that compete only at the level of behavioral output (Figure 8, gray box). As such, neurons for each pathway could theoretically project in parallel from the SOG to specific motor neurons for the execution of each response.
However, silencing the mushroom body disrupted both positional aversion and egg-laying attraction, suggesting that the neural circuits activated by both Gr66a pathways converge on this brain structure. Taken together with previous studies that implicate the mushroom body in other decision-making behaviors (Zhang et al. 2007; Krashes et al. 2009; Serway et al. 2009; Wu and Guo 2011), our results offer one alternative to the parallel pathway model, in which the mushroom body is a candidate integration center that receives and compares lobeline inputs from legs and pharynx, allowing the female fly to select a contextually relevant behavioral output (Figure 8, purple lines). Given that the mushroom body is divided into several neuronal subpopulations (Krashes et al. 2007; Kaun et al. 2011), future studies will be needed to determine whether stimuli from both the pharynx and the legs converge on the same subpopulation of neurons. Furthermore, although a neuroanatomical connection between the SOG and the mushroom body has been identified in other insects (Schroter and Menzel 2003), a link has yet to be discovered in D. melanogaster (Figure 8, dashed arrows). Given that the mushroom body is likely involved in complex spatial orientation (Zhang et al. 2007) and memory-related tasks (Krashes et al. 2009), silencing of this brain structure could disrupt both positional repulsion and egg-laying attraction at more global levels of informational processing, rather than acutely interfering with integration of signals from the two sensory pathways. Regardless of the exact mechanism, our findings show that the mushroom body plays an important role in both attractive and repulsive responses to lobeline.
In addition, our results that abdominal Gr66a neurons do not appear to play a primary role in determining egg-laying preference are curious in that past studies have attributed bristles on the Drosophila ovipositor and vagina as being necessary for egg-laying behaviors, largely based on classification of these sensilla as possessing a chemosensory-like morphology (Taylor 1989; Stocker 1994). However, electrophysiological and behavioral experiments testing the function of these bristles directly have not yet been performed in D. melanogaster. Furthermore, our observations and previous studies have noted that Gr66a abdominal neurons do not project to these bristles, and instead possess multidendritic neuron morphology (Thorne and Amrein 2008; Shimono et al. 2009; Park and Kwon 2011). Although we cannot eliminate the possibility that the ovipositor and vagina bristles are employed in other gustatory processes, our findings argue that Drosophila females can make taste-based evaluations about the quality of an egg-laying substrate by receiving input from pharynx neurons, presumably while they sample the quality of the substrate.
Characterization of the Drosophila gustatory system presents challenges, as single sensory neurons typically co-express combinations of several gustatory receptors (Thorne et al. 2004; Wang et al. 2004; Jiao et al. 2008). It has been postulated that for bitter compounds, this complex co-expression allows Drosophila to detect a multitude of potentially toxic substances and then indiscriminately execute a rejection response that is only modulated by the intensity of bitterness (Masek and Scott 2010). Previous studies have also implicated bitter-sensing Gr66a neurons in only aversive responses (Moon et al. 2006; Lee et al. 2009; Sellier et al. 2011), yet our findings that Gr66a neurons can produce an attractive response argue against such a simple model for the perception of and response toward bitter compounds. This separation of responses based on where lobeline is being detected by Gr66a corresponds with the findings that leg and pharyngeal sensory neurons project axons to different regions of the SOG (Miyazaki and Ito 2010).
Previous studies have shown that a single compound such as carbon dioxide or acetic acid can induce opposing responses. However, such behavioral divergences have been attributed to the compound being detected by different sensory modalities, such as the olfactory and gustatory systems (Suh et al. 2004; Fischler et al. 2007; Joseph et al. 2009), by multiple classes of receptors that sense different properties of a compound, such as odor vs. acidity (Ai et al. 2010) or by molecularly distinct receptor isoforms responding to two completely different stimuli, such as TrpA1-mediated chemical and thermal detection (Kang et al. 2011). In contrast, to the best of our knowledge, we describe an uncharacterized phenomenon in D. melanogaster, in which opposing attractive and repulsive responses to a single stimulus are induced by activation of neurons of the same sensory modality that are likely detecting the same chemical properties of the compound of interest.
Future studies will unravel the molecular mechanisms by which tissue-specific gustatory receptor expression produces divergent behavioral preferences. It has been postulated that Gr66a could be a member of a co-receptor complex required for bitter-signal transduction (Weiss et al. 2011) and that this complex may form multimers with additional gustatory receptors that then confer ligand specificity (Lee et al. 2009). Additionally, recent work has identified a family of ionotropic glutamate receptors involved in Drosophila sensory signaling (Benton et al. 2009); this novel family of receptors may be present in gustatory-related tissues in the adult fly (Croset et al. 2010). It will be interesting to investigate whether Gr66a neurons in the legs and the pharynx express identical or distinct subsets of taste receptors beyond the core co-receptor complex, and if different combinations of Gr66a and co-receptors determine whether a particular leg or pharynx neuron is wired into the aversive or attractive preference pathways, respectively.
In summary, we describe a previously uncharacterized strategy by which an organism utilizes a single sensory receptor in distinct anatomical locations to elicit opposing behavioral outputs in response to a single environmental cue.
Supplementary Material
Acknowledgments
The authors thank Kristin Scott and Michael D. Gordon for the tubulin-FRT-GAL80-FRT; UAS-TeTx; heat shock-FLP flies necessary for the clonal analysis experiments, as well as very helpful advice. We also thank Julie Simpson for the construction and kind gift of the TSH{GAL80} line, Scott Waddell for the MB{GAL80} lines, and Werner Boll and Markus Noll for both the pox-neuro flies and very useful input. Funding was provided by grants from National Institutes of Health/National Institute on Alcohol Abuse and Alcoholism. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Communicating editor: M. Wolfner
Literature Cited
- Ai M., Min S., Grosjean Y., Leblanc C., Bell R., et al. , 2010. Acid sensing by the Drosophila olfactory system. Nature 468(7324): 691–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amlou M., Moreteau B., David J. R., 1998. Genetic analysis of Drosophila sechellia specialization: oviposition behavior toward the major aliphatic acids of its host plant. Behav. Genet. 28: 455–464 [DOI] [PubMed] [Google Scholar]
- Aso Y., Grübel K., Busch S., Friedrich A. B., Siwanowicz I., et al. , 2009. The mushroom body of adult Drosophila characterized by GAL4 drivers. J. Neurogenet. 23(1–2): 156–172 [DOI] [PubMed] [Google Scholar]
- Awasaki T., Kimura K., 1997. Pox neuro is required for development of chemosensory bristles in Drosophila. J. Neurobiol. 32: 707–721 [DOI] [PubMed] [Google Scholar]
- Benton R., Vannice K. S., Gomez-Diaz C., Vosshall L. B., 2009. Variant ionotropic glutamate receptors as chemosensory receptors in Drosophila. Cell 136(1): 149–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boll W., Noll M., 2002. The Drosophila Pox neuro gene: control of male courtship behavior and fertility as revealed by a complete dissection of all enhancers. Development 129: 5667–5681 [DOI] [PubMed] [Google Scholar]
- Brembs B., 2009. Mushroom bodies regulate habit formation in Drosophila. Curr. Biol. 19(16): 1351–1355 [DOI] [PubMed] [Google Scholar]
- Chess K. F., Ringo J. M., 1985. Oviposition site selection by Drosophila melanogaster and Drosophila simulans. Evolution 39: 869–877 [DOI] [PubMed] [Google Scholar]
- Clyne J. D., Miesenböck G., 2008. Sex-specific control and tuning of the pattern generator for courtship song in Drosophila. Cell 133(2): 354–363 [DOI] [PubMed] [Google Scholar]
- Croset V., Rytz R., Cummins S. F., Budd A., Brawand D., et al. , 2010. Ancient protostome origin of chemosensory ionotropic glutamate receptors and the evolution of insect taste and olfaction. PLoS Genet. 6(8): e1001064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detzel A., Wink M., 1993. Attraction, deterrence or intoxication of bees (Apis mellifera) by plant allelochemicals. Chemoecology 4(1): 8–18 [Google Scholar]
- Dunipace L., Meister S., McNealy C., Amrein H., 2001. Spatially restricted expression of candidate taste receptors in the Drosophila gustatory system. Curr. Biol. 11(11): 822–835 [DOI] [PubMed] [Google Scholar]
- Eisses K. T., 1997. The influence of 2-propanol and acetone on oviposition rate and oviposition site preference for acetic acid and ethanol of Drosophila melanogaster. Behav. Genet. 127(3): 171–180 [DOI] [PubMed] [Google Scholar]
- Fischler W., Kong P., Marella S., Scott K., 2007. The detection of carbonation by the Drosophila gustatory system. Nature 448(7157): 1054–1057 [DOI] [PubMed] [Google Scholar]
- Fuyama Y., 1976. Behavior genetics of olfactory responses in Drosophila. I. Olfactometry and strain differences in D. melanogaster. Behav. Genet. 6: 407–420 [DOI] [PubMed] [Google Scholar]
- Galindo K., Smith D. P., 2001. A large family of divergent Drosophila odorant-binding proteins expressed in gustatory and olfactory sensilla. Genetics 159(3): 1059–1072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendre N., Lüer K., Friche S., Grillenzoni N., Ramaekers A., et al. , 2004. Integration of complex larval chemosensory organs into the adult nervous system of Drosophila. Development 131(1): 83–92 [DOI] [PubMed] [Google Scholar]
- Gordon M. D., Scott K., 2009. Motor control in a Drosophila taste circuit. Neuron 61(3): 373–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallem E. A., Ho M. G., Carlson J. R., 2004. The molecular basis of odor coding in the Drosophila antenna. Cell 117(7): 965–979 [DOI] [PubMed] [Google Scholar]
- Hekmat-Scafe D. S., Mercado A., Fajilan A. A., Lee A. W., Hsu R., et al. , 2010. Seizure sensitivity is ameliorated by targeted expression of K+-Cl− cotransporter function in the mushroom body of the Drosophila brain. Genetics 184(1): 171–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isono K., Morita H., 2010. Molecular and cellular designs of insect taste receptor system. Front. Cell Neurosci 4: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaenike J., 1982. Environmental modification of oviposition behavior in Drosophila. Am. Nat. 119: 784–802 [Google Scholar]
- Jiao Y., Moon S. J., Wang X., Ren Q., Montell C., 2008. Gr64f is required in combination with other gustatory receptors for sugar detection in Drosophila. Curr. Biol. 18(22): 1797–1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph R. M., Devineni A. V., King I. F., Heberlein U., 2009. Oviposition preference for and positional avoidance of acetic acid provide a model for competing behavioral drives in Drosophila. Proc. Natl. Acad. Sci. USA 106(27): 11352–11357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kable J. W., Glimcher P. W., 2009. The neurobiology of decision: consensus and controversy. Neuron 63(6): 733–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang K., Panzano V. C., Chang E. C., Ni L., Dainis A. M., et al. , 2011. Modulation of TRPA1 thermal sensitivity enables sensory discrimination in Drosophila. Nature 481(7379): 76–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaun K. R., Azanchi R., Maung Z., Hirsh J., Heberlein U., 2011. A Drosophila model for alcohol reward. Nat. Neurosci. 14(5): 612–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamoto T., 2001. Conditional modification of behavior in Drosophila by targeted expression of a temperature-sensitive shibire allele in defined neurons. J. Neurobiol. 47: 81–92 [DOI] [PubMed] [Google Scholar]
- Krashes M. J., Keene A. C., Leung B., Armstrong J. D., Waddell S., 2007. Sequential use of mushroom body neuron subsets during Drosophila odor memory processing. Neuron 53(1): 103–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krashes M. J., DasGupta S., Vreede A., White B., Armstrong J. D., et al. , 2009. A neural circuit mechanism integrating motivational state with memory expression in Drosophila. Cell 139(2): 416–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristan W. B., 2008. Neuronal decision-making circuits. Curr. Biol. 18(19): R928–R932 [DOI] [PubMed] [Google Scholar]
- Krochmal A., Wilken L., Chien M., 1972. Lobeline content of four Appalachian lobelias. Lloydia 35(3): 303–304 [PubMed] [Google Scholar]
- Larsson M. C., Domingos A. I., Jones W. D., Chiappe M. E., Amrein H., et al. , 2004. Or83b encodes a broadly expressed odorant receptor essential for Drosophila olfaction. Neuron 43(5): 703–714 [DOI] [PubMed] [Google Scholar]
- Lee Y., Moon S. J., Montell C., 2009. Multiple gustatory receptors required for the caffeine response in Drosophila. Proc. Natl. Acad. Sci. USA 106(11): 4495–4500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y., Kim S. H., Montell C., 2010. Avoiding DEET through insect gustatory receptors. Neuron 67(4): 555–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marella S., Fischler W., Kong P., Asgarian S., Rueckert E., et al. , 2006. Imaging taste responses in the fly brain reveals a functional map of taste category and behavior. Neuron 49(2): 285–295 [DOI] [PubMed] [Google Scholar]
- Masek P., Scott K., 2010. Limited taste discrimination in Drosophila. Proc. Natl. Acad. Sci. USA 107(33): 14833–14838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo T., Sugaya S., Yasukawa J., Aigaki T., Fuyama Y., 2007. Odorant-binding proteins OBP57d and OBP57e affect taste perception and host-plant preference in Drosophila sechellia. PLoS Biol. 5(5): e118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mery F., Kawecki T. J., 2002. Experimental evolution of learning ability in fruit flies. Proc. Natl. Acad. Sci. USA 99: 14274–14279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller P. M., Saltz J. B., Cochrane V. A., Marcinkowski C. M., Mobin R., et al. , 2011. Natural variation in decision-making behavior in Drosophila melanogaster. PLoS ONE 6(1): e16436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitri C., Soustelle L., Framery B., Bockaert J., Parmentier M. L., et al. , 2009. Plant insecticide L-canavanine repels Drosophila via the insect orphan GPCR DmX. PLoS Biol. 7(6): e1000147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki T., Ito K., 2010. Neural architecture of the primary gustatory center of Drosophila melanogaster visualized with GAL4 and LexA enhancer-trap systems. J. Comp. Neurol. 518(20): 4147–4181 [DOI] [PubMed] [Google Scholar]
- Moon S. J., Köttgen M., Jiao Y., Xu H., Montell C., 2006. A taste receptor required for the caffeine response in vivo. Curr. Biol. 16(18): 1812–1817 [DOI] [PubMed] [Google Scholar]
- Moreteau B., R’Kha S., David J. R., 1994. Genetics of a nonoptimal behavior: oviposition preference of Drosophila mauritiana for a toxic resource. Behav. Genet. 24: 433–441 [DOI] [PubMed] [Google Scholar]
- Park J. H., Kwon J. Y., 2011. A systematic analysis of Drosophila gustatory receptor gene expression in abdominal neurons which project to the central nervous system. Mol. Cells 32(4): 375–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond R. C., Gerking J. L., 1979. Oviposition site preference in Drosophila. Behav. Genet. 9: 233–241 [DOI] [PubMed] [Google Scholar]
- Ruiz-Dubreuil G., Burnet B., Connolly K., 1994. Behavioral correlates of selection for oviposition by Drosophila melanogaster females in a patchy environment. Heredity 73: 103–110 [DOI] [PubMed] [Google Scholar]
- Schroter U., Menzel R., 2003. A new ascending sensory tract to the calyces of the honeybee mushroom body, the subesophageal-calycal tract. J. Comp. Neurol. 465: 168–178 [DOI] [PubMed] [Google Scholar]
- Schwartz N. U., Zhong L., Bellemer A., Tracey W. D., 2012. Egg laying decisions in Drosophila are consistent with foraging costs of larval progeny. PLoS ONE 7(5): e37910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellier M. J., Reeb P., Marion-Poll F., 2011. Consumption of bitter alkaloids in Drosophila melanogaster in multiple-choice test conditions. Chem. Senses 36(4): 323–334 [DOI] [PubMed] [Google Scholar]
- Serway C. N., Kaufman R. R., Strauss R., de Belle J. S., 2009. Mushroom bodies enhance initial motor activity in Drosophila. J. Neurogenet. 23(1–2): 173–184 [DOI] [PubMed] [Google Scholar]
- Shimono K., Fujimoto A., Tsuyama T., Yamamoto-Kochi M., Sato M., et al. , 2009. Multidendritic sensory neurons in the adult Drosophila abdomen: origins, dendritic morphology, and segment- and age-dependent programmed cell death. Neural Dev. 4: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuai Y., Hu Y., Qin H., Campbell R. A., Zhong Y., 2011. Distinct molecular underpinnings of Drosophila olfactory trace conditioning. Proc. Natl. Acad. Sci. USA 108(50): 20201–20206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker R. F., 1994. The organization of the chemosensory system in Drosophila melanogaster: a review. Cell Tissue Res. 275(1): 3–26 [DOI] [PubMed] [Google Scholar]
- Stocker R. F., Schorderet M., 1981. Cobalt filling of sensory projections from internal and external mouthparts in Drosophila. Cell Tissue Res. 216(3): 513–523 [DOI] [PubMed] [Google Scholar]
- Suh G. S., Wong A. M., Hergarden A. C., Wang J. W., Simon A. F., et al. , 2004. A single population of olfactory sensory neurons mediates an innate avoidance behaviour in Drosophila. Nature 431(7010): 854–859 [DOI] [PubMed] [Google Scholar]
- Sweeney S. T., Broadie K., Keane J., Niemann H., O’Kane C. J., 1995. Targeted expression of tetanus toxin light chain in Drosophila specifically eliminates synaptic and causes behavioral defects. Neuron 14(2): 341–351 [DOI] [PubMed] [Google Scholar]
- Taylor B. J., 1989. Sexually dimorphic neurons in the terminalia of Drosophila melanogaster: I. Development of sensory neurons in the genital disc during metamorphosis. J. Neurogenet. 5(3): 173–192 [DOI] [PubMed] [Google Scholar]
- Thorne N., Chromey C., Bray S., Amrein H., 2004. Taste perception and coding in Drosophila. Curr. Biol. 14(12): 1065–1079 [DOI] [PubMed] [Google Scholar]
- Thorne N., Amrein H., 2008. Atypical expression of Drosophila gustatory receptor genes in sensory and central neurons. J. Comp. Neurol. 506(4): 548–568 [DOI] [PubMed] [Google Scholar]
- van Delden W., Kamping A., 1990. Genetic variation for oviposition behavior in Drosophila melanogaster. 2. Oviposition preferences and differential survival. Behav. Genet. 20: 661–673 [DOI] [PubMed] [Google Scholar]
- Wang Z., Singhvi A., Kong P., Scott K., 2004. Taste representations in the Drosophila brain. Cell 117(7): 981–991 [DOI] [PubMed] [Google Scholar]
- Weiss L. A., Dahanukar A., Kwon J. Y., Banerjee D., Carlson J. R., 2011. The molecular and cellular basis of bitter taste in Drosophila. Neuron 69(2): 258–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wink M., Schneider D., 1990. Fate of plant-derived secondary metabolites in three moth species (Syntomis mogadorensis, Syntomeida epilais, and Creatonotos transiens). J. Comp. Physiol. B 160(4): 389–400 [Google Scholar]
- Wu Z., Guo A., 2011. A model study on the circuit mechanism underlying decision-making in Drosophila. Neural Netw. 24(4): 333–344 [DOI] [PubMed] [Google Scholar]
- Xi W., Peng Y., Guo J., Ye Y., Zhang K., et al. , 2008. Mushroom bodies modulate salience-based selective fixation behavior in Drosophila. Eur. J. Neurosci. 27(6): 1441–1451 [DOI] [PubMed] [Google Scholar]
- Yang C. H., Belawat P., Hafen E., Jan L.-Y., Jan Y.-N., 2008. Drosophila egg-laying site selection as a system to study simple decision-making processes. Science 319(5870): 1679–1683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K., Guo J. Z., Peng Y., Xi W., Guo A., 2007. Dopamine-mushroom body circuit regulates saliency-based decision-making in Drosophila. Science 316(5833): 1901–1904 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.