Abstract
Purpose
In search for a new marker of preimplantation embryo viability the present study investigated oxygen consumption of individual cleavage stage murine embryos, and evaluated the predictive value regarding subsequent development to expanded blastocysts.
Methods
In all, 248 embryos were investigated from 2 cell stage until blastocyst stage with individual measurement of oxygen consumption and recording of developmental stage. Cleavage stage embryos and morula were divided in groups according to their oxygen consumption, and odds ratios (OR) for subsequent development to expanded blastocyst were calculated.
Results
Cleavage stage (2–8 cell) individual oxygen consumption was 0.16–0.20 nl O2 h−1, with a significant increase to 0.21–0.23 nl O2 h−1 at the morula stage followed by a more than twofold increase for the expanded blastocyst 0.47 nl O2 h−1. A significantly higher chance of reaching the expanded blastocyst stage was found in 4-cell embryos with high oxygen consumption, than embryos with low consumption (OR 2.25, 95% CI 1.04–4.90). Among 2-cell embryos the chance of low and high consumers was not significantly different. The method used in the present study somewhat compromised embryo development (51% blastocyst rate) compared to controls (80% blastocystrate) which could make our results less robust.
Conclusion
Preliminary data from the present study suggest that oxygen consumption in cleavage stage embryos may be an indicator, but a not a strong predictor, of subsequent development to expanded blastocysts.
Keywords: Murine embryos, Preimplantation development, Oxygen consumption
Introduction
In vitro fertilisation (IVF) and culture of preimplantation embryos is an established part of assisted reproduction, and has permitted over a million couples world wide to conceive. Yet, the method is limited by a rather low success rate with an overall average implantation rate of cleavage stage embryos in the order of 20–30% [1]. Characteristics of embryo morphology observable in the light microscope, in concert with kinetics of development, are still today the prime measures of embryo quality. Despite advances in the development of morphological criteria for embryo selection, and its undoubtedly strong association with developmental competence [2], additional indicators to assist selection of embryos with a particularly high implantation potential are still needed to improve the IVF success rate, especially in connection with elective single embryo transfers (eSET). Elective SET is performed more and more frequently, particularly in the Nordic countries, and will most likely increase the number of treatment cycles where a specific embryo must be chosen among morphologically equal peers. Elective SET also increases the frequency of frozen embryo replacement (FER) cycles. As the pregnancy rate per frozen-thawed embryo typically is reduced compared to fresh transfers, there may be a particular need for embryo viability assessment in these cycles also.
It is a common practice in IVF settings to transfer embryos on day 2 or day 3 following oocyte pick-up. Culture to the blastocyst stage demands significantly more laboratory resources, and requires surplus cleavage stage embryos to be meaningful in a clinical setting. Culture to the blastocyst stage is sometimes performed as the blastocyst development itself is a very strong viability indicator of the embryo [3]. However, although the individual blastocyst in general has a higher implantation rate than the cleavage stage (2–8 cells) embryo, it remains unclear whether a blastocyst transfer policy results in higher pregnancy rates per treatment cycle, than routine transfer of cleavage stage embryos [4]. This may in part be due to the fact that culture conditions still are not optimal. Hence, human preimplantation embryo viability remains compromised in vitro relative to in vivo, which supports a strategy of day 2 transfer [5]. Early viability indicators applicable at the cleavage stage are therefore of particular interest for the embryologist in a clinical setting. However, it should be considered that assessment of the cleavage stage embryo primarily is an assessment of the oocyte and the maternal competence, as the paternal effect mainly is evident after the 8 cell stage [6, 7], with a shift from maternal to embryo genomic control.
Non-invasive measures of embryo metabolism, such as amino acid profiles [8, 9] and utilisation of substrates for energy production [10–12] have been suggested as objective embryo quality criteria. Generation of energy is a prerequisite for development of the preimplantation embryo. The energy metabolism of preimplantation embryos significantly changes through development from fertilised oocyte to expanded blastocyst. The first embryonic cleavages are completely dependent on oxidative metabolism [8, 13], with about 95% of ATP production derived from oxidative phosphorylation, decreasing to 82% with compaction [12, 14]. From morula to blastocyst stage the metabolism changes towards an increasing contribution of ATP from aerobic glycolysis. Oxygen is the sole substrate of the oxidative energy metabolism where the embryo cannot fall back on its own reserves, so changes in mitochondrial activity should be directly reflected in altered fluxes of oxygen to the embryo. Early phases of cell death are associated with such changes in mitochondria [15–17] and altered energy metabolism may be reflected in the oxygen consumption before morphological changes becomes detectable. Therefore, individual embryo oxygen consumption appears the best available indicator of the overall energy metabolism of the cleavage stage embryo [5]. Yet, direct measures of oxygen consumption as a measure of ATP production may have to be interpreted with care. In a single study on mice it was found that as much as 70% of the cleavage stage oxygen consumption may be OXPHOS (mitochondrial oxidative phosphorylation) independent, decreasing to 30% at the blastocyst stage, due to a substantially increased mitochondrial OXPHOS dependent oxygen consumption between cleavage stage embryos and blastocysts [18]. Whether this is a general picture remains uncertain, and the nature and kinetics of the OXPHOS independent oxygen consumption as well as its implications for the embryo development needs to be studied in further detail.
In this study we applied a recently developed strong modification of existing technology to test whether oxygen consumption of the individual murine cleavage stage embryo (as an expression of a healthy oxidative energy metabolism) could predict the competence of the embryo to develop to the expanded blastocyst. For the first time, a coherent dataset of individual oxygen consumption and developmental stage throughout the preimplantation development was generated
Materials and methods
The oxygen consumption measurement principle
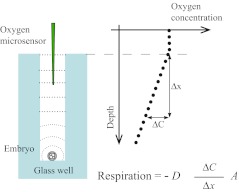
Individual embryo oxygen consumption was measured using an Embryo Respirometer prototype (www.unisense.com), capable of recording linear oxygen concentration gradients towards single embryos placed in a microwell. The basic measuring principle was substantially different from a previous method based on self referencing electrodes [18], as it overcomes the difficulty associated with spherical diffusion, is independent on exact knowledge of embryo sensor distance, and produce easily calculable absolute values of oxygen consumption. The method is validated and described in detail in [19]. Although based on exactly the same principle, the apparatus used for this experiment was technically different from the system used by [19], consisting of an automated unit capable of repeated oxygen consumption measurements by means of an oxygen microsensor and a specially devised culture dish. In brief, the embryo culture dish consisted of a series of small gas impermeable glass wells (depth 3 mm, diameter 0.7 mm). Each well was filled with culture medium (ISM1, Medicult A/S, Denmark) and the embryo was placed on the bottom of the well (Fig. 1). Oxygen supply to the embryo was maintained through molecular diffusion from the overlaying culture medium down through the well. As oxygen was consumed by the embryo at the bottom of the well, a linear oxygen concentration gradient was established. The oxygen flux towards the embryo was then calculated by measuring the oxygen concentration gradient from the top towards the bottom well. This was accomplished using an oxygen microsensor guided by microcontrollers capable of software controlled movements within and between the individual wells. Under steady state conditions the flux equals the oxygen consumption rate of the embryo at the bottom of the well as illustrated in Fig. 1 (Adapted from www.unisense.com with permission). Although the calculation of oxygen consumption was based on an oxygen gradient, the absolute change in oxygen partial pressure from the top towards the embryo at the bottom of the well was less than 1%, hence practically unchanged compared to normal culture conditions under an atmosphere of 5% CO2 in air. Each glass dish was designed with 36 wells, with four subunits of 3 * 3 wells with a common overlaying reservoir for oil. The respirometer was placed inside a Galaxy incubator.
Fig. 1.
Drawing of respirometer measuring principle
The reliability of the method was tested by continuous measurements in wells with culture media but without embryos. Average calculated oxygen consumption (95% CI) in wells filled with culture medium but without embryos was 0.01 (0.00–0.02) nl/h. Oxygen consumption estimates of individual embryos were obtained by subtracting actual blanks from actual measurements in each measurement session.
Morphology scoring
Individual embryo developmental stage and morphology was registered manually, and supported by advanced digital image recording (Fertimorph by IH-Medical).
Biological material
Embryo production in vivo
Three week old virgin mice (C57/Black) were superovulated by intraperitonal injection of 5 IU folligon (100 μl) pregnant mare serum gonadotropin (Intervet International B.V), followed by 5 IU (100 μl) Suigonan (serumgonadotropin (PMSG) and choriongonadotropin (HCG), Intervet International B.V) 48 h later. The mice were transferred to F1 C57/Black males, left overnight, and inspected for vaginal plug the following morning as indication of mating. The mice were killed by cervical dislocation and the oviduct, plus 2–3 mm of the uterine arm, was immediately removed and placed in pre-warmed (37°C) M2 flushing medium (Sigma-Aldridge). Embryos were retrieved by flushing the oviduct with M2 medium using a thin cannula, and washed twice in M2 medium before placed in an appropriate culture medium and incubated at 37°C under a 5% CO2 atmosphere (Galaxy incubator). The morning following mating was designated day 1 for the embryos. Fertilisation occurred from 24 h to about 3 h before day 1. Therefore, synchronized development of the embryos from batch to batch was not expected.
Embryo selection and loading procedure
Embryos (2PN if detectable) were selected for oxygen consumption measurement and loaded individually into the glass dishes, one in each well, using a denudation pipette (Swemed). Loading was done under a stereo microscope at 10–15 times magnification, followed by inspection from below using an inverted microscope, to ensure that the embryo actually reached the bottom of the well. Embryos in the glass dishes were cultured in a separate incubator, and only placed in the respirometer incubator during measurements, approximately 90 min daily. Continuous culture of embryos inside the respirometer was associated with >90% developmental arrest between 1 cell and expanded blastocyst and was therefore avoided.
Experimental design and data analysis
Oxygen consumption measurements and developmental stage identification was performed daily from day 2 until day 5. The first reliable steady state measurement of the oxygen consumption was performed on the morning of day 2 where the embryos were at the 2, 3 or 4 cell developmental stages. An embryo was considered arrested if there was no progress in developmental stage or increase in cell number, from one day to the next. In case of doubt, digital recorded images were used for evaluation of developmental progress. Oxygen consumption rates of embryos with detected arrest were not used in the data analysis.
Mean individual oxygen consumption according to developmental stage was determined. Individual cleavage stage oxygen consumption was determined and compared among embryos with subsequent arrested development, and embryos developing to expanded blastocysts. Individual cleavage stage embryos were grouped according to their oxygen consumption. OR for development to expanded blastocyst, among the groups, were determined by logistic regression. The effect of different cut points between low and high consumption groups was tested using ROC curve analysis for a range of cut points. Hence, the cut point resulting in the highest difference in Odds among low and high consumers, for development to expanded blastocysts, was used. Statistical calculations were performed using the statistical programme package Stata for windows (www.stata.com).
Results
A total 248 embryos were investigated. Of these, 200 embryos had their oxygen consumption recorded from day 2, and 48 embryos from day 3. Individual oxygen consumption rates are shown in Table 1. Oxygen consumption was low and relatively constant at the cleavage stage, and then rose slightly at the morale stage, followed by a significant increase at the expanded blastocyst stage. However once an embryo stopped developing (no increase in cell number, or no sign of blastulation for morulas within 24 h) it was excluded from the analysis thereafter. So values reported in Table 1, were based on rates from embryos, with ongoing development at the time of oxygen consumption measurement.
Table 1.
Mean individual oxygen consumption rates ± standard error of the estimate
| Developmental stage | Mean oxygen consumption (nl O2 h−1 embryo−1 ± SE) All embryos | Mean oxygen consumption (nl O2 h−1 embryo−1 ± SE) embryos reaching exp blast | Mean oxygen consumption (nl O2 h−1 embryo−1 ± SE) embryos arrested before exp blast |
|---|---|---|---|
| 2 cell | 0.162 ± 0.0043 (n = 93) | 0.162 ± 0.0073 (n = 20) | 0.162 ± 0.0052 (n = 73) |
| 4 cell | 0.166 ± 0.0038 (n = 128) | 0.172 ± 0.0053 (n = 55) | 0.161 ± 0.0053 (n = 73) |
| 7–8 cell | 0.197 ± 0.0089 (n = 33) | 0.218 ± 0.0115 (n = 15) | 0.179 ± 0.0120 (n = 18) |
| Morula | 0.234 ± 0.0056 (n = 142) | 0.261 ± 0.0070 (n = 71) | 0.207 ± 0.0074 (n = 71) |
| Expanded blastocyst | 0.464 ± 0.0185 (n = 69) | – | – |
N number of embryos with a recorded oxygen consumption.
Among embryos subsequently reaching the expanded blastocyst stage, there was a significant increase in oxygen consumption from the 4 cell stage (0.17 nl O2 h−1) to the 7–8 cell stage (0.22 nl O2 h−1) (p < 0.05) and to the morula stage (0.26 nl O2 h−1 embryo−1) (p < 0.05). A total increase of 53% from the 4 cell to the morula stage (p < 0.05) was observed. Embryos with arrested development had a smaller (30%) increase in oxygen consumption from 4 cell (0.16 nl O2 h−1) to the morula (0.21 nl O2 h−1 embryo−1) yet still significant. However, among embryos arresting before reaching the expanded blastocyst stage the increase in mean oxygen consumption from 4 cell to 7–8 cell, and then to morula was not significant for each step (p > 0.05). Cleavage stage mean oxygen consumption at each developmental stage (2, 4 and 7–8 cell) was not significantly different among embryos with subsequent arrest compared to embryos developing to expanded blastocysts.
Two and 4 cell embryos
Embryos at the 2 and 4 cell stage respectively were grouped into a high oxygen consumption group (>0.145 nl O2 h−1 embryo−1) and a low oxygen consumption group (>0.145 nl O2 h−1 embryo−1). The calculated OR for subsequent blastocyst development and its confidence interval (95% CI) was quite sensitive to which cut off point for high and low consumers that was used. Sensitivity analysis (data not shown) showed that using a cut point of 0.145 nl O2 h−1 embryo−1 for high and low consumption resulted in the most significant OR for the 2 and 4 cell group. For 2 cell embryos Odds Ratio for development into expanded blastocyst was 1.925 (CI 0.67–5.57, p = 0.227) in the high consumption group (n = 54) relative to the low consumption group (n = 39).
Odds Ratio for development into expanded blastocyst was for the 4 cell embryos in the high consumption (n = 85) group was 2.25 relative to the low consumption group (n = 43), with a 95% confidence interval 1.04–4.90, hence just significant (p = 0.040) Table 2.
Table 2.
Grouping of embryos according to their oxygen consumption, and Odds Ratios (with 95% confidence intervals) for development to expanded blastocysts among various consumption groups
| Developmental stage | Low consumption group nl O2 h−1 embryo−1 | High consumption group nl O2 h−1 embryo−1, (n) | OR (95% CI) High consumers for development to expanded blastocyst |
|---|---|---|---|
| 2 cell | <0.145 (n = 39) | >0.145 (n = 54) | 1.93 (0.67–5.57) |
| 4 cell | <0.145 (n = 43) | >0.145 (n = 85) | 2.25 (1.04–4.90) |
| 7–8 cell | <0.190 (n = 14) | >0.190 (n = 19) | 4.07 (0.85–19.4) |
| Morula | <0.230 (n = 72) | >0.230 (n = 70) | 3.18 (1.60–6.32) |
7–8 cells
The best OR for subsequent blastocyst development (according to sensitivity analysis) for a high consumption group (>0.19 nl O2 h−1 embryo−1) (n = 19) was 4.07 relative to the low consumption group (n = 14), (95% CI: 0.85–19.43), hence not significant. The number of embryos with 7–8 clearly individual cells was quite low as the embryos of the actual mouse strain, cultured under the given culture circumstances, often turned to a morula-like morphology, where the embryo appeared like a solid mass of indistinguishable cells, already immediately following the 5–6 cell stage.
Morula
Mean oxygen consumption for all morula was 0.23 nl O2 h−1 embryo−1. When divided into a low and high oxygen consumption group, best OR for development into expanded blastocyst in the high consumption group (>0.23 nl O2 h−1 embryo−1) (n = 70) was 3.18 relative to the low consumption group (<0.23 nl O2 h−1 embryo−1) (n = 72) (95% CI: 1.60–6.32, p = 0.001). Hence oxygen consumption above mean clearly indicated that the morula would continue development into expanded blastocyst.
Ordering embryos in three or more groups covering different consumption intervals, or using other cut off points for division into two groups, did not result in the identification of other oxygen consumption groups with a better prediction of development to expanded blastocyst (Data for less informative grouping of high and low consumers not shown). Additional analysis taking degree of fragmentation at each cleavage stage into account was conducted, but without significant change in results (data not shown).
The blastocyst rate during culture in the glass wells, with one daily oxygen consumption measurement, was 51% from 2 cell blastocyst. Blastocyst rate in control embryos in standard Nunc 4 well dishes was significantly higher 82%, although cultured in identical medium from the same batch.
Discussion
This study shows individual oxygen consumption rates from freshly in vivo produced murine embryos from day 2 following mating until the expanded blastocyst stage on day 5. Day 2 embryos were typically 2–4 cells, day 3 were 7–8 cells or morula, day 4 were morula or early blastocysts, and day 5 expanded or hatched blastocysts. Individual recording of developmental stage was performed daily; hence a coherent dataset of individual oxygen consumption and developmental stage throughout the preimplantation development, not previously reported, was generated. In general, oxygen consumption remained low until the blastocyst stage where a significant (p < 0.001) more than twofold increase was observed. This overall pattern is in accordance with several previous studies of preimplantation embryo oxygen consumption [12, 14, 18–20]. Trimarchi et al. [18] reported 0.1 nl O2 h−1 for cleavage stage murine embryos and 0.3 nl O2 h−1 for blastocysts using a self referencing oxygen electrode. Murine oxygen consumption rates reported in this study were remarkably similar to values reported by Houghton et al. [12] using closed respirometry, except for the morula stage where the present study indicate somewhat higher oxygen consumption rates (0.23 nl O2 h−1 embryo−1) compared to approximately 0.15 nl O2 h−1 embryo−1 as reported by Houghton et al. [12]. An early study by Mills and Brinster [20], using a Cartesian diver technique, actually observed an increase in oxygen consumption from the 2–8 cell stage to the morula stage quite similar to the observations in the present study. Differences in murine oxygen consumption rates reported, may relate to the different measuring techniques, different mouse strains as well as the use of varying culture media which may influence embryo metabolism [21].
Among embryos with a subsequent development to expanded blastocyst, the increase in oxygen consumption from 4 cell embryos (0.17 nl O2 h−1) to 7–8 cell embryos (0.22 nl O2 h−1), and again from 7–8 cell to morula (0.26 nl O2 h−1) was significant (p < 0.05). The significant increase may be associated with a successful rise in energy production required for the compactation and subsequent blastulation process as discussed by Trimarchi et al. [18] and Houghton et al. [12]. The observed increase in oxygen consumption among embryos which did not develop into expanded blastocysts was not significant from the 4 cell to the 7–8 cell stage or from the 7–8 cell to morula.
Investigation of association between oxygen consumption and developmental competence was done by grouping embryos in different consumption groups and calculating odds ratios for development to expanded blastocysts among the different consumption groups at a given developmental stage. The grouping of embryos reported in the present study yielded the best possible immediate prediction of development to expanded blastocyst, based on individual oxygen consumption. Taking degree of fragmentation at the 2, 4 and 7–8 cell stage into account did not add information about subsequent developmental competence. This was not surprising as the in vivo produced mouse embryos used in the present study were virtually unfragmented at the cleavage stage.
Several combinations of grouping embryos according to oxygen consumption at a given developmental stage were tested (data not shown), and it was empirically found that a simple division into a high and low consumption group yielded the best prediction of preimplantation developmental competence. The general trend was that embryos in the high consumption group had a higher probability of developing into expanded blastocysts compared to embryos grouped as low consumers, although not significantly so at the 2 cell stage and the 7–8 cell stage, and just significant at the 4 cell stage. Hence, oxygen consumption at the cleavage stage was a relatively poor predictor of preimplantation developmental competence. In contrast, oxygen consumption at the morula stage was a fair predictor with OR among high consumers being 3.18 relative to the low consumers, and highly significant (p < 0.001).
We acknowledge, in accordance with the manufacturer (www.unisense.com), that the current technology used in the present study was not truly non-disturbing, and resulted in reduced blastocyst rates compared to controls. However, it is hard to believe that embryos which would have arrested under optimal conditions should not also have arrested during the culture conditions in the present study, and still it was not possible clearly at the 2 or 4 cell stages to identify a specific group of embryos with subsequent preimplantation arrest, which it should have been if a specific oxygen consumption pattern predicts developmental arrest, or ongoing development. We therefore suggest that although the results possibly could have been clearer with optimal culture conditions, our conclusions remain valid.
Our main purpose of the study was to investigate if oxygen consumption among morphologically like embryos, at the early preimplantation stage (2 and 4 cells) could predict subsequent development into expanded blastocysts. As a selection tool in the clinical setting, we suggest that oxygen consumption measurements would be particularly useful to select among morphologically similar high quality embryos, equally well suited for transfer at a first glance. The murine embryos used in the present study were morphologically quite similar at each developmental stage, thus a suitable model reflecting a clinical situation, even if the general morphological variance among in vitro fertilised human embryos is higher than among in vivo produced murine embryos. High oxygen consumption relative to developmental stage indicated subsequent development to expanded blastocyst, although the results do not quite indicate that oxygen consumption of murine cleavage stage embryos is actually a strong predictor of development to expanded blastocyst. The actual usefulness of oxygen consumption at the cleavage stage, as a predictor of developmental competence, remains to be investigated in further detail, preferably with truly non disturbing equipment currently under development. Compared to the 2–8 cell stage, where the embryos exhibit a quiet metabolism with no growth in dry mass or protein content [5], and before onset of embryonic genome transcription [6, 7], it may be more promising to apply oxygen consumption measurements as a viability indicator at the blastocyst level, although it conflicts with the general idea of a very early marker.
In the present study we saw a significant predictive value of oxygen consumption at the morula stage. The expanded blastocyst is normally the latest developmental stage to be evaluated in vitro before transfer to the uterus. A key question there is whether a single measurement of oxygen consumption among morphologically like blastocysts could predict subsequent implantation and pregnancy. A truly non disturbing version of the technology used in the present study is currently under development (www.unisense.com). In general we suggest implantation and pregnancy as a better and clinically more relevant end point than blastocyst rate, and we recommend this to be applied in future studies. An important future study would therefore, with an improved method, be to investigate association between implantation potential of blastocysts and their oxygen consumption.
Acknowledgements
This work was supported by the Danish Medical Research Council, The Beckett Foundation, The Foundation of 17/12–1981, The Toyota Foundation, the A.P. Møller Foundation for the advancement of Medical Science, Karen Elise Jensens Foundation, Clinical Institute Aarhus University and Organon Denmark. Thanks to Morten Raaschou Image House Medical A/S for providing the Fertimorph equipment used in this study. Thanks to Puk Sandager for statistical advice.
Ethics
The use of experimental animals is approved and controlled by the Faculty Veterinary and follows the regulations of the National Committee of Experimental Animals.
References
- 1.Andersen AN, Gianaroli L, Felberbaum R, et al. Assisted reproductive technology in Europe, 2002. Results generated from European registers by ESHRE. Hum Reprod. 2006;21:1680–1697. doi: 10.1093/humrep/del075. [DOI] [PubMed] [Google Scholar]
- 2.Ziebe S, Petersen K, Lindenberg S, et al. Embryo morphology or cleavage stage: how to select the best embryos for transfer after in-vitro fertilization. Hum Reprod. 1997;12:1545–1549. doi: 10.1093/humrep/12.7.1545. [DOI] [PubMed] [Google Scholar]
- 3.Gardner DK, Lane M. Culture and selection of viable blastocysts: a feasible proposition for human IVF? Hum Reprod Updat. 1997;3:367–382. doi: 10.1093/humupd/3.4.367. [DOI] [PubMed] [Google Scholar]
- 4.Blake D, Proctor M, Johnson N, Olive D. Cleavage stage versus blastocyst stage embryo transfer in assisted conception 2005 Cochrane Database of Systematic Reviews, Issue 4. Art. No.: CD002118. doi:10.1002/14651858.CD002118.pub2. [DOI] [PubMed]
- 5.Leese HJ. What does an embryo need? Human Fertil. 2003;6:180–185. doi: 10.1080/1464770312331369463. [DOI] [PubMed] [Google Scholar]
- 6.Janny L, Menezo YJ. Evidence for a strong effect on human preimplantation embryo development and blastocystformation. Mol Reprod Dev. 1994;38(1):36–42. doi: 10.1002/mrd.1080380107. [DOI] [PubMed] [Google Scholar]
- 7.Miller JE, Smith TT. The effect of intracytoplasmic sperm injection and semen parameters on blastocyst development in vitro. Hum Reprod. 2001;16:918–924. doi: 10.1093/humrep/16.5.918. [DOI] [PubMed] [Google Scholar]
- 8.Brison DR, Leese HJ. Energy metabolism in late preimplantation rat embryos. J Reprod Fertil. 1991;93(1):245–251. doi: 10.1530/jrf.0.0930245. [DOI] [PubMed] [Google Scholar]
- 9.Houghton FD, Hawkhead JA, Humpherson PG, et al. Non-invasive amino acid turnover predicts human embryo developmental capacity. Hum Reprod. 2002;17:999–1005. doi: 10.1093/humrep/17.4.999. [DOI] [PubMed] [Google Scholar]
- 10.Gardner DK, Leese HJ. Assessment of embryo viability prior to transfer by the noninvasive measurement of glucose uptake. J Exp Zool. 1987;242:103–105. doi: 10.1002/jez.1402420115. [DOI] [PubMed] [Google Scholar]
- 11.Overstrom EW. Manipulation of early embryonic development. Anim Reprod Sci. 1992;28:277–285. doi: 10.1016/0378-4320(92)90114-S. [DOI] [Google Scholar]
- 12.Houghton FD, Thompson JG, Kennedy CJ, et al. Oxygen consumption and energy metabolism of the early mouse embryo. Mol Reprod Dev. 1996;44:476–485. doi: 10.1002/(SICI)1098-2795(199608)44:4<476::AID-MRD7>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 13.Kane MT, Buckley NJ. The effects of inhibitors of energy metabolism on the growth of one-cell rabbit ova to blastocysts in vitro. J Reprod Fertil. 1977;49(2):261–266. doi: 10.1530/jrf.0.0490261. [DOI] [PubMed] [Google Scholar]
- 14.Thompson JG, Partridge RJ, Houghton FD, Cox CI, Leese HJ. Oxygen uptake and carbohydrate metabolism by in vitro derived bovine embryos. J Reprod Fertil. 1996;106(2):299–306. doi: 10.1530/jrf.0.1060299. [DOI] [PubMed] [Google Scholar]
- 15.Liu L, Trimarchi JR, Keefe DL. Thiol oxidation-induced embryonic cell death in mice is prevented by the antioxidant dithiothreitol. Biol Reprod. 1999;61:1162–1169. doi: 10.1095/biolreprod61.4.1162. [DOI] [PubMed] [Google Scholar]
- 16.Crompton M. The mitochondrial permeability transition pore and its role in cell death. Biochem J 1999;341:233–49. [PMC free article] [PubMed]
- 17.Gross A, McDonnell JM, Korsmeyer SJ. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 1999;13:1899–1911. doi: 10.1101/gad.13.15.1899. [DOI] [PubMed] [Google Scholar]
- 18.Trimarchi JR, Lui L, Porterfield DM, et al. Oxidative phosphorylation dependent and independent oxygen consumption by individual preimplantation mouse embryos. Biol Reprod. 2000;62:1866–1874. doi: 10.1095/biolreprod62.6.1866. [DOI] [PubMed] [Google Scholar]
- 19.Lopes AS, Larsen LH, Rasming N, et al. Respiration rate of individual bovine in vitro produced embryos measured with a novel, non invasive and highly sensitive microsensor system. Reproduction. 2005;130:669–679. doi: 10.1530/rep.1.00703. [DOI] [PubMed] [Google Scholar]
- 20.Mills RM, Brinster RL. Oxygen consumption of preimplantation mouse embryos. Exp Cell Res. 1967;47:337–344. doi: 10.1016/0014-4827(67)90236-4. [DOI] [Google Scholar]
- 21.Barnett DK, Bavister BD. What is the relationship between the metabolism of preimplantation embryos and their developmental competence? Mol Reprod Dev. 1996;43(1):105–133. doi: 10.1002/(SICI)1098-2795(199601)43:1<105::AID-MRD13>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]