Abstract
Purpose
To evaluate the efficacy of using both urinary and recombinant FSH in a combined protocol for ovarian stimulation in an IVF treatment program.
Method
A total of 119 infertile couples undergoing ICSI treatment were randomized prospectively in this study. After a standard down-regulation with GnRH analogue, the patients were randomized in 2 groups 58 received combined urinary and recombinant FSH, starting with uFSH and then rFSH, and 61 controls received only recombinant FSH.
Result(s)
Pregnancy and implantation rates were significantly higher in the combined uFSH/rFSH group than the control (rFSH) group (43.9% vs 22.1% and 27.5% vs 13.2% respectively). Metaphase II oocyte and grade 1 embryos were significantly higher in favour of combined uFSH/rFSH group than the recombinant FSH group.
Conclusion(s)
This study shows that using a combination of both urinary and recombinant FSH for ovarian stimulation improves oocyte maturity and embryo cleavage, and increases pregnancy and implantation rates.
Keywords: Embryo, Oocyte, Ovarian stimulation, Recombinant human FSH, Urinary human FSH
Introduction
Controlled ovarian hyperstimulation is an integral part of in vitro fertilization and embryo transfer treatment. This is achieved by the administration of exogenous gonadotropins to increase follicular recruitment and oocyte yield. For such purpose, FSH preparations from different origin have been implemented in a variety of ovarian stimulation regimens with variable clinical outcomes. Until recently, gonadotropins used for ovarian stimulation have been extracted from the urine of postmenopausal women. With the advent of recombinant DNA technology, two pure FSH preparations have become available: follitropin-alpha and follitropin-beta, which lack LH activity or extraneous human proteins [1–3]. The purity and in vivo bioactivity of recombinant FSH are thought to confer safety, efficiency, and tolerability advantages over urine-derived FSH [4, 5].
Clinical trials have shown that recombinant FSH is effective in terms of number of oocytes retrieved, number of embryos obtained, and total gonadotropin dose needed, without increasing the risk for the ovarian hyperstimulation syndrome (OHSS) [6–8]. In addition, recombinant FSH has been shown to be as effective as urinary FSH or hMG with or without GnRH agonists [9–12]. Recently, the efficacy of rFSH compared to uFSH in terms of oocyte and embryo quality has been evaluated, and the results reported are highly in favour of urinary FSH. Some authors have attributed the difference to the presence of LH activity in the urinary FSH preparation which has a positive effect on oocyte maturation and embryo quality [13–15], while others assume that the difference between rFSH and uFSH may reside in the nature of FSH isoform activities [15, 16].
Gonadotropin isoforms influence a variety of biological activities, cellular growth and development, steroidogenesis and protein synthesis. Urinary FSH contains both acidic and mid-acidic isoforms whereas recombinant FSH contains a higher proportion of less acidic isoforms. Less acidic FSH isoforms exhibit a high in vitro bioactivity, but they have a faster clearance and thus a shorter circulatory half life than acidic FSH isoforms [17, 18]. Another study has shown that the slow clearance of the acidic isoforms results in more estrogenic follicles and follicular maturation and estradiol secretion [16].
Furthermore, several studies have documented the occurrence of significant changes in FSH heterogeneity during certain physiological conditions including puberty and the menstrual cycle [19–23]. Acidic FSH isoforms are produced during follicular and luteal phases when the E2 level is low whereas less acidic FSH isoforms are produced during mid-cycle i.e. when the E2 level is high. This shift towards the production and secretion of less acidic/sialylated FSH molecules in the mid-cycle and preovulatory phases of the cycle may be an important mechanism to regulate the intensity of the FSH stimulus during the final steps of follicular maturation [24].
Considering this evidence, in this study we sought to mimic the physiological cycle during ovarian stimulation by using a combined protocol of both uFSH and rFSH, starting with the urinary FSH preparation during the follicular phase and rFSH preparation during the mid-follicular phase until hCG administration. We evaluated the efficiency and the efficacy of this combined stimulation protocol on oocyte and embryo quality, and pregnancy and implantation rates as well.
Materials and methods
Patient selection
In a prospective, open, randomized study a total of 119 infertile couples undergoing their first ICSI treatment were enrolled for this study from June 2005 to March 2006. The women aged 27–38 years were included if they fulfilled the following criteria: (1) infertility attributable to tubal factor, male factor or idiopathic infertility; (2) serum hormonal profile (FSH and LH <12 mIU/ml, E2 <50 pg/ml and prolactin <30 ng/ml) within the normal range; (3) regular ovulatory menstrual cycles; (4) presence of normal uterine cavity; (5) body mass index (BMI) ≥20–≤26 kg/m2 and (6) first IVF treatment. The patients were excluded if they had previous poor response to gonadotropins, history of severe OHSS, or current polycystic ovarian syndrome or if the male partner had azoospermia or clinical signs of infection detected in semen analysis within 12 months before treatment.
Randomization was performed using a computer-generated random assignment schedule for each patient. Sealed and numbered envelopes were used to conceal the treatment allocation until randomization. The randomization took place after the confirmation of down-regulation and immediately before gonadotropin administration in order to minimize post-randomization withdrawals. All patients were counselled about the nature of the study and gave their written informed consent for their participation to the randomization procedure. Participating patients were registered in our local ethical committee register that approved the study. Only the first IVF patients that satisfied the inclusion criteria were enrolled in the study to reduce the heterogeneity of the patients and minimization confounding variables that may affect the results.
The primary end points were, clinical pregnancy and implantation rates. The secondary endpoints were total dose of FSH administered, total number of days of stimulation, serum estradiol levels and endometrial thickness on the day of hCG administration, number of mature oocytes retrieved, embryo quality, fertilization rate, embryo cleavage rate, live birth and miscarriage rates, cancellation rate, and incidence of moderate or severe OHSS. All end points except the cancellation rate and the incidence of OHSS were analyzed statistically.
Stimulation protocol
All patients underwent a standard down-regulation protocol with GnRH analogue hormone (triptroline, Decapeptyl 0.1 mg/day, Ipsen, Milan, Italy). The patients were randomized in two groups: group A (n = 58), patients that received 225 IU of urinary FSH (Fostimon, IBSA, Switzerland) for 6 days from the second day of the cycle and then 225 IU of recombinant FSH (Gonal-F; Serono, Rome, Italy) from the 7th day of stimulation until hCG administration, and group B, control group, (n = 61), patients that received 225 IU recombinant FSH alone from the second day of the cycle until hCG administration. The patients with a poor response to gonadotropin treatment were withdrawn from the study. Patients with excessive response to gonadotropins were counselled about the risk for OHSS and were advised to interrupt the stimulation cycle or to undergo oocyte retrieval with cryopreservation of any resultant embryos for replacement in the subsequent cycle.
Final oocyte maturation was triggered by the administration of 10,000 IU of human chorionic gonadotropin (hCG) (Gonasi HP IBSA, Switzerland) when the leading follicle was 18–19 mm and there were at least two follicles of 16–17 mm. Oocyte retrieval was performed 36 h after hCG administration and the harvested oocytes were denuded from their cumulus cell and were assessed for their maturity. The oocytes were then inseminated by ICSI, and the resultant embryos were scored according to established criteria [25, 26]. Of note, in Italy only three oocytes are permitted to be inseminated, therefore we performed ICSI as an in vitro fertilization technique of choice in order to select good quality oocytes for insemination. Ultrasound guided embryo transfer took place 48 h following insemination. The luteal phase was supported with the administration of 50 mg/day of progesterone.
Statistical analysis was performed using the JMP software (version 4.0.4; SAS, Cary, NC). The parameters were compared using the two tailed Student’s t-test for independent data and χ2-test, setting the significance level at p ≤ 0.05. ANOVA two-way test was also used to analyze continuous variables, including primary and secondary outcome parameters. Statistical power calculation was based on an α level of 0.05 (two-tailed test) with 80% power to detect a 20% difference with 50 evaluable patients per group The difference between treatments was evaluated using a two-sided, 95% confidence interval. All analyses were adjusted for age stratum in line with the study design. Correction for multiple comparison analysis was performed using either Bonferroni’s or Sidak’s adjustment methods by lowering the alpha for each test to 0.0039 with t value for double sided testing: ≥3.00. The difference had greater significance of pregnancy and implantation rates when linear mixed model, which controls for intrasubject variation was used to compare the data (p ≤ 0.001).
Results
Three patients were cancelled: one woman in group A and two women group B, because of excessive ovarian response leading to high risk for OHSS (1.7% and 3.2% of patients, respectively). This difference was not statistically significant. Of the119 studied patients, 116 underwent oocyte retrieval, 57 patients in group A and 59 in group B.
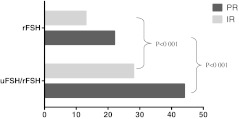
As depicted in Table 1, the two groups were comparable regarding demographic data, infertility factor distribution, duration of stimulation, estradiol level and endometrial thickness on the day of hCG administration. There was no significant difference observed between the two groups regarding the mean number of oocytes retrieved per patient. Considering oocyte maturation, a statistically higher proportion (p < 0.05) of MII oocytes and lower proportion of immature (GV) oocytes were observed in favour of group A compared to group B (65.1% vs 34.5% and 10.4% vs 37.5% respectively). Also significant differences (p < 0.05) were found in favour of group A with respect to group B in terms of grade 1 embryos (58.2% vs 37.3%). Significantly higher (p < 0.001) implantation rate (27.5% vs 13.2%) and pregnancy rate (43.9% vs 22.1%) was observed in favour of group A compared to group B (Table 2). Bonferroni’s or Sidak’s adjustment methods were used for multiple comparisons correction model by lowering the alpha value to 0.0039 in order to eliminate the false significance. Moreover, the Restricted Likelihood Method was used for linear mixed model analysis. Both evidenced a greater significance in favour of group A in terms of pregnancy and implantation rates (p < 0.001) (Fig. 1).
Table 1.
Demographic data and stimulation outcome
| uFSH/rFSH group A | rFSH group B | p value | |
|---|---|---|---|
| Patients (n) | 58 | 61 | |
| Mean age (years) ±SD | 34.1 ± 2.5 | 35.1 ± 3.1 | NS |
| Mean BMI±SD | 22.6 ± 1.8 | 23.6 ± 1.7 | NS |
| Mean duration of sterility (years) ±SD | 5.1 ± 1.2 | 4.1 ± 1.4 | NS |
| Primary infertility % (n) | 71.9 (41) | 74.6 (44) | NS |
| Tubal factor % (n) | 47.4 (27) | 44.1 (26) | NS |
| Male factor % (n) | 40.3 (23) | 40.6 (24) | NS |
| Unexplained infertility % (n) | 12.3 (7) | 15.3 (9) | NS |
| Duration of stimulation (days) | 11.4 ± 2.1 | 13.1 ± 2.2 | NS |
| Estradiol level on hCG day (pg/ml) | 2,056 ± 560 | 1,987 ± 699 | NS |
| Endometrial thickness on hCG day (mm) | 10.8 ± 2.1 | 11.2 ± 3.1 | NS |
NS: not significant
Table 2.
Embryological characteristics and clinical outcome
| uFSH/rFSH group A | rFSH group B | p value | |
|---|---|---|---|
| No. of patients underwent egg retrieval | 57 | 59 | |
| Cancelled patients | 1 | 2 | NS |
| Mean number of retrieved oocytes ±SD | 10.5 ± 1.4 | 12.2 ± 2.9 | NS |
| Mature ocytes (MII) % | 65.1 | 34.5 | 0.05 |
| Mature oocytes (MI) % | 30.5 | 28 | NS |
| Immature oocytes (GV) % | 10.4 | 37.5 | 0.05 |
| Mean number of embryos transferred/patient ±SD | 2.7 ± 1.0 | 2.6 ± 0.9 | NS |
| Grade I embryos (%) | 58.2 | 37.3 | 0.05 |
| Grade II embryos (%) | 31.6 | 45.6 | NS |
| Grade III embryos (%) | 9.0 | 15.0 | NS |
| Grade IV embryos (%) | 1.2 | 2.1 | NS |
| Pregnancy rate % (n) | 43.9 (25) | 22.1 (13) | 0.001 |
| Implantation rate (%) | 27.5 | 13.2 | 0.001 |
| Abortion rate % (n) | 12 (3) | 15.3 (2) | NS |
NS: not significant
Fig. 1.
Comparison of pregnancy and implantation rates between groups A and B. PR pregnancy rate, IR implantation rate. Statistically higher pregnancy and implantation rates (p < 0.001) in favour of uFSH/rFSH group compared to rFSH group
Discussion
Recombinant FSH has introduced an alternative to urine-derived FSH for ovarian stimulation regimens. Several comparison studies have shown that recombinant FSH is more effective than urinary FSH (HMG or highly purified FSH) and the absence of LH activity in rFSH does not affect follicular growth [6–8]. However, recent reports demonstrate that urinary FSH is considerably better than recombinant FSH in terms of oocyte and embryo quality and pregnancy and implantation rates, although the number of retrieved oocytes is higher in favour of rFSH [13–15].
Of the factors that affect oocyte quality in stimulated cycles, the most important appear to be patient age, basal hormonal profile, profound suppression of LH during down-regulation and estradiol concentration per growing follicle. There is some evidence that estradiol appears to have a key role in oocyte maturation [27–29]. Tesarik and Mendoza [30, 31] reported that estradiol exerts a beneficial effect on cytoplasmic maturation via a non-genomic calcium-mediated mechanism, which contributes to oocyte capacitation for fertilization and early post-fertilization development. Significantly higher pregnancy rates have been reported in women with an intermediate estradiol/oocyte ratio between 70 and 140 pg/ml [32].
Additionally, profound suppression of LH during the down-regulation protocols affects oocyte quality and clinical outcome. It has been reported that suppression of LH below the level <0.5 IU/l is associated with a reduced cohort of embryos and a reduced estradiol/oocyte ratio [33, 34]. On the other hand, other studies have shown that a low concentration of endogenous LH (<3 mIU/ml) in the late follicular phase is associated with lower fertilization rates and higher biochemical pregnancy rates. It has been suggested that when using recombinant FSH only, it may be of clinical benefit to add LH in the late follicular phase or to further reduce the dose of GnRH analogue [33–36]. Conversely, it has been reported that patients with very suppressed LH levels respond similarly to those moderately suppressed, and only 6% of patients would benefit from exogenous LH administration [32]. Recombinant FSH lacks any LH activity by definition; nevertheless it remains highly effective in stimulating follicle growth and maturation.
Another factor that could affect oocyte maturity and development may be the nature of FSH isoforms used for ovarian stimulation. It has been shown that gonadotropin isoforms influence a variety of biological activities, cellular growth and development, steroidogenesis and protein synthesis [37–39]. Because of their structural differences, FSH isoforms differ in their ability to bind to target cell receptors surviving in the circulation and induce a biological response in vivo and in vitro [40–44]. Evident differences between recombinant and urinary FSH were recognized, rFSH contains a higher proportion of less acidic isoforms, whereas urinary FSH contains a higher proportion of acidic forms. This difference reflects their biological bioactivity, rate of clearance and biological function. It has been suggested that the less acidic isoforms have a faster circulatory clearance and, thus, a shorter circulatory half-life [17] than the acidic isoforms [45, 46]. However, a more recent study has shown that the slow clearance of the acidic isoform results in better follicular maturation and estradiol secretion than the less acidic isoform [16].
In our study the estradiol level at HCG day was slightly higher though not statistically significant (2,056 ± 560 vs 1,987 ± 699) in the combined uFSH/rFSH compared to rFSH group. Although the number of retrieved oocytes does not significantly differ between the two groups, significant differences were observed in favour of the combined protocol compared to the rFSH group in terms of the proportion of mature and immature oocytes (65.1% vs 34.5% and 10.4% vs 37.55 respectively). Also statistically higher grade I embryos (58.2% vs 37.3%) was found in group A compared to group B. Although the mean number of replaced embryos was similar in both groups, statistically higher pregnancy (43.9% vs 22.1%) and implantation rates (27.5% vs 13.2%) were observed in group A than in group B. This could partially explain the differences found between the two groups in terms of pregnancy and implantation rates, and can reflect the fact that the embryos derived from the combined protocol group have a high proportion of potentially good quality embryos.
This observation might be also explained by the fact that the combined protocol may mimic the physiological cycle, where more acidic FSH isoforms prevail during the follicular phase of the menstrual cycle and shift to less acidic FSH isoforms in the mid cycle i.e. preovulatory phase. Some studies reported significant differences related to in-vitro biological potency among the various intrapituitary FSH isoforms and strongly suggests that the shifts towards the production and secretion of more basic or acidic FSH molecules occurring in certain specific physiological conditions (e.g. puberty and menstrual cycle), may represent an important mechanism through which the anterior pituitary regulates gonadal function [24, 47]. Other studies have previously shown that almost all stored FSH isoforms may be released from the pituitary gland with few or no modifications in their number and pH values and that the charge distribution of the circulating isoforms changes according to the phase of the menstrual cycle [21, 23, 48]. The shift towards the production and secretion of less acidic/sialylated FSH molecules during this cycle phase may be an important mechanism to regulate the intensity of the FSH stimulus during the final steps of follicular maturation.
Although our result seems to show a significant difference between the two groups in terms of oocyte and embryo quality and clinical outcome in favour of the combined protocol an additional investigation on large number of patients is needed to further characterize the impact of different FSH isoforms on oocyte follicular development and oocyte maturation, and its implication in stimulation regimens.
References
- 1.Chaple E, Kelton C, Nugent N. Expression of human gonadotrophins by recombinant DNA methods. In: Genazzani AR, Petraglia F, editors. Proceedings of the 3rd world congress on gynaecological endocrinology. Carnforth, United Kingdom: Parthenon; 1992. pp. 179–184. [Google Scholar]
- 2.Loumaye E, Campbell R, Salat-Baroux J. Human follicle stimulating hormone produced by recombinant DNA technology: a review for clinicians. Hum Reprod Update. 1995;1:188–199. doi: 10.1093/humupd/1.2.188. [DOI] [PubMed] [Google Scholar]
- 3.Olijve W, Boer W, Mulders JWM, Wezenbeek PM. Molecular biology and biochemistry of human recombinant follicle stimulating hormone (Puregon) Mol Hum Reprod. 1996;2:371–382. doi: 10.1093/molehr/2.5.371. [DOI] [PubMed] [Google Scholar]
- 4.Redfearn A, Hughes EG, O’Connor M, Dolovich J. Delayed-type hypersensitivity to human gonadotropin: case report. Fertil Steril. 1995;64:479–482. doi: 10.1016/s0015-0282(16)57865-0. [DOI] [PubMed] [Google Scholar]
- 5.Albano C, Smitz J, Camus M, Bennink HC, Steirteghem A, Devroey P. Pregnancy and birth in an in-vitro fertilization cycle after controlled ovarian stimulation in a woman with a history of allergic reactions to human menopausal gonadotrophin. Hum Reprod. 1996;11:1632–1634. doi: 10.1093/oxfordjournals.humrep.a019459. [DOI] [PubMed] [Google Scholar]
- 6.Out HJ, Mannaerts BMJL, Driessen SGAJ, Coelingh-Bennink HJT. A prospective, randomized, assessor-blind, multicentre study comparing recombinant and urinary follicle stimulating hormone (Puregon vs. Metrodin) in in-vitro fertilization. Hum Reprod. 1995;10:2534–2540. doi: 10.1093/oxfordjournals.humrep.a135740. [DOI] [PubMed] [Google Scholar]
- 7.Out HJ, Mannaerts BMJL, Driessen SGAJ, Coelingh-Bennink HJT. Recombinant follicle stimulating hormone (recombinant FSH, Puregon) in assisted reproduction: more oocytes, more pregnancies. Results from five comparative studies. Hum Reprod. 1996;2:162–171. doi: 10.1093/humupd/2.2.162. [DOI] [PubMed] [Google Scholar]
- 8.Bergh C, Howles CM, Borg K, Hamberger L, Josefsson B, Nilsson L, et al. Recombinant human follicle stimulating hormone (r-FSH, Gonal-F) vs. highly purified urinary FSH (Metrodin highly purified): results of a randomized comparative study in women undergoing assisted reproductive techniques. Hum Reprod. 1997;12:2133–2139. doi: 10.1093/humrep/12.10.2133. [DOI] [PubMed] [Google Scholar]
- 9.Devroey P, Mannaerts B, Smitz J, Coelingh-Bennink H, Steirteghe Clinical outcome of a pilot efficacy study on recombinant human follicle-stimulating hormone (Org 32489) combined with various gonadotrophin-releasing hormone agonist regimens. Hum Reprod. 1994;9:1064–1069. doi: 10.1093/oxfordjournals.humrep.a138634. [DOI] [PubMed] [Google Scholar]
- 10.Hedon B, Out HJ, Hugues JN, Camier B, Cohen J, Lopes P, et al. Efficacy and safety of recombinant follicle stimulating hormone (Puregon) in infertile women pituitary-suppressed with triptorelin undergoing in-vitro fertilization: a prospective, randomized, assessor-blind, multicentre trial. Hum Reprod. 1995;10:3102–3106. doi: 10.1093/oxfordjournals.humrep.a135866. [DOI] [PubMed] [Google Scholar]
- 11.Out HJ, Reimitz PE, Coelingh-Bennink HJT. A prospective, randomized study to assess the tolerance and efficacy of intramuscular and subcutaneous administration of recombinant follicle-stimulating hormone (Puregon) Fertil Steril. 1997;67:278–283. doi: 10.1016/S0015-0282(97)81911-5. [DOI] [PubMed] [Google Scholar]
- 12.Jansen CAM, Os HC, Out HJ, Coelingh-Bennink HJT. A prospective, randomized clinical trial comparing recombinant follicle stimulating hormone (Puregon) and human menopausal gonadotrophins (Humegon) in non-down-regulated in-vitro fertilization cycles. Hum Reprod. 1998;13:2995–2999. doi: 10.1093/humrep/13.11.2995. [DOI] [PubMed] [Google Scholar]
- 13.Ng EHY, Lau EYL, Yeung WSBY, Ho PC. HMG is as good as recombinant human FSH in terms of oocyte and embryo quality: a prospective randomized trial. Hum Reprod. 2001;2:319–325. doi: 10.1093/humrep/16.2.319. [DOI] [PubMed] [Google Scholar]
- 14.Strehler E, Abt M, El-Danasouri I, Santo M, Sterzik K. Impact of recombinant follicle-stimulating hormone and human menopausal gonadotrophins on in vitro fertilization outcome. Fertil Steril. 2001;75:332–336. doi: 10.1016/S0015-0282(00)01696-4. [DOI] [PubMed] [Google Scholar]
- 15.Selman HA, Santo M, Sterzik K, Coccia E, Danasouri I. Effect of highly purified urinary follicle-stimulating hormone on oocyte and embryo quality. Fertil Steril. 2002;78:1061–1067. doi: 10.1016/S0015-0282(02)04202-4. [DOI] [PubMed] [Google Scholar]
- 16.West CR, Carlson NE, Lee JS, McNeilly AS, Sharma TP, Ye W, et al. Acidic mix of FSH isoforms are better facilitators of ovarian follicular maturation and E2 production than the less acidic. Endocrinology. 2002;143:107–116. doi: 10.1210/en.143.1.107. [DOI] [PubMed] [Google Scholar]
- 17.Antonio MD, Borrelli F, Datola A, Bucci R, Mascia M, Polletta P, et al. Biological characterization of recombinant human follicle stimulating hormone isoforms. Hum Reprod. 1999;14:1160–1167. doi: 10.1093/humrep/14.5.1160. [DOI] [PubMed] [Google Scholar]
- 18.Vitt UA, Kloosterboer HJ, Rose UM, Mulders JW, Kiesel PS, Bete S, et al. Isoforms of human recombinant follicle-stimulating hormone: comparison of effects on murine follicle development in vitro. Biol Reprod. 1998;59:854–861. doi: 10.1095/biolreprod59.4.854. [DOI] [PubMed] [Google Scholar]
- 19.Padmanabhan V, Sairam MR, Hassing JM, Brown MB, Ridings JW, Beitins IZ. Follicle-stimulating hormone signal transduction: role of carbohydrate in aromatase induction in immature Sertoli cells. Mol Cell Endocrinol. 1991;79:119–128. doi: 10.1016/0303-7207(91)90102-X. [DOI] [PubMed] [Google Scholar]
- 20.Wide L. Follicle-stimulating hormones in anterior pituitary gland from children and adults differ in relation to sex and age. J Endocrinol. 1989;123:519–529. doi: 10.1677/joe.0.1230519. [DOI] [PubMed] [Google Scholar]
- 21.Wide L, Bakos O. More basic forms of both human follicle-stimulating hormone and luteinizing hormone in serum at midcycle compared with the follicular and luteal phase. J Clin Endocrinol Metab. 1993;76:885–889. doi: 10.1210/jc.76.4.885. [DOI] [PubMed] [Google Scholar]
- 22.Phillips DJ, Wide L. Serum gondotropin isoforms become more basic after an exogenous challenge of gonadotropin-releasing hormone in children undergoing pubertal development. J Clin Endocrinol Metab. 1994;79:814–819. doi: 10.1210/jc.79.3.814. [DOI] [PubMed] [Google Scholar]
- 23.Zambrano E, Olivares A, Mendez JP, Guerrero L, Díaz-Cueto L, Veldhuis JD, et al. Dynamics of basal and gonadotropin-releasing hormone-releasable serum follicle-stimulating hormone charge isoform distribution throughout the human menstrual cycle. J Clin Endocrinol Metab. 1995;80:1647–1656. doi: 10.1210/jc.80.5.1647. [DOI] [PubMed] [Google Scholar]
- 24.Ulloa-Aguirre A, Damian-Matsumura P, Jimenez M, Zambrano E, Díaz-Sánchez V. Biological characterization of the isoforms of urinary human follicle-stimulating hormone contained in a purified commercial preparation. Hum Reprod. 1992;7:1371–1378. doi: 10.1093/oxfordjournals.humrep.a137576. [DOI] [PubMed] [Google Scholar]
- 25.Veeck LL. An atlas of human gametes and conception. London: Parthenon; 1999. [Google Scholar]
- 26.Veeck LL. Oocyte assessment and biological performance. Ann N Y Acad Sci. 1988;541:259–274. doi: 10.1111/j.1749-6632.1988.tb22263.x. [DOI] [PubMed] [Google Scholar]
- 27.Zelsinki-Wooten MB, Hess DL, Wolf D, Stouffer R. Steroid production during ovarian stimulation impairs oocyte fertilization but not folliculogenesis in rhesus monkey. Fertil Steril. 1994;61:1147–1154. [PubMed] [Google Scholar]
- 28.Wu TJ, Wang L, Wan YY. Detection of estrogen receptor messenger ribonucleic acid in human oocyte and cumulus oocyte complexes using reverse transcriptase polymerase chain reaction. Fertil Steril. 1993;59:54–59. [PubMed] [Google Scholar]
- 29.Hild-Petito S, Stouffer RL, Brenner RM. Immunocytochemical localization of estrogen and progesterone receptors in the monkey ovary throughout the menstrual cycle. Endocrinology. 1988;123:2896–2905. doi: 10.1210/endo-123-6-2896. [DOI] [PubMed] [Google Scholar]
- 30.Tesarik J, Mendoza C. Nongenomic effects of 17β-estradiol on maturing human oocytes: relationship to oocyte developmental potential. J Clin Endocrinol Metab. 1995;80:1438–1443. doi: 10.1210/jc.80.4.1438. [DOI] [PubMed] [Google Scholar]
- 31.Tesarik J, Mendoza C. Direct non-genomic effects of follicular steroids on maturing human oocytes: oestrogen versus androgen antagonism. Hum Reprod Update. 1997;3:95–100. doi: 10.1093/humupd/3.2.95. [DOI] [PubMed] [Google Scholar]
- 32.Loumaye E, Engrand P, Howles CM, O’Dea L. Assessment of the role of serum luteinizing hormone and estradiol response to follicle-stimulating hormone on in vitro fertilization treatment. Fertil Steril. 1998;67(5):889–899. doi: 10.1016/S0015-0282(97)81402-1. [DOI] [PubMed] [Google Scholar]
- 33.Westergaard LG, Laursen SB, Andersen CY. Increased risk of early pregnancy loss by profound suppression of luteinizing hormone during ovarian stimulation in normogonadotrophic women undergoing assisted reproduction. Hum Reprod. 2000;15:1003–1008. doi: 10.1093/humrep/15.5.1003. [DOI] [PubMed] [Google Scholar]
- 34.Fleming R, LIoyd F, Herbert M, Fenwick J, Griffiths T, Murdoch A. Effects of profound suppression of luteinizing hormone during ovarian stimulation on follicular activity, oocyte and embryo function in cycles stimulated with purified follicle stimulating hormone. Hum Reprod. 1998;13:1788–1792. doi: 10.1093/humrep/13.7.1788. [DOI] [PubMed] [Google Scholar]
- 35.Fleming R, Rehka P, Deshpande N, Jamieson ME, Yates RWS, Lyall HL. Suppression of LH during ovarian stimulation effects differ in cycles stimulated with purified urinary FSH and recombinant FSH. Hum Reprod. 2000;15:1440–1445. doi: 10.1093/humrep/15.7.1440. [DOI] [PubMed] [Google Scholar]
- 36.Esposito MA, Barnhart KT, Coutifaris C, Patrizio P. Role of periovulatory luteinizing hormone concentrations during assisted reproductive technology cycles stimulated exclusively with recombinant follicle-stimulating hormone. Fertil Steril. 2001;75:519–524. doi: 10.1016/S0015-0282(00)01745-3. [DOI] [PubMed] [Google Scholar]
- 37.Sairam MR, Bhargavi GN. A role for glycosylation of the alpha subunit in transduction of biological signal in glycoprotein hormones. Science. 1985;229:65–67. doi: 10.1126/science.2990039. [DOI] [PubMed] [Google Scholar]
- 38.Bishop LA, Robertson DM, Cahir N, Schofield PR. Specific roles for the asparagine-linked carbohydrate residues of recombinant human follicle-stimulating hormone in receptor binding and signal transduction. Mol Endocrinol. 1994;8:722–731. doi: 10.1210/me.8.6.722. [DOI] [PubMed] [Google Scholar]
- 39.Davis D, Liu X, Segaloff DL. Identification of the sites of N-linked glycosylation on the follicle-stimulating hormone (FSH) receptor and assessment of their role in receptor function. Mol Endocrinol. 1995;9:159–170. doi: 10.1210/me.9.2.159. [DOI] [PubMed] [Google Scholar]
- 40.Chappel SC, Ulloa-Aguirre A, Ramaley J. Sexual maturation in female rats: time-related changes in the isoelectric focusing pattern of anterior pituitary follicle-stimulating hormone. Biol Reprod. 1983;28:196–205. doi: 10.1095/biolreprod28.1.196. [DOI] [PubMed] [Google Scholar]
- 41.Blum WF, Gupta D. Heterogeneity of rat FSH by chromatofocusing: studies on serum FSH, hormone released in vitro and metabolic clearance rates of its various forms. J Endocrinol. 1985;105:29–37. doi: 10.1677/joe.0.1050029. [DOI] [PubMed] [Google Scholar]
- 42.Wide L. The regulation of metabolic clearance rate of human FSH in mice by variation of the molecular structure of the hormone. Acta Endocrinol. 1986;112:336–344. doi: 10.1530/acta.0.1120336. [DOI] [PubMed] [Google Scholar]
- 43.Wide L, Hobson BM. Influence of the assay method used on the selection of the most active forms of FSH from the human pituitary. Acta Endocrinol. 1986;113:17–22. doi: 10.1530/acta.0.1130017. [DOI] [PubMed] [Google Scholar]
- 44.Ulloa-Aguirre A, Cravioto A, Damian-Matsumura P, Jimenez M, Zambrano E, Diaz-Sanchez V. Biological characterization of the naturally occurring analogues of intrapituitary human follicle-stimulating hormone. Hum Reprod. 1992;7:23–30. doi: 10.1093/oxfordjournals.humrep.a137550. [DOI] [PubMed] [Google Scholar]
- 45.Flack MR, Bennet AP, Froehlich J, Anasti JN, Nisula BC. Increased biological activity due to basic isoforms in recombinant human follicle-stimulating hormone produced in a human cell line. J Clin Endocrinol Metab. 1994;79:756–760. doi: 10.1210/jc.79.3.756. [DOI] [PubMed] [Google Scholar]
- 46.Galway AB, Hsueh AJ, Keene JL, Yamoto M, Fauser BC, Boime I. In vitro and in vivo bioactivity of recombinant human follicle-stimulating hormone and partially deglycosylated variants secreted by transfected eukaryotic cell lines. Endocrinology. 1990;127:93–100. doi: 10.1210/endo-127-1-93. [DOI] [PubMed] [Google Scholar]
- 47.Zambrano E, Barrios-de-Tomasi J, Cardenas M, UIIoa-Aguirre1 A. Studies on the relative in-vitro biological potency of the naturally-occurring isoforms of intrapituitary follicle stimulating hormone. Mol Hum Reprod. 1996;2:563–571. doi: 10.1093/molehr/2.8.563. [DOI] [PubMed] [Google Scholar]
- 48.Padmanabhan V, Lang LL, Sonstein J, Kelch RP, Beitins IZ. Modulation of serum follicle-stimulating hormone bioactivity and isoform distribution by estrogenic steroids in normal women and in gonadal dysgenesis. J Clin Endocrinol Metab. 1988;67:465–473. doi: 10.1210/jcem-67-3-465. [DOI] [PubMed] [Google Scholar]