Abstract
Purpose
In this work, we describe a system for the morphological scoring of human oocytes prior to fertilisation and use this system to test whether oocyte morphology is an indicator of fertilisation, embryo development and implantation potential.
Methods
The study is a prospective trial of the use of oocyte morphological scores in 822 patients undergoing their first cycle of ICSI. Analyses of oocytes were performed prior to ICSI procedures and the scores compared with fertilisation rates, embryo quality and clinical results.
Results
‘Top quality’ oocytes had a significantly higher level of fertilisation (96%) as compared to low scoring oocytes (25.6%). Where top quality oocytes formed top quality embryos, we noted a clinical success rate of 63.4%.
Conclusions
Clinical success rates were increased in cases where top quality oocytes formed top quality embryos after ICSI. The analysis of oocyte morphology may represent a positive selection feature during ICSI.
Keywords: Human oocyte, Morphological analysis, Intracytoplasmic sperm injection, Assisted reproduction, Pregnancy
Introduction
The aim of human in vitro fertilisation (IVF) protocols is to enable patients to achieve an acceptable singleton pregnancy rate with the transfer of a minimal number of embryos into the uterus [1–4]. This is usually achieved either by the extended culture of human embryos to the blastocyst stage [5, 6] or by the morphological analysis and selection of the highest quality embryos for transfer [7–22]. More recently, the assessment of zygote quality has been applied [23–32]. Although these techniques have greatly assisted in achieving an acceptable pregnancy rate with the minimum number of embryos transferred into the uterus, one aspect of these protocols is that non-transferred embryos are discarded or cryopreserved. In recent years, countries such as Italy, Germany and Switzerland have effectively banned this practice on ethical grounds [33–35]. In Italy, it is now forbidden to produce more than three embryos in a single IVF cycle, effectively limiting to three the number of oocytes inseminated. Therefore, the assessment of zygote and embryo quality scores alone can no longer be used as a selection criterion in these countries. A standardised method for the morphological assessment of metaphase-II stage human oocytes prior to ICSI could prove to be an extremely useful tool in selecting three oocytes for insemination, not only for countries in which embryo selection is prohibited, but as a general addition to the selection criteria during human in vitro fertilisation protocols.
Human metaphase II oocytes have distinct morphological characteristics that may be indicative of quality and therefore used to predict implantation potential [21, 36–43]. For example, regular patterns of granularity have been observed in the oocyte cytoplasm and it has been suggested that these patterns correlate with oocyte quality [44, 45]. Human oocytes may contain inclusions or vacuoles which may be detrimental to the implantation potential of this material [37, 38, 41–43]. The oocyte plasma membrane response to penetration with an ICSI pipette has also been suggested to be a useful determinant of oocyte quality [46]. Many other parameters have also been analysed in oocytes such as zona pellucida thickness, spindle birefringence and extracellular dysmorphisms [36, 47, 48]. Results vary as to whether distinct oocyte dysmorphisms have any relation to fertilisation and development rates, probably due to a discordance in the scoring of these dysmorphisms [50], although an effect on pregnancies is often noted [21, 36, 47, 50].
We previously demonstrated that a simple, combined zygote and embryo quality assessment could be used to select material for transfer during cycles of in vitro fertilisation [51]. In this report, we have used three simple parameters of oocyte morphology that can be determined by light microscopy in the absence of any additional equipment prior to ICSI to test whether the analysis of oocyte morphology is applicable to the selection of oocytes for insemination. We tested whether the use of the specific oocyte morphologies tested as a selection criterion could increase the accuracy of the predictability of previously assessed scores and therefore be applied to the selection of oocytes for microinjection.
Materials and methods
Patients
Patients were attending IVF clinics in Italy for in vitro fertilisation protocols. All patients included in the present study were undergoing their first cycle of ICSI. Patients were prepared using standard controlled ovarian hyperstimulation protocols including downregulation of the pituitary gland with a GnRH agonist (Decapeptyl, Ipsen, Italy) followed by ovarian stimulation with exogenous FSH (Gonal-F, Serono, Italy). A single member of the medical staff co-ordinated all stimulation protocols, ensuring standardisation. Oocyte retrieval was performed 36 h after the administration of 10,000 UI hCG when two to three follicles of 18–20 mm diameter were observed by ultrasound examination, and blood 17β-oestradiol levels reached 150–200 pg/ml/follicle over 18 mm. All oocytes in the present project were treated with ICSI 3 h after oocyte retrieval (60 min after removal of the cumulus complex). A single team of biologists coordinated all biological work, ensuring that both culture protocols and embryo assessment were standardised. Oocytes were processed for ICSI using commercial IVF medium (Medicult, Copenhagen, Denmark) pre-equilibrated to 37°C and 5% CO2. According to Italian Law [35], no more than three embryos can be produced during a single IVF procedure. Therefore, oocyte quality was assessed immediately prior to the ICSI procedure, and where possible, three oocytes of equivalent grade selected for insemination and cultured individually to enable further assessment. Zygote quality was scored 16–17 h after ICSI. Embryo quality on day 2 was assessed 40–41 h after insemination. Embryos were transferred in all cases on the second day after oocyte retrieval. The establishment of a pregnancy was considered as a positive β-hCG test of over 60 IU/l 14 days after embryo transfer. The implantation rate was calculated by the observation of foetal heartbeats after ultrasound analysis, 8 weeks after the establishment of pregnancy.
Study design
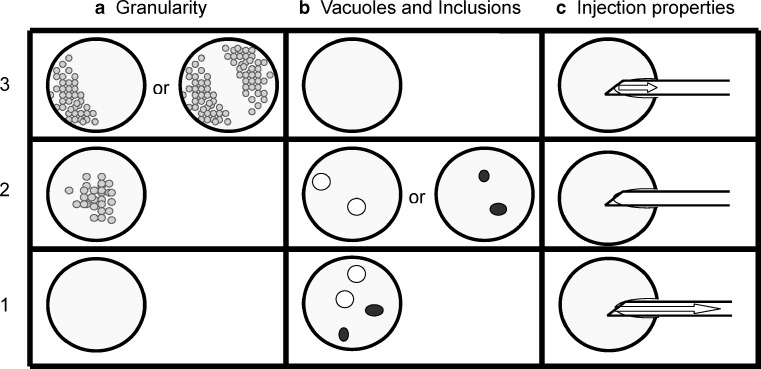
The study is a prospective trial to test the effect of specific morphological assessment of oocytes from 1,364 patients treated in Italy between January 2004 and December 2005. Patients were unselected for age, BMI and treatment protocol. Patients diagnosed for endometriosis and polycystic ovarian syndrome were excluded from the study due to the possible influence of these factors on oogenesis or endometrial receptivity. Furthermore, semen samples were always fresh ejaculates in order to exclude the influence of non-ejaculated or cryopreserved semen on the clinical results. In the 822 patients selected for oocyte morphological assessment, all oocytes of a cohort were examined and, where possible, three oocytes of equivalent grade selected for fertilisation. The oocyte scoring system was developed based on data from published literature [36–44, 46, 49]. The choice of parameters was based on three parameters easily recognised and scored without the need for additional equipment within the laboratory. Briefly, three parameters were considered. Oocyte granularity refers to the presence of heterogeneous areas of cytoplasm. These are mostly observed either to one side of the cytoplasm or in the centre. Oocyte granularity is correlated with the localisation of mitochondria and may represent domains of high ATP request that are necessary for the normal development of embryos [52, 53]. However, granularity focused in the centre of the oocyte is considered a dysmorphism [50]. For the purpose of the present analysis, we considered granular cytoplasm to one side of the oocyte to indicate top quality and therefore awarded such oocytes three points. Granularity in the centre of the oocyte was awarded two points and oocytes lacking granularity in the cytoplasm one point (Fig. 1a). The presence of vacuoles or inclusions within the cytoplasm was also considered because the presence of these dysmorphisms is considered an abnormality [42–44, 49]. Basically, oocytes lacking both vacuoles and inclusions were awarded three points, oocytes with either small amounts of vacuoles or inclusions two points, and oocytes containing both vacuoles and inclusions one point (Fig. 1b). For the consideration of injection parameters, we awarded three points to oocytes in which the ICSI pipette encountered some resistance and which the removal of the pipette left a visible ‘funnel’ for at least 30 s; oocytes in which a considerable amount of resistance was encountered before plasma membrane rupture were awarded two points and oocytes in which no resistance to the ICSI pipette was encountered were awarded one point ([46], Fig. 1c).
Fig. 1.
Morphological features selected for analysis. The figure is a representative figure of morphological parameters selected for analysis. a granularity describes the observation of granules in the cytoplasm. When these are polarised to one, or both sides of the oocytes, a score of 3 points is given. Granularity localised to the centre of the oocyte is scored as 2 points, and oocytes with a complete absence of granularity awarded 1 point. b The presence of vacuoles and inclusions (consisting of any deformity of the oocyte cytoplasm) was scored, 3 points awarded to oocytes not containing any features, 2 points if the oocyte cytoplasm contained either vacuoles or inclusions, and 1 point in cases where the oocyte contains both features. c Injection properties of the oocyte were scored during the ICSI procedure. In cases where a small amount of pressure was required to rupture the oocyte plasma membrane (the membrane did not stretch further than the natural limit of the plasma membrane - see arrow) 3 points were awarded. In cases where the membrane ruptured without aspiration pressure, 2 points were awarded and where an excessive pressure was required for the rupture of the plasma membrane (see arrow), 1 point was awarded.
Statistics
All data were plotted as mean ± standard deviation unless stated. All plots and statistical analysis was calculated using the Sigma Plot and Sigma Stat software packages (SPSS, Erkrath, Germany). Regression lines were calculated by the method of least squares and the significance of the regression lines was tested with the Pearson product-moment test. The z test with Yates correction was used to test the significance of proportions where necessary.
Results
Correlation between oocyte scores and fertilisation of oocytes after ICSI
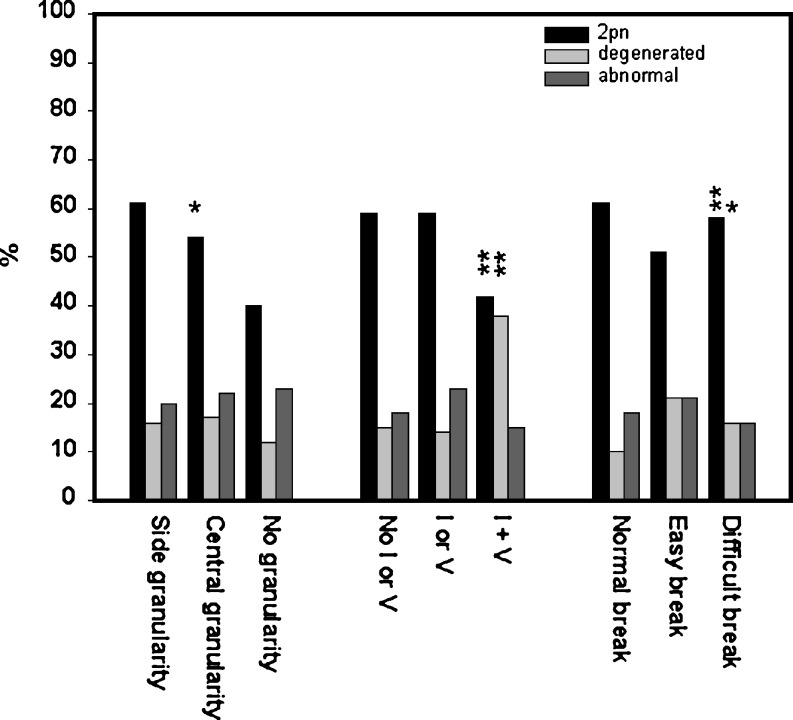
In total, data of oocyte morphology was obtained from 6,397 metaphase-II oocytes retrieved from 822 patients attending for ICSI cycles between 2004 and 2005 (Table 1). The Italian Law of 2004 [35] restricts to three the number of embryos to be formed during an IVF procedure. Therefore, we noted morphology scores to in the three oocytes selected for ICSI. Two thousand and seventeen of the 2,460 oocytes in which oocyte selection criteria were applied fertilised (82%, Table 1). We examined the relationship between the separate oocyte scores and fertilisation and development characteristics of embryos obtained during these procedures. Initially, the scores were considered independently to evaluate the effects of the distinct morphological features on fertilisation. The first parameter—oocyte granularity—was divided into three groups: granularity observed localised to one side of the oocyte (three points), granularity observed in the centre of the oocyte (two points) and oocytes with homogenous cytoplasm lacking granular features (one point). Of the 6,397 oocytes examined, 2,460 oocytes were selected for ICSI after the evaluation of morphology (Table 1). Of these, 1,845 oocytes had score 3 cytoplasm, 492 oocytes had a cytoplasmic score of two points and 123 oocytes had score 1 cytoplasm. We noted distinct fertilisation responses correlated with the morphology of the oocyte cytoplasm. Of the 1,845 oocytes scoring three points for cytoplasmic granularity, 1,580 fertilised normally (85.6%, Fig. 1). The percentage of oocytes that normally fertilised diminished as the morphological score decreased. Oocytes scoring two points were characterised by a fertilisation rate of 74.4% (366/492 oocytes, Fig. 1). Of oocytes with no cytoplasmic granular features (one point score), 71/123 fertilised normally (57.7%, Fig. 1), significantly less than that of top-scoring oocytes (p < 0.001, z test). Levels of abnormally fertilised oocytes and degenerated oocytes did not show any correlation between the three groups.
Table 1.
Patient characteristics
| Parameters | Values |
|---|---|
| Patients | 822 |
| Mean age (years) | 34.2 ± 3.4 |
| Number oocytes (mean ± sd) | 6397 (8.6 ± 4.1) |
| Number oocytes selected for ICSI (mean ± SD) | 2460 (2.9 ± 0.2a) |
| Number fertilised (% fertilisation) | 2017 (82%) |
| Number transferred (mean ± SD) | 2017 (2.4 ± 0.2a) |
| Number pregnancies (pregnancy rate %) | 345 (41.9%) |
| Number gestational sacs (implantation rate %) | 453 (20.0%) |
| Number live births | 442 |
| Number patients with triplets | 28 |
| Number patients with twins | 41 |
| Number singleton births | 276 |
Data are actual values with percentages in parentheses where required. Data shown are mean ± standard deviation.
The presence of vacuoles or cytoplasmic inclusions in the oocyte cytoplasm was also considered in the 2,460 oocytes selected for ICSI. Of 1730 oocytes with no cytoplasmic defects (score three points), 1,490 fertilised normally (86.1%, Fig. 1). This result was not significantly different in oocytes in which a single defect was present (two point score, 442/528 oocytes fertilising normally, 83.6%, Fig. 1). Where two defects were present in the oocyte cytoplasm (one point), a strong reduction in the level of fertilisation was noted (85/202 oocytes, 42.0%, Fig. 1) together with a strong increase in the level of oocyte degeneration after ICSI (76/202 oocytes degenerated, 37.6%, Fig. 1), suggesting that these oocytes are severely compromised.
The third morphological score examined the response of the oocyte plasma membrane to the ICSI pipette. Here, three points were awarded to ‘normal break’ oocytes (oocytes in which some suction is required to rupture the plasma membrane), two points to ‘easy break’ oocytes (oocytes in which the plasma membrane breaks immediately upon the pressure of the ICSI pipette) and one point to ‘difficult break’ oocyte patterns (oocytes in which excessive suction is required to rupture the oocyte plasma membrane). Again, a correlation was noted between injection pattern and the fertilisation potential of individual oocytes. Of the 2,460 oocytes selected for ICSI, ‘Normal break’ oocytes (score 3) were characterised by a fertilisation rate of 86.0% (1,547/1,799 oocytes, Fig. 1). ‘Easy break’ (score 2) oocytes had a significantly lower level of fertilisation. In the present data, 194/325 ‘easy break’ oocytes fertilised (72.9%, Fig. 1). This was coupled with a level of oocyte degeneration and abnormal fertilisation after ICSI significantly higher than that of normal break oocytes (89/325 oocytes, 21%, Fig. 1). Interestingly, neither the level of fertilisation nor levels of degeneration of ‘difficult break’ oocytes were significantly different to the levels observed in ‘normal break’ oocytes. Here, 276/336 oocytes fertilised normally (82.1%, Fig. 1).
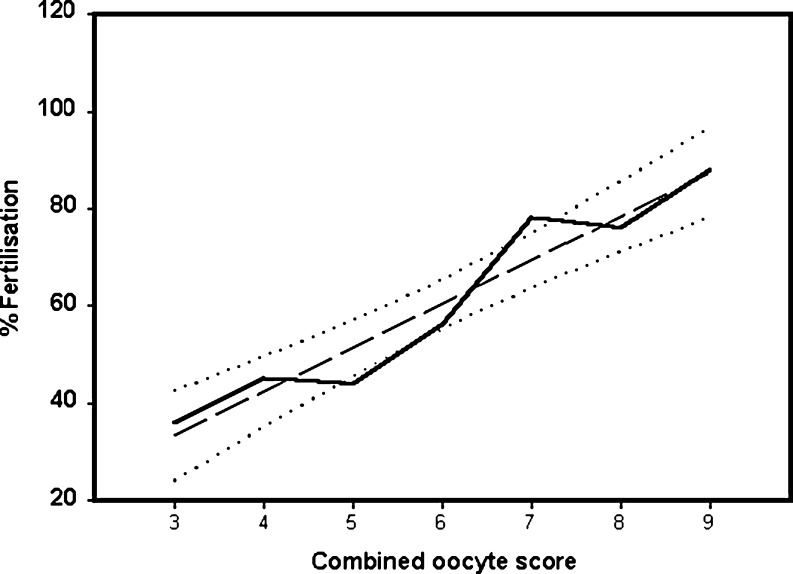
We examined whether combinations of scores could more precisely determine the fertilisation rate (see Table 2). Fertilisation rates were affected by combinations of scores (Table 2). We noted that oocytes in which the presence of top scores for both morphology and injection parameters were noted were characterised by a higher fertilisation rate than these scores alone (Table 2). Furthermore, the presence of vacuoles and vesicles in the oocyte cytoplasm influenced the fertilisation characteristics of oocytes negatively, even in the presence of top scores for other morphological characteristics (Table 2). When the three parameters were assessed together, oocytes considered ‘top quality’ according to the present scoring scheme (i.e. with nine points) achieved a fertilisation rate of 96% (1,620/1,688 oocytes fertilised, Fig. 2). Lowest quality scoring oocytes were characterised by a fertilisation rate of 25.6% (11/43 oocytes, Fig. 2). The percentage of fertilised oocytes was highly correlated with the oocyte score (r = 0.99, p < 0.001, Fig. 2). These data suggest that the top scoring oocytes are correlated with the highest fertilisation rate.
Table 2.
Correlation between oocyte morphology scores and fertilisation rates
| Granularity | Vesicles/vacuoles | Injection properties | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall fertilisation rate | 3 | 2 | 1 | 3 | 2 | 1 | 3 | 2 | 1 | ||
| 85 | 74 | 57 | 86 | 84 | 42 | 86 | 59 | 82 | |||
| Granularity | 3 | 85 | 88 | 84 | 57* | 95** | 78** | 88 | |||
| 2 | 74 | 74 | 70** | 43* | 68** | 59** | 86 | ||||
| 1 | 57 | 58 | 47* | 42** | 57* | 42** | 58* | ||||
| Vesicles/vacuoles | 3 | 86 | 88 | 74 | 58* | 86 | 69** | 73** | |||
| 2 | 84 | 84 | 70** | 47* | 94** | 68** | 76 | ||||
| 1 | 42 | 57* | 43* | 42* | 45* | 44** | 42* | ||||
| Injection properties | 3 | 86 | 95** | 68** | 57* | 86 | 94** | 45* | |||
| 2 | 59 | 78** | 59** | 42** | 69** | 68** | 44** | ||||
| 1 | 82 | 88 | 86 | 58* | 73** | 76 | 42* | ||||
Figures in bold indicate the overall fertilisation rates scored for single morphological scores without the consideration of other scores. Italicised figures indicate fertilisation rates for combinations of scores. The z test was used to test for significant differences between proportions.
*p < 0.001
**p < 0.05
Fig. 2.
Biological results after morphological analysis of human oocytes during ICSI cycles. Results show percentages of normal and abnormal fertilisation rates and percentage of oocyte lysis after intracytoplasmic sperm injection in oocytes in which morphological analysis preceded the ICSI procedure. Black bars are percentages of normal fertilisation, light grey bars percentages of oocyte degeneration and dark grey bars percentages of abnormal fertilisation (i.e. one pronucleus). The subtitles are the single morphological parameters observed. I Inclusions, V vacuoles. Asterisk denotes data significantly different from first parameter (p < 0.05, two-tailed t test). Two asterisks represent data highly significantly different from first parameter (p < 0.001, two-tailed t test). The data is derived from a total of 2,017 oocytes in which fertilisation was verified after the analysis of morphology
Correlation between oocyte and embryo scores
In collaboration with other groups, we have previously applied a scoring system to human zygotes and embryos to test the relationship between this and clinical outcome after IVF [49]. The previous work suggested that a combination of zygote and embryo scores and growth rate of preimplantation embryos could be used to select embryos with high implantation potential for transfer. In the present work, we applied this scoring system to oocytes fertilised after the analysis of oocyte morphology to test whether oocyte scores could increase the accuracy of this system. We first tested whether that the previously noted correlations were present in the present data. The correlation coefficient between zygote and day 2 embryo scores in the present work was 0.25 (p < 0.001, n = 3323). This was not significantly different from the previous work, demonstrating that the scores are reproducible.
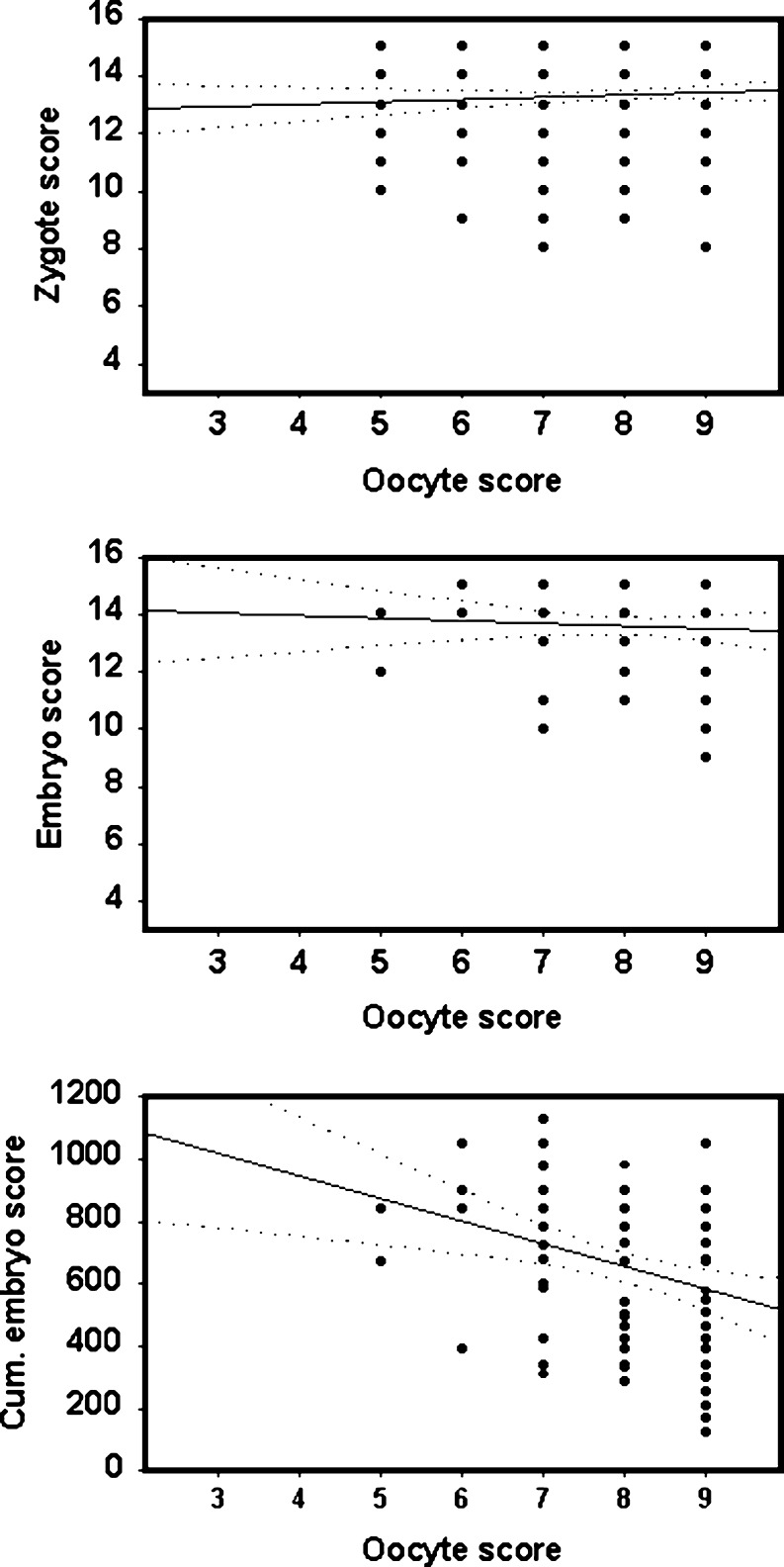
We examined whether the development of a top quality embryo according to the previous morphological assessment could be predicted from the morphological analysis of oocytes prior to ICSI. Zygote and embryo morphology scores were registered in all 2,017 oocytes fertilised after the evaluation of oocyte. Oocyte morphology scores were found to have some correlation with zygote quality assessments (r = 0.25, p < 0.001, n = 2,017, Fig. 3). The assessment of simple embryo morphology did not correlate with oocyte morphology. Of the 2,017 oocytes fertilised after morphological assessment, the correlation between oocyte and embryo morphology was absent (r = 0.07, p > 0.05, n = 2017, Fig. 3). However, if a cumulative score incorporating zygote and embryo morphology together with growth rate [51] was applied to embryo development, a correlation between oocyte morphology and embryo morphology was present (r = 0.16, p = 0.01, n = 2,017, Figs. 3 and 4).
Fig. 3.
Correlation between normal fertilisation rates and combinations of oocyte morphological scores. Oocyte morphological scores can vary between three and nine. Fertilisation rates at each level of morphological analysis are shown. Long dashed line is the correlation coefficient and dotted lines are the 95% confidence intervals. The data is derived from a total of 2,017 oocytes in which fertilisation was verified after the analysis of morphology
Fig. 4.
Correlations between oocyte morphological scores and morphological scores applied during the first two days of development. Top Correlation between oocyte and zygote morphology. Centre Correlation between oocyte morphology and embryo morphology. Embryo morphology was assessed on day 2 (40–41 h after fertilisation). Bottom Correlation between oocyte morphology and cumulative embryo score. Zygote, embryo and cumulative scores were assessed according to previous work [51]. Continuous line shows correlation coefficient and dotted lines are the 95% confidence intervals. The data is derived from a total of 2,017 oocytes in which fertilisation was verified after the analysis of morphology
Can oocyte score be used to increase the predictability of outcome after ICSI?
In total, out of the 822 transfers performed after the application of oocyte morphology, 345 patients achieved a clinical pregnancy (41.9%, Tables 1 and 3 lower right). Although the highest quality oocytes were used from a cohort of a particular patient, this did not necessarily mean that top quality (i.e. score 9) oocytes were always selected for insemination. Furthermore, top quality embryos did not always result from the fertilisation of top quality oocytes. We analysed whether the observation of top quality oocytes alone was indicative of clinical success. Of the 822 patients examined, all oocytes retrieved in 674 cases had highly similar morphological scores (i.e. differing by a single point). Of these cases, oocytes from 485 patients were classed as ‘top quality’ according to the morphological score (Table 3, upper right). We examined the results obtained in patients in which top quality embryos were derived from these oocytes. The 145 patients in which top quality embryos resulted from the fertilisation of top quality oocytes achieved a pregnancy rate of 63.4% (92 patients, Table 3, upper left). The implantation rate was similarly high in this group (115/406 embryos implanted, 28.3%, Table 3). These results were a significant improvement over the background pregnancy rate where all embryo qualities were assessed (248/485 pregnancies, 51.1%, p < 0.05 and implantation rate 19.6%, p < 0,001, Table 3, lower right). The results are also significantly higher than results obtained when all oocyte qualities were calculated, but top quality embryos resulted (Table 3, lower left).
Table 3.
Effect of top quality oocyte morphology on clinical results
| Embryo quality >600 | All embryos | |
|---|---|---|
| Top quality oocytes (score = 9) | 63.4%** (28.3%*) n = 145 | 51.1% (19.6%) n = 485 |
| All morphology-assessed oocytes | 52.6%* (22.1%) n = 322 | 41.9% (20.0%) n = 822 |
Data shows pregnancy rates with implantation rates in parentheses. The z test was used to test for significant differences between proportions.
*p < 0.001
**p < 0.05
Discussion
The present data suggests that individual morphological assessments are correlated with oocyte quality. The combination of results suggests that an oocyte with cytoplasmic granularity to one side of the oocyte, with neither vacuoles nor vesicles or other inclusions, and a plasma membrane that requires a small amount of pressure from the ICSI pipette before rupture, is of the highest quality. In fact, in the present data, 96% (1,620/1,688 oocytes) of such oocytes fertilised after ICSI.
Our previous reports suggested that zygote and embryo quality were correlated and that the use of the combined quality scores improved clinical outcome after ICSI [51]. In the present work, we first confirmed the relationship between zygote and embryo morphology, suggesting that the previously applied scoring system is reproducible. The present data suggests that oocyte morphology correlates well with both zygote morphology and the previously determined cumulative assessment of embryo quality on day 2 [51]. We did not examine the correlation between oocyte morphology and day 3 cumulative scores in the present work because we cannot select embryos and therefore it is of little value to culture these to day 3.
Our previous clinical results suggested that the cumulative zygote and embryo score on day 2 after insemination was correlated with clinical results [51]. In the present data, we tested whether the addition of oocyte morphology could increase the clinical success rate, and therefore predictability, of the previous analysis. We therefore examined whether the pregnancy rate in cases in which top quality embryos on day 2 were derived from top quality oocytes was improved over cases in which top quality embryos were derived from all oocytes. Global data from oocyte morphology assessment produced a pregnancy rate of 52.6% for top quality embryos on day 2 (Table 3, lower left). A significantly higher pregnancy rate of 63.4% was achieved when top quality embryos were derived from top quality oocytes (Table 3, upper left), presumably because of the selection of the best quality oocytes for ICSI in a cohort retrieved after aspiration. The pregnancy rates for all embryos transferred when derived from top quality oocytes (51.1%) was lower than the cases in which top quality embryos were formed, (Table 3, upper right) suggesting that oocyte quality is not determinant in the achievement of pregnancy without the development of top quality embryos. However, the fact that pregnancy rates in cases of top quality oocytes and embryos was higher than the global pregnancy rates (Table 3, lower right) suggests that the selection of both oocyte and embryo quality has a positive effect over the use of a single scoring system, although the implantation rates were not always significantly higher (Table 3). These data suggest that the morphological analysis of oocyte quality to select top quality oocytes can increase the pregnancy rate in patients undergoing ICSI procedures.
The use of oocyte morphology is therefore a useful addition to the tool of a laboratory of in vitro fertilisation. The application of oocyte morphology should have a positive effect not only in countries in which embryo selection is restricted or banned, but in all laboratories. The analysis of oocyte morphology should assist in the goal during ICSI procedures of the reduction of the number of embryos transferred in a cycle to a single top quality embryo.
Acknowledgements
We thank Vincenzo Monfrecola for his assistance in the completion of this work.
Footnotes
Capsule
A system for scoring oocyte morphology is presented. Data suggests that this system can be used to select oocytes for insemination during ICSI cycles.
References
- 1.Coetsier T, Dhont M. Avoiding multiple pregnancies in in-vitro fertilisation: who’s afraid of single embryo transfer? Hum Reprod. 1998;10:2663–2670. doi: 10.1093/humrep/13.10.2663. [DOI] [PubMed] [Google Scholar]
- 2.Pennings G. Multiple pregnancies: a test case for the moral quality of assisted reproduction. Hum Reprod. 2000;15:2466–2469. doi: 10.1093/humrep/15.12.2466. [DOI] [PubMed] [Google Scholar]
- 3.Gleicher N, Barad D. The relative myth of elective single embryo transfer. Hum Reprod. 2006;21:1337–1344. doi: 10.1093/humrep/del026. [DOI] [PubMed] [Google Scholar]
- 4.Wang J, Lane M, Norman RJ. Reducing multiple pregnancy from assisted reproduction treatment: educating patients and medical staff. Med J Aust. 2006;184:180–181. doi: 10.5694/j.1326-5377.2006.tb00181.x. [DOI] [PubMed] [Google Scholar]
- 5.Gardner DK, Vella P, Lane M, Wagley L, Schlenker T, Schoolcraft W. Culture of human blastocysts increases implantation rates and reduces the need for multiple embryo transfers. Fertil Steril. 1998;69:84–88. doi: 10.1016/S0015-0282(97)00438-X. [DOI] [PubMed] [Google Scholar]
- 6.Gardner DK, Schoolcraft W. Elimination of higher order multiple gestations by blastocyst culture and transfer. In: Shoham Z, Howles C, Jacobs H, editors. Elimination of higher order multiple gestations by blastocyst culture and transfer. London: Martin Dunitz; 1999. pp. 267–274. [Google Scholar]
- 7.Edwards RG, Fishel SB, Cohen J, Fehilly CB, Purdy JM, Slater JM, et al. Factors influencing the success of in vitro fertilisation for alleviating human infertility. J In Vitro Fertil Embryo Transf. 1984;1:3–23. doi: 10.1007/BF01129615. [DOI] [PubMed] [Google Scholar]
- 8.Cummins JM, Breen TM, Harrison KL, Shaw JM, Wilson LM, Hennessey JF. A formula for scoring human embryo growth rates in in vitro fertilisation: its value in predicting pregnancy and in comparison with visual estimates of embryo quality. J In Vitro Fertil Embryo Transf. 1986;3:284–295. doi: 10.1007/BF01133388. [DOI] [PubMed] [Google Scholar]
- 9.Puissant F, Rysselberge M, Barlow P, Dewesze J, Leroy F. Embryo scoring as a prognostic tool in IVF treatment. Hum Reprod. 1987;2:705–708. doi: 10.1093/oxfordjournals.humrep.a136618. [DOI] [PubMed] [Google Scholar]
- 10.Visser D, Fourie F. The applicability of cumulative embryo score selection and quality control in an in-vitro fertilisation/embryo transfer programme. Hum Reprod. 1993;8:1719–1722. doi: 10.1093/oxfordjournals.humrep.a137922. [DOI] [PubMed] [Google Scholar]
- 11.Giorgetti C, Terriou P, Auquier P, Hans E, Spach JL, Salzmann J, et al. Embryo score to predict implantation after in vitro fertilisation: based on 957 single embryo transfers. Hum Reprod. 1995;10:2427–2431. doi: 10.1093/oxfordjournals.humrep.a136312. [DOI] [PubMed] [Google Scholar]
- 12.Ziebe S, Petersen K, Lindenberg S, Andersen AG, Gabrielsen A, Andersen AN. Embryo morphology or cleavage stage: how to select the best embryos for transfer after in-vitro fertilisation. Hum Reprod. 1997;12:1545–1549. doi: 10.1093/humrep/12.7.1545. [DOI] [PubMed] [Google Scholar]
- 13.Rjinders P, Jansen C. The predictive value of day 3 embryo morphology regarding blastocysts formation, pregnancy and implantation rate after day 5 transfer following in-vitro fertilisation or intracytoplasmic sperm injection. Hum Reprod. 1998;13:2869–2873. doi: 10.1093/humrep/13.10.2869. [DOI] [PubMed] [Google Scholar]
- 14.Hu Y, Maxson WS, Hoffman DI, Ory SJ, Eager S, Dupre J, et al. Maximising pregnancy rates and limiting high-order multiple conceptions by determining the optimal number of embryos to transfer based on quality. Fertil Steril. 1998;69:650–657. doi: 10.1016/S0015-0282(98)00024-7. [DOI] [PubMed] [Google Scholar]
- 15.Alikani M, Calderon G, Tomkin G, Garrisi J, Kokot M, Cohen J. Cleavage anomalies in early human embryos and survival after prolonged culture in vitro. Hum Reprod. 2000;15:2634–2643. doi: 10.1093/humrep/15.12.2634. [DOI] [PubMed] [Google Scholar]
- 16.Alikani M, Cohen J, Tomkin G, Garrisi J, Mack C, Scott RT. Human embryo fragmentation in-vitro and its effects for human pregnancy and implantation. Fertil Steril. 1999;71:836–842. doi: 10.1016/S0015-0282(99)00092-8. [DOI] [PubMed] [Google Scholar]
- 17.Desai N, Goldstein J, Rowland D, Goldfarb J. Morphological evaluation of human embryos and derivation of an embryo quality scoring system specific for day 3 embryos: a preliminary study. Hum Reprod. 2000;15:2190–2196. doi: 10.1093/humrep/15.10.2190. [DOI] [PubMed] [Google Scholar]
- 18.Racowsky C, Jackson KV, Cekleniak NA, Fox JH, Hornstein MD, Ginsberg EJ. The number of eight-cell embryos is a key determinant for selecting day 3 or day 5 transfer. Fertil Steril. 2000;73:558–564. doi: 10.1016/S0015-0282(99)00565-8. [DOI] [PubMed] [Google Scholar]
- 19.Hardarson T, Hanson C, Sjogren A, Lundin K. Human embryos with unevenly sized blastomeres have lower pregnancy and implantation rates: indications for aneuploidy and multinucleation. Hum Reprod. 2001;16:313–318. doi: 10.1093/humrep/16.2.313. [DOI] [PubMed] [Google Scholar]
- 20.Langley MT, Marek DM, Gardner DK, Doody KM, Doody KJ. Extended embryo culture in human assisted reproduction treatments. Hum Reprod. 2001;16:902–908. doi: 10.1093/humrep/16.5.902. [DOI] [PubMed] [Google Scholar]
- 21.Hamamah S. Oocyte and embryo quality: is their morphology a good criterion? J Gynecol Obstet Biol Reprod (Paris) 2005;34:5S38–5S41. [PubMed] [Google Scholar]
- 22.Edwards RG, Steptoe PC, Purdy JM. Establishing full-term human pregnancies using cleaving embryos grown in vitro. Br J Obstet Gynaecol. 1980;87:737–756. doi: 10.1111/j.1471-0528.1980.tb04610.x. [DOI] [PubMed] [Google Scholar]
- 23.Sadowy S, Tomkin G, Munne S, Ferrara-Congedo T, Cohen J. Impaired development of zygotes with uneven pronuclear size. Zygote. 1998;6:137–142. doi: 10.1017/S0967199498000057. [DOI] [PubMed] [Google Scholar]
- 24.Tesarik J, Greco E. The probability of abnormal preimplantation development can be predicted by a single static observation on pronuclear stage morphology. Hum Reprod. 1999;14:1318–1323. doi: 10.1093/humrep/14.5.1318. [DOI] [PubMed] [Google Scholar]
- 25.Scott L, Alvero R, Leondires M, Miller B. The morphology of human pronuclear embryos is positively related to blastocysts development and implantation. Hum Reprod. 2000;15:2394–2403. doi: 10.1093/humrep/15.11.2394. [DOI] [PubMed] [Google Scholar]
- 26.Tesarik J, Junca AM, Hazout A, Aubriot FX, Nathan C, Cohen-Bacrie P, et al. Embryos with high implantation potential after intracytoplasmic sperm injection can be recognised by a simple, non-invasive examination of pronuclear morphology. Hum Reprod. 2000;15:1396–1399. doi: 10.1093/humrep/15.6.1396. [DOI] [PubMed] [Google Scholar]
- 27.Wittemer C, Bettahar-Lebugle K, Ohl J, Rongieres C, Nisand I, Gerlinger P. Zygote evaluation: an efficient tool for embryo selection. Hum Reprod. 2000;15:2591–2597. doi: 10.1093/humrep/15.12.2591. [DOI] [PubMed] [Google Scholar]
- 28.Ludwig M, Schopper B, Al-Hasani S, Diedrich K. Clinical use of a pronuclear stage score following intracytoplasmic sperm injection: impact on pregnancy rates under the conditions of the German embryo law. Hum Reprod. 2000;15:325–329. doi: 10.1093/humrep/15.2.325. [DOI] [PubMed] [Google Scholar]
- 29.Dale B, Fiorentino A, Simone ML, Matteo L, Scotto di Frega A, Wilding M, et al. Zygote versus embryo transfer: a prospective randomized multicenter trial. J Assist Reprod Genet. 2002;19:456–461. doi: 10.1023/A:1020354318164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Payne JF, Raburn DJ, Couchman GM, Price TM, Jamison MG, Walmer DK. Relationship between pre-embryo pronuclear morphology (zygote score) and standard day 2 or 3 embryo morphology with regard to assisted reproductive technique outcomes. Fertil Steril. 2005;84:900–909. doi: 10.1016/j.fertnstert.2005.04.047. [DOI] [PubMed] [Google Scholar]
- 31.Chen C, Kattera S. Comparison of pronuclear zygote morphology and early cleavage status of zygotes as additional criteria in the selection of day 3 embryos: a randomized study. Fertil Steril. 2006;85:347–352. doi: 10.1016/j.fertnstert.2005.07.1319. [DOI] [PubMed] [Google Scholar]
- 32.Senn A, Urner F, Chanson A, Primi MP, Wirthner D, Germond M. Morphological scoring of human pronuclear zygotes for prediction of pregnancy outcome. Hum Reprod. 2006;21:234–239. doi: 10.1093/humrep/dei282. [DOI] [PubMed] [Google Scholar]
- 33.Gesetz zum Schutz von Embryonen. In ‘Fassung der Bekanntmachung’ 13. Dezember 1990–BGBl. I S. 2747.
- 34.LPAM Legge federale del 18 Dicembre 1998 concernente la procreazione con assistenza medica (Legge sulla medicina della procreazione). RU 2000 3055.
- 35.Legge 40 “Norme in materia di procreazione medicalmente assistita”. Gazzetta Ufficiale Repubblica Italiana 2004, 45 del 24 Febbraio.
- 36.Sutter P, Dozortsev D, Qian C, Dhont M. Oocyte morphology does not correlate with fertilization rate and embryo quality after intracytoplasmic sperm injection. Hum Reprod. 1996;11:595–597. doi: 10.1093/humrep/11.3.595. [DOI] [PubMed] [Google Scholar]
- 37.Sathananthan AH. Ultrastructure of the human egg. Hum Cell. 1997;10:21–38. [PubMed] [Google Scholar]
- 38.Serhal PF, Ranieri DM, Kinis A, Marchant S, Davies M, Khadum IM. Oocyte morphology predicts outcome of intracytoplasmic sperm injection. Hum Reprod. 1997;12:1267–1270. doi: 10.1093/humrep/12.6.1267. [DOI] [PubMed] [Google Scholar]
- 39.Loutradis D, Drakakis P, Kallianidis K, Milingos S, Dendrinos S, Michalas S. Oocyte morphology correlates with embryo quality and pregnancy rate after intracytoplasmic sperm injection. Fertil Steril. 1999;72:240–244. doi: 10.1016/S0015-0282(99)00233-2. [DOI] [PubMed] [Google Scholar]
- 40.Suppinyopong S, Choavaratana R, Karavakul C. Correlation of oocyte morphology with fertilization rate and embryo quality after intracytoplasmic sperm injection. J Med Assoc Thail. 2000;83:627–632. [PubMed] [Google Scholar]
- 41.Plachot M, Selva J, Wolf JP, Bastit P, Mouzon J. Consequences of oocyte dysmorphy on the fertilization rate and embryo development after intracytoplasmic sperm injection. A prospective multicenter study. Gynecol Obstet Fertil. 2002;30:772–779. doi: 10.1016/S1297-9589(02)00437-X. [DOI] [PubMed] [Google Scholar]
- 42.Otsuki J, Okada A, Morimoto K, Nagai Y, Kubo H. The relationship between pregnancy outcome and smooth endoplasmic reticulum clusters in MII human oocytes. Hum Reprod. 2004;19:1591–1597. doi: 10.1093/humrep/deh258. [DOI] [PubMed] [Google Scholar]
- 43.Ebner T, Moser M, Tews G. Is oocyte morphology prognostic of embryo developmental potential after ICSI? Reprod Biomed Online. 2006;12:507–512. doi: 10.1016/S1472-6483(10)62006-8. [DOI] [PubMed] [Google Scholar]
- 44.Blerkom J, Henry G. Oocyte dysmorphism and aneuploidy in meiotically mature human oocytes after ovarian stimulation. Hum Reprod. 1992;7:379–390. doi: 10.1093/oxfordjournals.humrep.a137655. [DOI] [PubMed] [Google Scholar]
- 45.Stalf T, Herrero J, Mehnert C, Manolopoulos K, Lenhard A, Gips H. Influence of polarization effects in ooplasma and pronuclei on embryo quality and implantation in an IVF program. J Assist Reprod Genet. 2002;19:355–362. doi: 10.1023/A:1016300703430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Palermo GD, Alikani M, Bertoli M, Colombero LT, Moy F, Cohen J, et al. Oolemma characteristics in relation to survival and fertilization patterns of oocytes treated by intracytoplasmic sperm injection. Hum Reprod. 1996;11:172–176. doi: 10.1093/oxfordjournals.humrep.a019012. [DOI] [PubMed] [Google Scholar]
- 47.Alikani M, Palermo G, Adler A, Bertoli M, Blake M, Cohen J. Intracytoplasmic sperm njection in dysmorphic human oocytes. Zygote. 1995;3:283–288. doi: 10.1017/S0967199400002707. [DOI] [PubMed] [Google Scholar]
- 48.Shen Y, Stalf T, Mehnert C, Santis L, Cino I, Tinneberg HR, et al. Light retardance by human oocyte spindle is positively related to pronuclear score after ICSI. Reprod Biomed Online. 2006;12:737–751. doi: 10.1016/S1472-6483(10)61086-3. [DOI] [PubMed] [Google Scholar]
- 49.Meriano JS, Alexis J, Visram-Zaver S, Cruz M, Casper RF. Tracking of oocyte dysmorphisms for ICSI patients may prove relevant to the outcome in subsequent patient cycles. Hum Reprod. 2001;16:2118–2123. doi: 10.1093/humrep/16.10.2118. [DOI] [PubMed] [Google Scholar]
- 50.Balaban B, Urman B. Effect of oocyte morphology on embryo development and implantation. Reprod Biomed Online. 2006;12:608–615. doi: 10.1016/S1472-6483(10)61187-X. [DOI] [PubMed] [Google Scholar]
- 51.Placido G, Wilding M, Strina I, Alviggi E, Alviggi C, Mollo A, et al. High outcome predictability after IVF using a combined score for zygote and embryo morphology and growth rate. Hum Reprod. 2002;17:2402–2409. doi: 10.1093/humrep/17.9.2402. [DOI] [PubMed] [Google Scholar]
- 52.Blerkom J. Mitochondria in human oogenesis and preimplantation embryogenesis: engines of metabolism, ionic regulation and developmental competence. Reproduction. 2004;128:269–280. doi: 10.1530/rep.1.00240. [DOI] [PubMed] [Google Scholar]
- 53.Wilding M, Dale B, Marino M, Matteo L, Alviggi C, Pisaturo ML, et al. Mitochondrial aggregation patterns and activity in human oocytes and preimplantation embryos. Hum Reprod. 2001;16:909–917. doi: 10.1093/humrep/16.5.909. [DOI] [PubMed] [Google Scholar]