Abstract
Background
There has been controversy over the role of FSH in the regulation of preantral follicle development. LH is a survival and differentiation factor that increases oocyte maturation in FSH-supplemented cultures of mouse preantral follicles. However, little information exists on the action of LH and FSH in the developmental competence of porcine preantral follicle oocytes in vitro.
Materials and methods
Porcine preantral follicles were cultured for 3 days in the presence or absence of FSH or LH. Oocytes from these follicles were then matured, fertilized in vitro, and embryos were cultured. Estradiol secretion and histological analysis of cultured follicles were also carried out.
Results
FSH or combined LH and FSH significantly enhanced follicular growth compared to LH alone or the controls. Combined LH and FSH treatment of preantral follicles significantly increased the percentage (59 ± 5%) of oocytes competent to undergo cleavage to the two-cell stage after fertilization. A significant effect was seen on oocyte competence to develop from the two-cell to the blastocyst stage (30 ± 6%) compared to FSH alone treatment (45 ± 7 and 14 ± 5%, respectively). The amount of estradiol on days 2 and 3 of culture was significantly higher in follicles cultured with FSH (48.75 ± 17, 70.5 ± 14 pg/ml) or combined LH and FSH (63.25 ± 16, 72.5 ± 12 pg/ml) than that cultured with the untreated controls (16 ± 10, 5.66 ± 4 pg/ml).
Conclusions
The results indicated that FSH is essential for the in vitro growth of porcine preantral follicles, estradiol secretion, and for oocytes to acquire competence to resume meiosis and undergo fertilization and embryonic development. LH with FSH treatment of porcine preantral follicles can improve the quality of oocytes by promoting growth and a higher frequency of embryonic development.
Keywords: Developmental competence, FSH, Growth, LH, Porcine preantral follicles
Introduction
Development of the ovarian follicle is a dynamic process involving important morphological and functional changes in thecal and granulosa cells (proliferation, steroidogenesis and gonadotropin sensitivity), and in oocytes (cytoplasmic and nuclear maturation). The gonadotropins follicle-stimulating hormone (FSH) and luteinizing hormone (LH) are heterodimeric glycoproteins produced within the adenohypophysis that, in the female, act primarily at the level of the ovarian follicle. The actions of gonadotropins are targeted to ovarian somatic cells through specific cell-surface receptors. These receptors, namely the LH receptor (LHR) and the FSH receptor (FSHR), are members of the G protein-coupled receptor superfamily.
In sheep, FSHR mRNA can be observed in granulosa cells of early preantral follicles with one or two cell layers, and its gene expression continues throughout folliculogenesis [1]. FSH is an essential survival hormone for the prevention of the programmed demise of early antral follicles in rodents [2–4]. Moreover, FSH plays an important role in the final differentiation of granulosa cells in antral and preovulatory follicles, to allow the biosynthesis of estrogens and to prepare the preovulatory follicles for ovulation [5]. However, there has been controversy over the role of FSH in the regulation of preantral follicle development.
In vivo, preantral follicles are considered to be gonadotropin-independent because animal or human preantral follicles can develop to the antral stage in conditions with minimal circulating gonadotropins [6–8], but several studies have suggested that development of early follicles is under the influence of gonadotropin. In vitro, although several studies have demonstrated an important role for FSH in preantral follicle growth [9–12], other studies have indicated that treatment with FSH does not enhance preantral follicle growth [13, 14].
LH receptors are expressed on theca cells from preantral and antral follicles, and on granulosa cells from large antral follicles [15–18]. LH may play multiple roles throughout follicular development, but most studies have focused on the action in late-stage follicles and during the periovulatory period. In contrast, the action of LH in preantral follicle development has received less attention. Cortvrindt et al. [19, 20] have suggested that LH is a survival and differentiation factor that increases oocyte maturation in FSH-supplemented cultures of mouse preantral follicles, and LH has a stage-limited effect on mouse preantral follicle development in vitro. However, little information exists on the action of LH and FSH in the developmental competence of porcine preantral follicle oocytes in vitro.
The objective of this study was to evaluate the effects of FSH and/or LH on in vitro growth of porcine preantral follicles, estradiol secretion, antrum formation, oocyte maturation, and subsequent embryonic development.
Materials and methods
Animal and tissue collection
Ovaries were collected from prepubertal gilts at a local abattoir and transported to our laboratory in Dulbecco’s phosphate-buffered saline (DPBS; Gibco 11500-030, Grand Island, NY, USA) supplemented with 3 mg/ml bovine serum albumin (BSA; A-8022, fraction V, Sigma, St. Louis, MO, USA) maintained at 30–37°C. Serum was collected from prepubertal gilts and stored in aliquots at −20°C until they were used.
Preantral follicles and in vitro culture
The preantral follicles were collected and cultured as previously described [21, 22]. The ovaries were cut into small pieces (1–3 mm) and preantral follicles were isolated mechanically using watchmaker’s forceps in DPBS with 3 mg/ml BSA.
Preantral follicles 296 ± 8 μm in diameter were collected into four-well multidishes (Nunclon; Nunc, IL, USA) containing the collecting medium NCSU23 supplemented with 3 mg/ml BSA. The follicles were transferred from the collecting medium into the culture medium that consisted of NCSU23 supplemented with 3.5 μg/ml insulin (I5523, Sigma), 10 μg/ml transferrin (T5391, Sigma), 100 μg/ml l-ascorbic acid (A4544, Sigma), and 7.5% porcine serum. Depending on the experiment, culture medium was supplemented with 1.5 ng/ml ovine FSH (OFSH-20, 4,453 IU/mg; National Hormone and Pituitary Program of National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), Torrance, CA, USA), and/or ovine LH (OLH-26; National Hormone and Pituitary Program of NIDDK) at various concentrations. The follicles were randomly distributed to different experimental groups and cultured for 3 days in 24-well cell culture cluster plates (3524; Costar, Corning, NY, USA), with three follicles per well in 280 μl culture medium. The culture was carried out at 38.5°C in 5% CO2 in air. Culture medium was changed every day with freshly prepared medium. The diameters of follicles were measured using a stereomicroscope with an ocular scale at a magnification of ×50.
Histological investigation of follicles
Histological investigation of follicles was completed using a protocol described by Wu et al. [20]. In brief, cultured follicles were fixed in 3% paraformaldehyde and 2.5% glutaraldehyde in phosphate buffer. After washing, the fixed follicles were dehydrated in increasing concentration of ethanol. The follicles were pre-infiltrated in 1:1 mixture of 100% ethanol and Technovit 7100 together with Hardener I (Heraeus Kulzer, Germany). Infiltration was then carried out by placing the follicles into Technovit 7100 with Hardener I. For embedding, the follicles were transferred to Beem capsules in Technovit 7100 with Hardener I and II. Serial 1-μm sections were cut through the follicles, and the sections were placed on glass slides and stained with methylene blue solution.
Measurement of estradiol
Estradiol in culture media was measured using an ELISA method (Serono Diagnostics, Woking, Surrey). The inter- and intra-assay coefficients of variation were ≤5% and the sensitivity was <20 pmol/ml.
In vitro maturation of oocyte–cumulus complexes (OCCs)
Maturation of OCCs was evaluated as described by Wu et al. [21] After the culture was completed, the follicles were opened using two needles and the OCCs were flushed into DPBS supplemented with 3 mg/ml BSA. After washing three times in NCSU23 medium supplemented with 0.23 mM pyruvate and 10% porcine serum, the OCCs were cultured for 48 h in the same medium, which was further supplemented with 0.12 μg/ml OFSH, 2.5 μg/ml OLH, 20 ng/ml epidermal growth factor (EGF), 50 μg/ml L-ascorbic acid and 10–20 antral follicular shell pieces.
In vitro fertilization (IVF) and embryo culture
After maturation, oocytes were washed three times with IVF medium consisting of modified Tris-buffered medium, 2 mM caffeine and 2 mg/ml BSA (A7888, Sigma). The oocytes were then transferred to 50 μl IVF medium that was covered with warm mineral oil in a 35 × 10-mm2 tissue culture dish (Corning, Corning, NY, USA). The dishes were kept in a CO2 incubator for about 30 min until spermatozoa were added for fertilization.
Porcine sperm (SGI, Cambridge, MA, USA) was prepared as described previously [21, 23]. The prepared sperm suspension (50 μl) was added to 50 μl oocyte-containing medium (final concentration of 5 × 105 cells/ml). The oocytes were incubated with spermatozoa for 5–6 h at 38.5°C in an atmosphere of 5% CO2 in air. After insemination, the oocytes were washed three times in embryo culture medium (NCSU23 containing 3 mg/ml BSA and 0.5% (v/v) minimum essential medium amino acids), and cultured in 500 μl embryo culture medium in a 60 × 15 mm center-well organ culture dish (Becton Dickinson, Falcon, NJ, USA) until examination. At 48 and 168 h after IVF, cleavage rate and blastocyst formation were evaluated under a stereomicroscope. Fertilization rate was calculated by adding the cleaved embryos and one-cell oocytes that had been penetrated by spermatozoa. One-cell oocytes and blastocytes were fixed in 25% (v/v) acetic acid in ethanol, stained with 1% (w/v) orcein in 45% (v/v) acetic acid, and examined under a phase-contrast microscope.
Results
Role of FSH and/or LH in porcine follicular growth
Without FSH or LH (0 control), follicles grew slowly to a final size of 357 ± 5 μm, which was significantly smaller than that of follicles cultured with FSH (471 ± 7 μm) (Table 1). Antrum formation was not observed in these control cultures. With LH alone, follicles reached a final size of 366 ± 7–378 ± 8 μm but did not develop to the antral stage. The three different amounts of LH (15, 30 or 60 ng/ml) did not produce significantly different final sizes of follicles. FSH significantly enhanced follicular growth compared to LH alone or the controls. Combined LH and FSH produced rapid growth of follicles with a mean final diameter of 479 ± 10–481 ± 9 μm. However, the final follicle sizes were not significantly larger than those achieved with FSH alone.
Table 1.
Effect of FSH, LH or LH with FSH on development of porcine preantral follicles in vitro
| Variable concentration (ng/ml) | Follicle diameter(mean+SEM, μm) | Antrum formation (%) | |
|---|---|---|---|
| Start size (day 0)a | End size (day 3) | ||
| 0 (Control) | 296 ± 8 | 357 ± 5* | 0 |
| FSH 1.5 | 297 ± 9 | 471 ± 7 | 89 ± 2 |
| LH 15 | 297 ± 6 | 366 ± 7 | 0 |
| 30 | 297 ± 8 | 377 ± 6 | 0 |
| 60 | 296 ± 8 | 378 ± 8 | 0 |
| FSH 1.5 + LH | |||
| 15 | 296 ± 7 | 479 ± 10 | 86 ± 4 |
| 30 | 298 ± 6 | 485 ± 11 | 90 ± 2 |
| 60 | 297 ± 7 | 481 ± 9 | 87 ± 3 |
aThe diameters between conditions were not different on day 0.
*p < 0.05 as compared with other conditions.
Effect of FSH or combination of LH and FSH on developmental competence of oocytes from preantral follicles
After culture of preantral follicles with FSH or LH (30 ng/ml) and FSH, the capacity of the oocytes to mature, fertilize and develop into embryos was evaluated. Oocytes from preantral follicles cultured with FSH or LH and FSH reached the metaphase II stage in nearly the same proportion (52 ± 8 and 53 ± 6%, respectively) (Table 2). For fertilization, no significant difference was observed between FSH alone and LH and FSH treatment. Surprisingly, combined LH and FSH treatment of preantral follicles significantly increased the percentage (59 ± 5%) of oocytes competent to undergo cleavage to the two-cell stage after fertilization, the most profound, a significant effect was seen on oocyte competence to develop from the two-cell to the blastocyst stage (30 ± 6%), compared to FSH alone treatment (45 ± 7 and 14 ± 5%, respectively).
Table 2.
Maturation, fertilization and embryonic development of oocytes from porcine preantral follicles under the different conditions
| Conditions (ng/ml) | Maturation(%) | Fertilization (%) | 2 cells(%) | Blastocyst(%) |
|---|---|---|---|---|
| FSH 1.5 | 52 ± 8* | 54 ± 6* | 45 ± 7** | 14 ± 5** |
| FSH 1.5 + LH | ||||
| 15 | 51 ± 7 | 55 ± 7 | 51 ± 6 | 23 ± 6 |
| 30 | 53 ± 6 | 57 ± 5 | 59 ± 5 | 30 ± 6 |
| 60 | 52 ± 6 | 55 ± 6 | 56 ± 5 | 27 ± 5 |
*p > 0.05 as compared with other conditions.
**p < 0.05 as compared with other conditions.
Role of FSH or combination of LH and FSH in induction of estradiol secretion by follicles
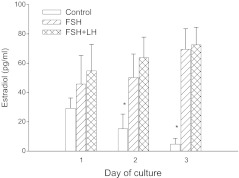
Estradiol secretion by follicles was assessed in cultures with FSH, combined LH (30 ng/ml) and FSH, or in untreated controls. Without FSH or LH (30 ng/ml) supplementation, follicles secreted progressively lower amounts of estradiol. In contrast, follicles cultured with FSH or LH and FSH supplementation, released progressively larger amounts of estradiol. Moreover, the amount of estradiol on days 2 and 3 of culture was significantly higher in follicles cultured with FSH (48.75 ± 17, 70.5 ± 14 pg/ml) or LH and FSH (63.25 ± 16, 72.5 ± 12 pg/ml) than that in with the untreated controls (16 ± 10, 5.66 ± 4 pg/ml). However, there was no significant difference in estradiol secretion between follicle culture with FSH and combined LH and FSH (Fig. 1).
Fig. 1.
Comparison of the effects of medium containing FSH, LH and FSH, or no gonadotropins (control) on production of estradiol by follicles in vitro. *Indicates P<0.05 as compared with other conditions
Histological observations of cultured follicles
Histological examination of sections of follicles cultured for 3 days with three different conditions (control, FSH, and combined LH and FSH) showed that oocytes were at the germinal vesicle (GV) stage and the cytoplasm had a homogeneous structure. In the controls, a few granulosa cells had proliferated and no antrum formation was observed (Fig. 2a). With FSH, oocytes were healthy and surrounded by one or two layers of granulosa cells (cumulus cells). Granulosa cells had proliferated, and normal organization and antrum formation was seen (Fig. 2b). With combination of LH and FSH, oocytes were surrounded by one layer of granulosa cells (cumulus cells). Granulosa cells had proliferated and showed normal organization and antrum formation (Fig. 2c).
Fig. 2.
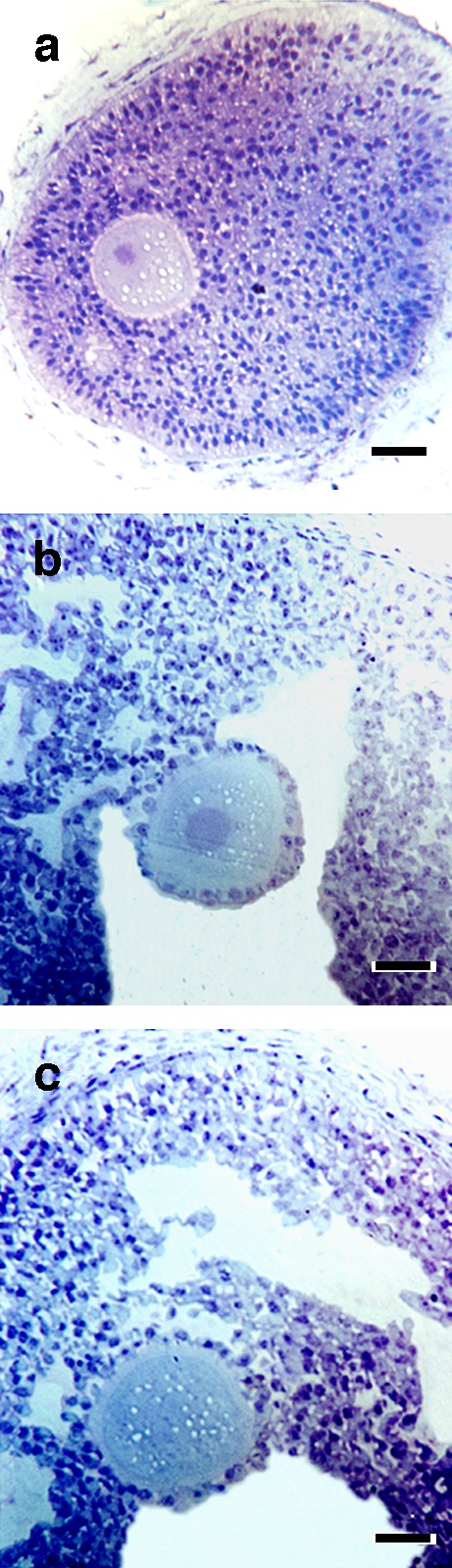
Examples of porcine follicles fixed after 3 days of culture under different conditions. a Controls (no gonadotropins); b FSH; c Combined FSH and LH (30 ng/ml). Bar 50 mm
Discussion
Processes occurring during follicular oocyte development establish the foundation for embryogenesis. Transcripts essential for early embryo development are produced, and stored in dormant from throughout oocyte growth, and are activated and translated during meiotic maturation and late embryo development. Oocyte growth and development occur in an ovarian follicular environment involving highly coordinated proliferation and differentiation of theca and granulosa cells. This coordination can be offset by exogenous gonadotropins or other factors.
This study tested the hypothesis that FSH or combined LH and FSH treatment of porcine preantral follicles improves the quality of oocytes by promoting growth and higher frequency of embryonic developmental competence. The results supported this hypothesis. When FSH was added to the culture medium, preantral follicles grew rapidly to the antral stage, half of their oocytes matured, and subsequently developed to the blastocyst stage after IVF. In contrast, without FSH, preantral follicles failed to grow to the antral stage and none became mature oocytes. These results support previous studies [9, 10, 21, 24–27] suggesting that FSH plays an important role in the development of preantral follicles in vitro. FSH promotes preantral follicle growth by inducing granulosa cell proliferation and differentiation, because granulosa cell growth accounts for the majority of this follicle expansion [28]. Moreover, FSH may indirectly drive the theca to make the androgen substrate that is converted to estrogen by the granulosa cell aromatase. FSH and secreted estrogen coordinate to induce antral formation in cultured preantral follicles. In contrast, it is considered that FSH may not be a required element for in vitro follicular development [13, 14]. Recently, Eppig et al. [29] have reported that FSH treatment of cultured oocyte–granulosa cell complexes does not significantly affect oocyte growth, oocyte competence to resume meiosis or undergo fertilization, and preimplantation development. Furthermore, treatment of the complexes with both FSH and insulin produces a highly deleterious effect on competence to undergo development from the two-cell to blastocyst stage.
As compared with FSH treatment alone, combined LH and FSH treatment of preantral follicles significantly increased the percentage of their oocytes competent to undergo cleavage to the two-cell stage after IVF. Moreover, there was a significant effect on oocyte competence to develop from the two-cell to the blastocyst stage. LH acts synergistically with FSH in promoting follicular development and function. An example of this synergic action is estrogen production via the two-cell, two-gonadotropin model, where LH drives the theca to make the androgen substrate that is converted to estrogen by granulosa cell aromatase, under the influence of FSH. Steroids might be important regulators of the essential oocyte cytoplasmic changes required for normal fertilization [30]. This synergic action is also supported by previous work [31] showing that bovine embryonic development to the blastocyst stage is significantly higher when oocytes mature with FSH combined with a high concentration of LH, rather than FSH or LH alone.
In this study, combined LH and FSH produced rapid growth of follicles and resulted in a final follicle size that was significantly larger than that achieved with LH alone. Recent immunohistochemical studies have demonstrated that LHRs and FSHRs are also expressed in cumulus cells during follicular development, suggesting that LH and FSH might interfere during the oocytes’ entire growth phase [17, 32–35]. In this study, histological observations revealed different effects of combined LH and FSH on granulose cell differentiation. The number of cells forming the cumulus mass in LH and FSH combination cultures was lower than that with FSH alone. This result is consistent with previous work from Cortvrindt et al. [19], suggesting more pronounced differentiation of the granulosa cells in the presence of LH. However, the exact mechanisms by which gonadotropins influence embryonic development are unknown. FSH and LH have previously been shown to influence the protein synthetic capacity of oocytes [36]. Recent work from Anderiesz et al. [31] has suggested that gonadotropins may improve oocyte viability by influencing oocyte protein content, stimulating synthesis in oocytes. This protein content might be essential for embryonic development. Additionally, combination of LH and FSH may be able to improve embryonic development by modulating the oocyte’s nutritional or appropriate steroid environment, or other effects which are concurrent with the LH and FSH-induced effects on follicle differentiation. However, these were not directly investigated. The result of the present study showed that combined LH and FSH significantly increased embryo developmental competence. This indicates that combination of LH and FSH can improve cytoplasmic maturation of porcine oocytes required for further development.
In summary, this study shows that FSH is essential for in vitro growth of porcine preantral follicles, antral formation, estradiol secretion, and for oocytes acquiring competence to resume meiosis and undergo fertilization and embryonic development. Perhaps for first time, combined LH and FSH treatment of porcine preantral follicles can improve the quality of oocytes by promoting growth and a higher frequency of embryonic developmental competence.
Acknowledgments
We thank the National Hormone and Pituitary Program, NIDOK, for its generous donation of FSH and LH. This work was supported and sponsored by the Shanghai Pujiang Program (to J.W.) and the Project for the Returned Overseas Chinese Scholars sponsored by the Scientific Research Foundation of the State Education Ministry (to J.W.).
References
- 1.Tisdall DJ, Watanabe K, Hudson NL, Smith P, McNatty KP. FSH receptor gene expression during ovarian follicle development in sheep. J Mol Endocrinol. 1995;15:273–281. doi: 10.1677/jme.0.0150273. [DOI] [PubMed] [Google Scholar]
- 2.Hirshfield AN. Development of follicles in the mammalian ovary. Int Rev Cyt. 1991;124:43–101. doi: 10.1016/S0074-7696(08)61524-7. [DOI] [PubMed] [Google Scholar]
- 3.Hsueh AJ, Billig H, Tsafriri A. Ovarian follicle atresia: a hormonally controlled apoptotic process. Endocr Rev. 1994;15:707–724. doi: 10.1210/er.15.6.707. [DOI] [PubMed] [Google Scholar]
- 4.Chun SY, Eisenhauer KM, Minami S, Billig H, Perlas E, Hsueh AJ. Hormonal regulation of apoptosis in early antral follicles: follicle-stimulating hormone as a major survival factor. Endocrinology. 1996;137:1447–1456. doi: 10.1210/en.137.4.1447. [DOI] [PubMed] [Google Scholar]
- 5.Hsueh AJ, Adashi EY, Jones PB, Welsh TH., Jr Hormonal regulation of the differentiation of cultured ovarian granulose cells. Endocr Rev. 1984;5:76–127. doi: 10.1210/edrv-5-1-76. [DOI] [PubMed] [Google Scholar]
- 6.Gulyas BJ, Hodgen GD, Tullner WW, Ross GT. Effects of fetal or maternal hypophysectomy on endocrine organs and body weight in infant rhesus monkeys (Macaca mulatta): with particular emphasis on oogenesis. Biol Reprod. 1977;16:216–227. doi: 10.1095/biolreprod16.2.216. [DOI] [PubMed] [Google Scholar]
- 7.Hillier SG. Current concepts the roles of follicle stimulating hormone and luteinizing hormone in folliculogenesis. Hum Reprod. 1994;9:188–191. doi: 10.1093/oxfordjournals.humrep.a138480. [DOI] [PubMed] [Google Scholar]
- 8.Halpin DM, Jones A, Fink G, Charlton HM. Postnatal ovarian follicle development in hypogonadal (hpg) and normal mice and associated changes in the hypothalamic–pituitary ovarian axis. J Reprod Fertil. 1986;77:287–296. doi: 10.1530/jrf.0.0770287. [DOI] [PubMed] [Google Scholar]
- 9.Nayudu PL, Osborn SM. Factors influencing the rate of preantral and antral growth of mouse ovarian follicles in vitro. J Reprod Fertil. 1992;95:349–362. doi: 10.1530/jrf.0.0950349. [DOI] [PubMed] [Google Scholar]
- 10.Roy SK, Greenwald GS. Hormonal requirements for the growth and differentiation of hamster preantral follicles in long-term culture. J Reprod Fertil. 1989;87:103–114. doi: 10.1530/jrf.0.0870103. [DOI] [PubMed] [Google Scholar]
- 11.Cain LS, Chatterjee A, Collins TJ. In vitro folliculogenesis of rat preantral follicles. Endocrinology. 1995;136:3369–3377. doi: 10.1210/en.136.8.3369. [DOI] [PubMed] [Google Scholar]
- 12.McGee EA, Spears N, Minami S, Hsu S, Chun SY, Billig H, et al. Preantral ovarian follicles in serum-free culture: suppression of apoptosis following activation of the cGMP pathway and stimulation of growth and differentiation by FSH. Endocrinology. 1997;138:2417–2424. doi: 10.1210/en.138.6.2417. [DOI] [PubMed] [Google Scholar]
- 13.Li R, Phillips DM, Mather JP. Activin promotes ovarian follicle development in vitro. Endocrinology. 1995;136:849–856. doi: 10.1210/en.136.3.849. [DOI] [PubMed] [Google Scholar]
- 14.Boland NI, Humpherson PG, Leese H, Gosden RG. Pattern of lactate production and steroidogenesis during growth and maturation of mouse ovarian follicles in vitro. Biol Reprod. 1993;48:798–806. doi: 10.1095/biolreprod48.4.798. [DOI] [PubMed] [Google Scholar]
- 15.Eckery DC, Moeller CL, Nett TM, Sawyer HR. Localization and quantification of binding sites for follicle-stimulating hormone, luteinizing hormone, growth hormone, and insulin-like growth factor I in sheep ovarian follicles. Biol Reprod. 1997;57:507–513. doi: 10.1095/biolreprod57.3.507. [DOI] [PubMed] [Google Scholar]
- 16.Johnson AL, Bridgham JT, Wagner B. Characterization of a chicken luteinizing hormone receptor (cLH-R) complementary deoxyribonucleic acid, and expression of cLH-R messenger ribonucleic acid in the ovary. Biol Reprod. 1996;55:304–309. doi: 10.1095/biolreprod55.2.304. [DOI] [PubMed] [Google Scholar]
- 17.Bao B, Garverick HA, Smith GW, Smith MF, Salfen BE, Youngquist RS. Changes in messenger ribonucleic acid encoding luteinizing hormone receptor, cytochrome p450-side chain cleavage, and aromatase are associated with recruitment and selection of bovine ovarian follicles. Biol Reprod. 1997;56:1158–1168. doi: 10.1095/biolreprod56.5.1158. [DOI] [PubMed] [Google Scholar]
- 18.Meduri G, Hai MTV, Jolivet A, Takemori S, Kominami S, Driancourt MA, et al. Comparison of cellular distribution of LH receptors and steroidogenic enzymes in the porcine ovary. J Endocrinol. 1996;148:435–446. doi: 10.1677/joe.0.1480435. [DOI] [PubMed] [Google Scholar]
- 19.Cortvrindt R, Hu Y, Smitz J. Recombinant luteinizing hormone as a survival and differentiation factor increases oocyte maturation in recombinant follicle stimulating hormone-supplemented mouse preantral follicle culture. Hum Reprod. 1998;13:1292–1302. doi: 10.1093/humrep/13.5.1292. [DOI] [PubMed] [Google Scholar]
- 20.Wu J, Nayudu PL, Kiesel PS, Michelmann HW. Luteinizing hormone has a stage-limited effect on preantral follicle development in vitro. Biol Reprod. 2000;63:320–327. doi: 10.1095/biolreprod63.1.320. [DOI] [PubMed] [Google Scholar]
- 21.Wu J, Emery BR, Carrell DT. In vitro growth, maturation, fertilization, and embryonic development of oocytes from porcine preantral follicles. Biol Reprod. 2001;64:375–381. doi: 10.1095/biolreprod64.1.375. [DOI] [PubMed] [Google Scholar]
- 22.Wu J, Carrell DT, Wilcox AL. Development of in vitro-matured oocytes from porcine preantral follicles following intracytoplasmic sperm injection. Biol Reprod. 2001;65:1579–1585. doi: 10.1095/biolreprod65.5.1579. [DOI] [PubMed] [Google Scholar]
- 23.Yang JG, Chen WY, Li PS. Effects of glucocorticoids on maturation of porcine oocytes and their subsequent fertilizing capacity in vitro. Biol Reprod. 1999;60:929–936. doi: 10.1095/biolreprod60.4.929. [DOI] [PubMed] [Google Scholar]
- 24.Gore-Langton RE, Daniel SAJ. Follicle-stimulating hormone and estradiol regulate antrum-like reorganization of granulosa cells in rat preantral follicle cultures. Biol Reprod. 1990;43:65–72. doi: 10.1095/biolreprod43.1.65. [DOI] [PubMed] [Google Scholar]
- 25.Hirao Y, Nagai T, Kubo M, Miyano T, Miyake M, Kato S. In vitro growth and maturation of porcine oocytes. J Reprod Fertil. 1994;100:333–339. doi: 10.1530/jrf.0.1000333. [DOI] [PubMed] [Google Scholar]
- 26.Spears N, Murray AA, Allison V, Boland NI, Gosden RG. Role of gonadotrophins and ovarian steroids in the development of mouse follicles in vitro. J Reprod Fertil. 1998;113:19–26. doi: 10.1530/jrf.0.1130019. [DOI] [PubMed] [Google Scholar]
- 27.Cortvrindt R, Smitz J, Steirteghem AC. Assessment of the need for follicle stimulating hormone in early preantral mouse follicle culture in vitro. Hum Reprod. 1997;12:759–768. doi: 10.1093/humrep/12.4.759. [DOI] [PubMed] [Google Scholar]
- 28.Gougeon A. Rate of follicular growth in the human ovary. In: Hall EV, Hillier SG, McNatty KP, Schoemaker J, editors. Follicular Maturation and Ovulation. Amsterdam, North Holland: Elsevier; 1982. pp. 155–169. [Google Scholar]
- 29.Eppig JJ, O’Brien MJ, Pendola FL, Watanabe S. Factors affecting the developmental competence of mouse oocytes grown in vitro: follicle-stimulating hormone and insulin. Biol Reprod. 1998;59:1445–1453. doi: 10.1095/biolreprod59.6.1445. [DOI] [PubMed] [Google Scholar]
- 30.Zhang X, Armstrong DT. Effects of follicle-stimulating hormone and ovarian steroids during in vitro meiotic maturation on fertilization of rat oocytes. Gamete Res. 1989;23:267–277. doi: 10.1002/mrd.1120230304. [DOI] [PubMed] [Google Scholar]
- 31.Anderiesz A, Ferraretti AP, Magli C, Fiorentino A, Fortini D, Gianaroli L, et al. Effect of recombinant human gonadotrophins on human, bovine and murine oocyte meiosis, fertilization and embryonic development in vitro. Hum Reprod. 2000;15:1140–1148. doi: 10.1093/humrep/15.5.1140. [DOI] [PubMed] [Google Scholar]
- 32.Bukovsky A, Chen TT, Wimalasena J, Caudle MR. Cellular localization of luteinizing hormone receptor immunoreactivity in the ovaries of immature, gonadotropin-primed and normal cycling rats. Biol Reprod. 1993;48:1367–1382. doi: 10.1095/biolreprod48.6.1367. [DOI] [PubMed] [Google Scholar]
- 33.Channing CP, Bae I, Stone S, Anderson LD, Edelson S, Fowler SC. Porcine granulosa and cumulus cell properties LH/hCG receptors, ability to secrete progesterone and ability to respond to LH. Mol Cell Endocrinol. 1981;22:359–370. doi: 10.1016/0303-7207(81)90044-7. [DOI] [PubMed] [Google Scholar]
- 34.Lawrence TS, Dekel N, Beers WH. Binding of human chorionic gonadotrophin by rat cumuli oophori and granulose cells: a comparative study. Endocrinology. 1980;106:1114–1118. doi: 10.1210/endo-106-4-1114. [DOI] [PubMed] [Google Scholar]
- 35.Shima K, Kitayama S, Nakano R. Gonadotrophin binding sites in human ovarian follicles and corpora lutea during the menstrual cycle. Obstet Gynecol. 1987;69:800–806. [PubMed] [Google Scholar]
- 36.Moor RM, Osborn JC, Crosby IM. Gonadotrophin-induced abnormalities in sheep oocytes after superovulation. J Reprod Fertil. 1985;74:167–172. doi: 10.1530/jrf.0.0740167. [DOI] [PubMed] [Google Scholar]