Abstract
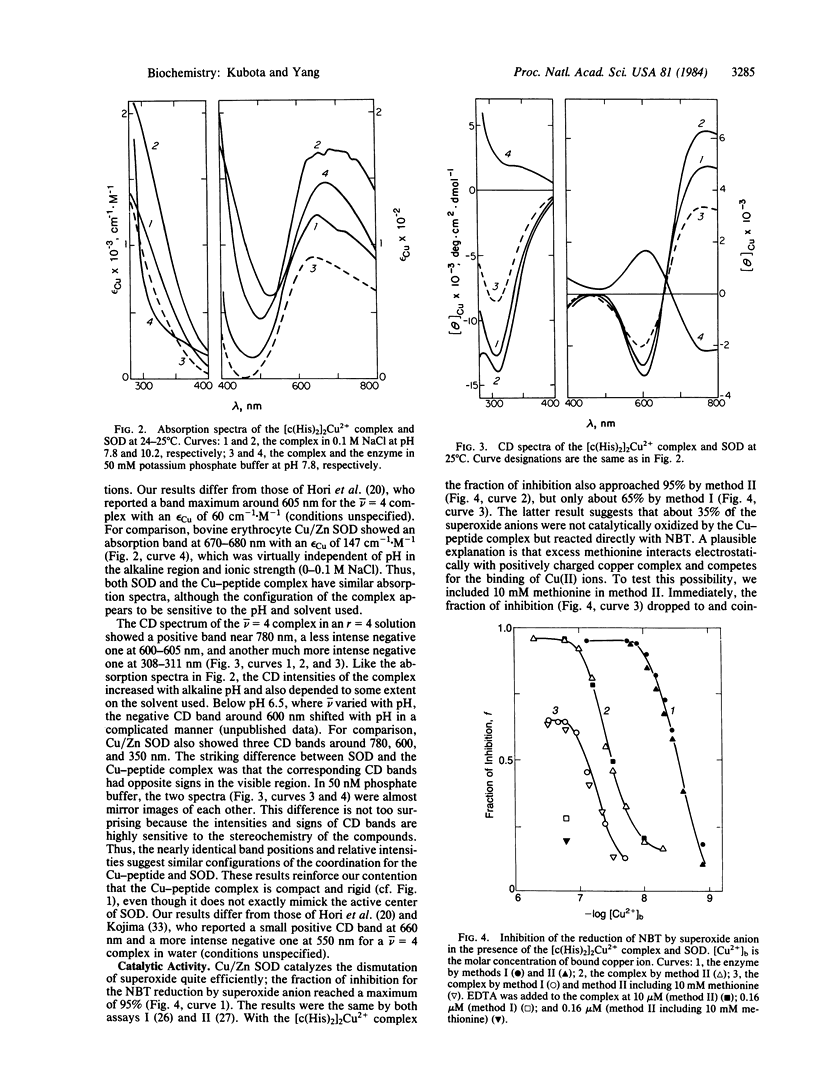
The formation of copper complexes with bis[cyclo( histidylhistidine )] copper(II) was determined potentiometrically; the maximum coordination number was four deprotonated histidine residues per Cu(II) ion. This complex mimicks the active center of Cu/Zn superoxide dismutase (superoxide:superoxide oxidoreductase, EC 1.15.1.1). Its absorption spectrum showed a broad band above 600 nm as compared with the 670- to 680-nm band of the enzyme. Its CD spectrum had a negative 600- to 605-nm band and a more intense positive band near 780 nm. The band positions were identical with, and the intensities were higher than, those of the enzyme, but the corresponding bands had opposite signs. This can be attributed to the difference in the distorted planar structure between the complex and enzyme. The complex catalyzed the dismutation of superoxide anion; the activity was 1/10th that of the enzyme on a molar basis, but about three times that of the enzyme on a weight basis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beauchamp C., Fridovich I. A mechanism for the production of ethylene from methional. The generation of the hydroxyl radical by xanthine oxidase. J Biol Chem. 1970 Sep 25;245(18):4641–4646. [PubMed] [Google Scholar]
- Beauchamp C., Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971 Nov;44(1):276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Boden N., Holmes M. C., Knowles P. F. Binding of water to "types I and II" Cu2+ in proteins. Biochem Biophys Res Commun. 1974 Apr 8;57(3):845–848. doi: 10.1016/0006-291x(74)90623-8. [DOI] [PubMed] [Google Scholar]
- Carson S., Vogin E. E., Huber W., Schulte T. L. Safety tests of orgotein, an antiinflammatory protein. Toxicol Appl Pharmacol. 1973 Oct;26(2):184–202. doi: 10.1016/0041-008x(73)90252-4. [DOI] [PubMed] [Google Scholar]
- FELSENFELD G. The determination of cuprous ion in copper proteins. Arch Biochem Biophys. 1960 Apr;87:247–251. doi: 10.1016/0003-9861(60)90168-5. [DOI] [PubMed] [Google Scholar]
- Forman H. J., Fridovich I. On the stability of bovine superoxide dismutase. The effects of metals. J Biol Chem. 1973 Apr 25;248(8):2645–2649. [PubMed] [Google Scholar]
- Fridovich I. Superoxide dismutases. Annu Rev Biochem. 1975;44:147–159. doi: 10.1146/annurev.bi.44.070175.001051. [DOI] [PubMed] [Google Scholar]
- Gaber B. P., Brown R. D., Koenig S. H., Fee J. A. Nuclear magnetic relaxation dispersion in protein solutions. V. Bovine erythrocyte superoxide dismutase. Biochim Biophys Acta. 1972 Jun 22;271(1):1–5. doi: 10.1016/0005-2795(72)90126-2. [DOI] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- Oberley L. W., Buettner G. R. Role of superoxide dismutase in cancer: a review. Cancer Res. 1979 Apr;39(4):1141–1149. [PubMed] [Google Scholar]
- Richardson J. S., Thomas K. A., Richardson D. C. Alpha-carbon coordinates for bovine Cu,Zn superoxide dismutase. Biochem Biophys Res Commun. 1975 Apr 21;63(4):986–992. doi: 10.1016/0006-291x(75)90666-x. [DOI] [PubMed] [Google Scholar]
- Richardson J., Thomas K. A., Rubin B. H., Richardson D. C. Crystal structure of bovine Cu,Zn superoxide dismutase at 3 A resolution: chain tracing and metal ligands. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1349–1353. doi: 10.1073/pnas.72.4.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson P., Jr, Fridovich I. Does copper-D-penicillamine catalyze the dismutation of O2-? Arch Biochem Biophys. 1980 Sep;203(2):830–831. doi: 10.1016/0003-9861(80)90246-5. [DOI] [PubMed] [Google Scholar]
- Rotilio G., Calabrese L., Bossa F., Barra D., Agrò A. F., Mondovì B. Properties of the apoprotein and role of copper and zinc in protein conformation and enzyme activity of bovine superoxide dismutase. Biochemistry. 1972 May 23;11(11):2182–2187. doi: 10.1021/bi00761a027. [DOI] [PubMed] [Google Scholar]
- Valentine J. S., Curtis A. B. A convenient preparation of solutions of superoxide aniom and the reaction of superoxide anion with a copper (II) complex. J Am Chem Soc. 1975 Jan 8;97(1):224–226. doi: 10.1021/ja00834a058. [DOI] [PubMed] [Google Scholar]