Abstract
Purpose: To investigate the impact of oxidative stress on pregnancy success by monitoring malondialdehyde levels in follicular fluid.
Methods: Forty five couples were enrolled in this prospective study. Following long protocol of GnRH analogues and r-FSH treatment, oocyte retrieval and intracytoplasmic sperm injection were performed. Malondialdehyde levels were assayed by thiobarbutiric acid reacting substances test. Student's t-test and χ2 test were used for statistical analysis.
Results: Patients were divided into two groups; group I (pregnancy positive, n = 20), group II (pregnancy negative, n = 25). There was no statistical significant difference in terms of age, infertility period, FSH levels on the third day, number of oocytes retrieved and fertilization rates between the two groups. Pregnancy rates were found to be decreasing in higher malondialdehyde levels.
Conclusion: Malondialdehyde can be used as a marker of oxidative stress and a potential marker in predicting assisted reproductive techniques outcome.
KEYWORDS: Assisted reproductive techniques, follicular fluid, malondialdehyde, oxidative stress, reactive oxygen species.
INTRODUCTION
Cells living under aerobic conditions are continuously subject to paradoxic effects of oxygen. Oxygen is vital for cell survival, however the reactive oxygen species (ROS) which are metabolites of oxygen may alter the functions of the cells and/or even threaten their lives (1). ROS therefore should be inactivated at all times and be kept in minimum amounts necessary to maintain normal cell functions. Excessive production of ROS and deficiency in antioxidant defense mechanisms result as oxidative stress (OS) (2).
Oxidative stress (OS) is believed to afflict reproductive functions. Follicular fluid represents a very important microenviroment which harbors oocytes and plays a role in fertilization and embryogenesis. This microenviroment is a metabolically active system in which oocytes live in and contains steroid hormones, growth factors, cytokines, granulosa cells and leucocytes. Having a lipid rich membrane similar to spermatozoa may cause oocytes to face lipid peroxidation and hazardous effects of ROS (1,3). The clinical result will be patients facing infertility problems. Diagnosing such patients and predicting the outcomes of the treatment and developing an antioxidant treatment regimen may become very important in the near future.
Malondialdehyde (MDA) is a by-product of lipid peroxide decomposition and has been used to monitor the degree of the peroxidative damage on cells (4). Biochemical assays of MDA have been proven to be very important in showing impaired sperm functions in terms of motility and capacity for sperm-oocyte fusion (3).
In our study we aimed to find out the impact of oxidative stress (OS) on pregnancy success by monitoring MDA levels in follicular fluid.
MATERIALS AND METHODS
Forty five couples (45 females, 45 males) were enrolled in the study who had been followed prospectively in our clinic's assisted reproductive techniques (ARTs) department. Age of the patients ranged from 20 to 40 years in the female group and from 22 to 49 years in the male group. To rule out the male factor, all patients were selected from the intracytoplasmic sperm injection (ICSI) performed group. Patient ages, infertility periods, and levels of FSH on the 3rd day of menstrual cycle (basal FSH) had been recorded for each patient.
In order to suppress the menstrual cycle long protocol GnRH analogue had been used. On the 21st day of the cycle 0.5 mg Leuprolid Acetate sc had been administered. The estradiol (E2) levels less than 50 pg/mL and endometrium thickness less than 4 mm on the 2nd day of mensturation had been considered as down regulation, and gonadotropin treatment had been introduced.
The dosage of gonadotropin had been calculated individually for each patient considering the age and the basal FSH levels. Varying doses of r-FSH (150–450 IU/day) had been used. After achieving to develop minimum three follicules of 18 mm or more, E2 levels were assayed. 10000 IU HCG (im) had been administered and follicular aspiration (OPU) had been performed in the following 34–36 h. Mature oocytes had been counted and extracted from the follicular fluid and been implanted on the IVF-G1 and G2 culture mediums. Since ICSI had been performed to all patients, cumulus cells had been removed 4–6 h after oocyte formation and microinjection had been performed. They had been examined for the presence of two pro-nuclei 16–18 h after ICSI. The transfer of the embryos was performed 48–72 h after ICSI. Prior to transfer the embryos were evaluated microscopically in order to be 6–8 cells in each embryo of grade I–II. Maximum three embryos were transferred. On the 12th–14th days of the transfer a urine β-HCG test was performed to confirm pregnancy.
The aim of this study was to show the levels of MDA in follicular fluids of infertile women. The follicular fluids which had been collected on the ovum pick-up (OPU) day had been stored at −20°C in the laboratory. Thiobarbutiric acid reacting substances (TBARS) test was performed to assess the MDA levels in follicular fluid. This test detects all the substances including MDA which reacts to thiobutiric acid and is therefore called TBARS. Biological fluid which reacted to a combination of 15% TCA (trichloroacetic acid), 0.375% TBA (thiobutiric acid), 0.25 M HCl reactives was incubated in boiling water for 20 min and cooled later on. The fluid was then centrifuged at 3500 rpm for 10 min and absorbance readings were taken on the spectrophotometer at 535 nm. The result is multiplied by 6 and divided by 0156 and the final result is read as μmol/MDA or TBARS.
SPSS for Windows 11.5 program had been used for statistical analysis. Student's t-test and χ2 tests were used to compare the quantitative statistical data. Also ROC curves were used to evaluate the cut-off values of MDA levels in follicular fluid. p < 0.05 was considered to be statistically significant.
RESULTS
Twenty pregnancies were recorded among the 45 couples enrolled in the study: 1 being biochemical pregnancy, 12 being singletons, 6 being twins and 1 being triplet. Patients were divided into two groups; group I (pregnancy positive) and group II (pregnancy negative).
The two groups had been compared statistically in terms of age, infertility period, and level of FSH on 3rd day of menstrual cycle (basal FSH). The mean age was 30.15 (±4.19) years in the pregnancy positive group (group I) and 32.48(±4.47) years in the pregnancy negative group (group II) showing no significant difference (p > 0.05). There was no statistical significant difference in terms of infertility period and levels of FSH between the two groups (Table I).
Table I.
Comparison of Patient Age, Infertility Period, Basal FSH, E2 Levels on HCG Administration Day, Number of Collected Oocytes and Follicular Fluid MDA Levels
| Pregnancy positive (Group I) (n = 20) | Pregnancy negative (Group II) (n = 25) | p | |
|---|---|---|---|
| Age (years) | 30.15 (±4.19) | 32.48 (±4.47) | NS |
| Infertility period (years) | 7.6 (±3.2) | 8.9 (±4.3) | NS |
| FSH (mIU/mL) level on 3rd day | 6.60 (±2.22) | 6.81 (±2.47) | NS |
| E2(pg/mL) level on HCG day | 2076 (±1060) | 2363 (±1307) | NS |
| Number of oocyte collected | 10.7 (±4.8) | 9.4 (±4) | NS |
| Fertilization rate (%) | 78.6 (±13.8) | 68 (±21.5) | NS |
| Follicular fluid MDA level (μmol/L) | 5.12 (±1.8) | 6.79 (±2.96) | <0.05 |
NS: not significant; p < 0.05; significant.
Following controlled ovarian hyperstimulation (COS) E2 levels on HCG administration day, the number of oocytes retrieved, fertilization rates and follicular fluid MDA levels were compared between the two groups. Pregnancy rates were found to be decreasing in higher MDA levels. The mean MDA level of the 20 pregnant patients was 5.12 (±1.85) μmol/L whereas it was 6.79 (±2.96) μmol/L in the non-pregnant 25 patients showing statistical significance (p < 0.05). The other parameters did not show any statistical significant difference between the two groups (Table I, Fig. 1).
Fig. 1.
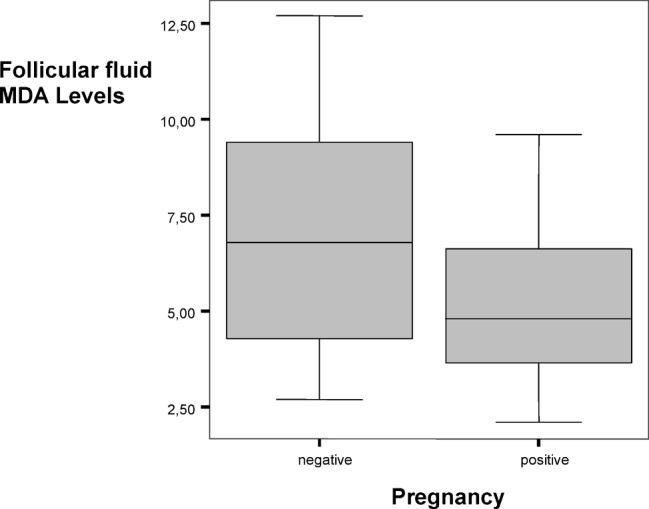
Follicular fluid MDA levels (μmol/L) and pregnancy relations.
ROC curves were used to evaluate the efficiency of predicting the probability to conceive using follicular MDA levels, age, 3rd day FSH levels, E2 levels on HCG administration day (Fig. 2). The most valuable parameters were found to be follicular fluid MDA levels and age respectively. For a cut-off value of 5.25 μmol/L for follicular fluid MDA, it showed 60% sensitivity and 64% specificity (AUC: Area Under Curve = 0.673).
Fig. 2.

ROC curves showing pregnancy relation with different parameters.
DISCUSSION
Oxidative stress (OS) causes damage in all cells of mammals. For this reason it can afflict reproductive functions of spermatozoa and oocytes. OS results from excessive production of ROS and/or impaired antioxidant defense mechanisms. In literature there are many studies considering OS as a major reproductive failure factor especially in males. Aitken and Irvine stated that sperm disfunction, motility reduction and DNA damage may be related to high levels of ROS. Sikka and Lenzi reported that only impaired oxidant-antioxidant balance due to systemic predisposition or pathologies could be held responsible for male infertility. Sharma and Saleh indicated that presence of leucocytes in semen is associated with OS and therefore causes male infertility (5–12).
The impact of OS on female reproductive system has been a little investigated issue. Agarwal et al. in their study tried to define the relationship between different female infertility problems and ROS levels in peritoneal fluids. ROS were present in peritoneal fluids of patients with endometriosis and idiopathic infertility. Levels of ROS were not significantly different in patients with endometriosis and control group, but there was a significant difference between patients with idiopathic infertility and control group. Therefore ROS were considered to play a role in idiopathic infertility (3).
Same authors in another study had investigated the relationship between ROS levels in follicular fluid and oocyte maturation, fertilization, and pregnancy. As a result they have reported that follicular fluid ROS levels may be a potential marker for predicting success in ARTs (13).
Jozwick et al. compared levels of OS markers in serum and pre-ovulatory follicular fluid and found lower levels in follicular fluid. In their study no significant difference was found in OS marker levels in follicular fluid between pregnant and nonpregnant women. They also reported a lack of relationship between fertilization rate and OS marker levels (14). Similarly in our study we could not find a relationship between follicular fluid MDA levels and fertilization rates.
Oyawaye et al. investigated follicular fluid ROS levels in IVF cases and reported significant lower levels of OS marker levels in successfully fertilized and transferred oocytes. They stated that ROS play an important role in the female reproductive system (15).
The impact of OS both on male and female reproductive systems had been investigated in different studies. Is OS really a cause of infertility? Can we use ROS levels as a marker in predicting ARTs success? Is there a future for antioxidant therapy for infertility?
As seen in the previous studies and in ours, the effects of ROS on female reproductive functions are not still clear, however OS is believed to play an important role in unexplained infertility cases. Although MDA has a relatively poor predictive value, lower follicular fluid MDA levels in pregnancy positive group (Group I) indicate that MDA levels may be used as a predictive marker in ARTs success. Compared to other parameters such as age, basal FSH and E2 level on HCG administration day, follicular fluid MDA level is found to be more predictive.
CONCLUSION
OS afflicts reproductive systems of both sexes. OS causes sperm motility and function failure in males. It plays a role in some cases of unexplained female infertility. We can use MDA which is a by-product of lipid peroxidation process as a marker of OS. MDA levels may also be used as a potential marker in predicting ARTs outcome. On the bases of these findings, we believe that antioxidant therapy regimens will be discussed more for the treatment of unexplained infertility cases in the near future.
REFERENCES
- 1.de Lamirande E, Gagnon C. Impact of reactive oxygen species on spermatozoa: A balancing act between beneficial and detrimental effects. Hum Reprod. 1995;10:15–21. doi: 10.1093/humrep/10.suppl_1.15. [DOI] [PubMed] [Google Scholar]
- 2.Sikka SC. Relative impact of oxidative stress on male reproductive function. Curr Med Chem. 2001;8:851–862. doi: 10.2174/0929867013373039. [DOI] [PubMed] [Google Scholar]
- 3.Agarwal A, Saleh RA, Bedaiwy MA. Role of oxygen species in the pathophysiology of human reproduction. Fertil Steril. 2003;79:829–843. doi: 10.1016/S0015-0282(02)04948-8. [DOI] [PubMed] [Google Scholar]
- 4.Aitken RJ, Fisher H. Reactive oxygen species generation and human spermatozoa: The balance of benefit and risk. Bioassays. 1994;16:259–267. doi: 10.1002/bies.950160409. [DOI] [PubMed] [Google Scholar]
- 5.Aitken RJ, Clarkson JS, Hargreave TB, Irvine DS, Wu FC. Analysis of the relationship between defective sperm function and the generation of reactive oxygen species in cases of oligozoospermia. J Androl. 1989;10(3):214–220. doi: 10.1002/j.1939-4640.1989.tb00091.x. [DOI] [PubMed] [Google Scholar]
- 6.Aitken RJ. A free radical theory of male infertility. Reprod Fertil Dev. 1994;6:19–24. doi: 10.1071/RD9940019. [DOI] [PubMed] [Google Scholar]
- 7.Aitken RJ, Krausz C. Oxidative stress, DNA damage and the Y chromosome (Review) Reproduction. 2001;122:497–506. doi: 10.1530/rep.0.1220497. [DOI] [PubMed] [Google Scholar]
- 8.Irvine DS, Twigg JP, Gordon EL, Fulton N, Milne PA, Aitken RJ. DNA integrity in human spermatozoa: Relationships with semen quality. J Androl. 2000;21(1):33–44. [PubMed] [Google Scholar]
- 9.Sikka SC. Oxidative stress and role of antioxidants in normal and abnormal sperm function. Front Biosci. 1996;1:78–86. doi: 10.2741/a146. [DOI] [PubMed] [Google Scholar]
- 10.Lenzi A, Gandini L, Picardo M, Tramer F, Sandri G, Panfili E. Lipoperoxidation damage of spermatozoa polyunsaturated fatty acids (PUFA): Scavenger mechanisms and possible scavenger therapies. Front Biosci. 2000;5:E1–E15. doi: 10.2741/Lenzi. [DOI] [PubMed] [Google Scholar]
- 11.Sharma RK, Pasqualotto E, Nelson DR, Thomas AJ, Jr, Agarwal A. Relationship between seminal white blood cell counts and oxidative stress in men treated at an infertility clinic. J Androl. 2001;22(4):575–583. [PubMed] [Google Scholar]
- 12.Saleh RA, Agarwal A, Kandirali E, Sharma RK, Thomas AJ, Nada EA, Evenson DP, Alvarez JG. Leukocytospermia is associated with increased reactive oxygen species production by human spermatozoa. Fertil Steril. 2002;78(6):1215–1224. doi: 10.1016/S0015-0282(02)04237-1. [DOI] [PubMed] [Google Scholar]
- 13.Attaran M, Pasqualotto E, Falcone T, Goldberg JM, Miller KF, Agarwal A, Sharma RK. The effect of follicular fluid reactive oxygen species on the outcome of in vitro fertilization. Int J Fertil Wom Med. 2000;45(5):314–320. [PubMed] [Google Scholar]
- 14.Jozwik Ma, Wolczynski S, Jozwik Mi, Szamatowicz M. Oxidative stress markers in preovulatory follicular fluid in humans. Mol Hum Rep. 1999;5(5):409–413. doi: 10.1093/molehr/5.5.409. [DOI] [PubMed] [Google Scholar]
- 15.Oyawoye O, Abdel GA, Garner A, Constantinovici N, Perret C, Hardiman P. Antioxidants and reactive oxygen species in follicular fluid of women undergoing IVF: Relationship to outcome. Hum Reprod. 2003;18(11):2270–2274. doi: 10.1093/humrep/deg450. [DOI] [PubMed] [Google Scholar]


