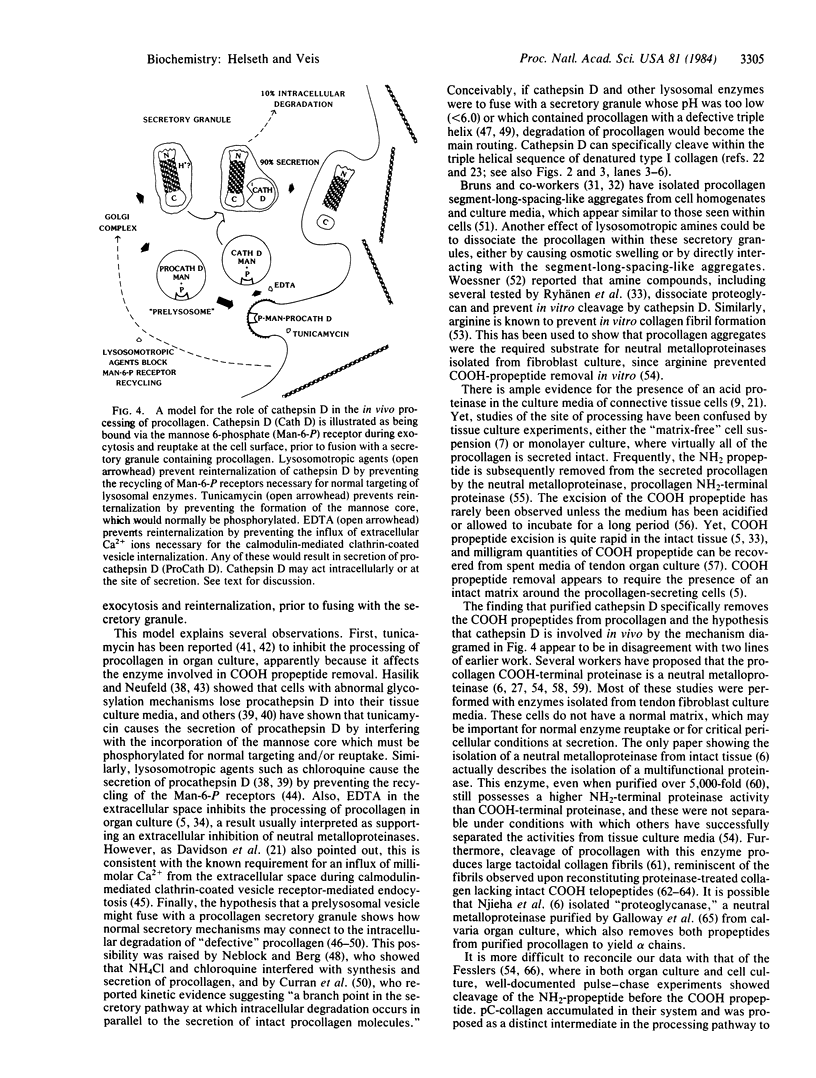
Abstract
The proteolytic removal of the extension COOH-terminal propeptide from procollagen has been examined in vitro. A crude enzyme activity was identified in a whole-chicken-embryo extract that acted at acid pH and appeared to be similar to one identified previously [Davidson, J. M., McEneany , L. S. G. & Bornstein , P. (1979) Eur. J. Biochem. 100, 551-558]. This activity was inhibitable by pepstatin but not by leupeptin, suggesting that it might be cathepsin D. Cathepsin D was purified 907-fold from chicken livers by affinity chromatography on pepstatin-aminohexyl-Sepharose 4B and was found to remove the COOH propeptides from procollagen. At pH 6.0, the site of cleavage appeared to shift from the COOH telopeptide to the COOH telopeptide/propeptide junction, based upon the difference in electrophoretic migration of the cleavage products, although determining the actual cleavage site will require end-group analysis. A model for the involvement of cathepsin D in the in vivo processing of procollagen is presented.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrett A. J., Kirschke H. Cathepsin B, Cathepsin H, and cathepsin L. Methods Enzymol. 1981;80(Pt 100):535–561. doi: 10.1016/s0076-6879(81)80043-2. [DOI] [PubMed] [Google Scholar]
- Barrett A. J. Lysosomal acid proteinase of rabbit liver. Biochem J. 1967 Aug;104(2):601–608. doi: 10.1042/bj1040601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg R. A., Schwartz M. L., Crystal R. G. Regulation of the production of secretory proteins: intracellular degradation of newly synthesized "defective" collagen. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4746–4750. doi: 10.1073/pnas.77.8.4746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienkowski R. S., Baum B. J., Crystal R. G. Fibroblasts degrade newly synthesised collagen within the cell before secretion. Nature. 1978 Nov 23;276(5686):413–416. doi: 10.1038/276413a0. [DOI] [PubMed] [Google Scholar]
- Bruns R. R., Hulmes D. J., Therrien S. F., Gross J. Procollagen segment-long-spacing crystallites: their role in collagen fibrillogenesis. Proc Natl Acad Sci U S A. 1979 Jan;76(1):313–317. doi: 10.1073/pnas.76.1.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capaldi M. J., Chapman J. A. The C-terminal extrahelical peptide of type I collagen and its role in fibrillogenesis in vitro. Biopolymers. 1982 Nov;21(11):2291–2313. doi: 10.1002/bip.360211115. [DOI] [PubMed] [Google Scholar]
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Davidson J. M., McEneany L. S., Bornstein P. Procollagen processing. Limited proteolysis of COOH-terminal extension peptides by a cathepsin-like protease secreted by tendon fibroblasts. Eur J Biochem. 1979 Oct 15;100(2):551–558. doi: 10.1111/j.1432-1033.1979.tb04201.x. [DOI] [PubMed] [Google Scholar]
- Dean R. T., Barrett A. J. Lysosomes. Essays Biochem. 1976;12:1–40. [PubMed] [Google Scholar]
- Diegelmann R. F., Peterkofsky B. Collagen biosynthesis during connective tissue development in chick embryo. Dev Biol. 1972 Jul;28(3):443–453. doi: 10.1016/0012-1606(72)90028-0. [DOI] [PubMed] [Google Scholar]
- Duksin D., Bornstein P. Impaired conversion of procollagen to collagen by fibroblasts and bone treated with tunicamycin, an inhibitor of protein glycosylation. J Biol Chem. 1977 Feb 10;252(3):955–962. [PubMed] [Google Scholar]
- Duksin D., Davidson J. M., Bornstein P. The role of glycosylation in the enzymatic conversion of procollagen to collagen: studies using tunicamycin and concanavalin A. Arch Biochem Biophys. 1978 Jan 30;185(2):326–332. doi: 10.1016/0003-9861(78)90174-1. [DOI] [PubMed] [Google Scholar]
- Erickson A. H., Conner G. E., Blobel G. Biosynthesis of a lysosomal enzyme. Partial structure of two transient and functionally distinct NH2-terminal sequences in cathepsin D. J Biol Chem. 1981 Nov 10;256(21):11224–11231. [PubMed] [Google Scholar]
- Fessler L. I., Morris N. P., Fessler J. H. Procollagen: biological scission of amino and carboxyl extension peptides. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4905–4909. doi: 10.1073/pnas.72.12.4905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fietzek P. P., Kühn K. The primary structure of collagen. Int Rev Connect Tissue Res. 1976;7:1–60. doi: 10.1016/b978-0-12-363707-9.50007-1. [DOI] [PubMed] [Google Scholar]
- Fleischmajer R., Olsen B. R., Timpl R., Perlish J. S., Lovelace O. Collagen fibril formation during embryogenesis. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3354–3358. doi: 10.1073/pnas.80.11.3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischmajer R., Timpl R., Tuderman L., Raisher L., Wiestner M., Perlish J. S., Graves P. N. Ultrastructural identification of extension aminopropeptides of type I and III collagens in human skin. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7360–7364. doi: 10.1073/pnas.78.12.7360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller F., Boedtker H. Sequence determination and analysis of the 3' region of chicken pro-alpha 1(I) and pro-alpha 2(I) collagen messenger ribonucleic acids including the carboxy-terminal propeptide sequences. Biochemistry. 1981 Feb 17;20(4):996–1006. doi: 10.1021/bi00507a054. [DOI] [PubMed] [Google Scholar]
- GROSS J., KIRK D. The heat precipitation of collagen from neutral salt solutions: some rate-regulating factors. J Biol Chem. 1958 Aug;233(2):355–360. [PubMed] [Google Scholar]
- Galloway W. A., Murphy G., Sandy J. D., Gavrilovic J., Cawston T. E., Reynolds J. J. Purification and characterization of a rabbit bone metalloproteinase that degrades proteoglycan and other connective-tissue components. Biochem J. 1983 Mar 1;209(3):741–752. doi: 10.1042/bj2090741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg B., Taubman M. B., Radin A. Procollagen peptidase: its mode of action on the native substrate. Cell. 1975 Jan;4(1):45–50. doi: 10.1016/0092-8674(75)90132-4. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Noriega A., Grubb J. H., Talkad V., Sly W. S. Chloroquine inhibits lysosomal enzyme pinocytosis and enhances lysosomal enzyme secretion by impairing receptor recycling. J Cell Biol. 1980 Jun;85(3):839–852. doi: 10.1083/jcb.85.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasilik A., Neufeld E. F. Biosynthesis of lysosomal enzymes in fibroblasts. Phosphorylation of mannose residues. J Biol Chem. 1980 May 25;255(10):4946–4950. [PubMed] [Google Scholar]
- Hasilik A., Neufeld E. F. Biosynthesis of lysosomal enzymes in fibroblasts. Synthesis as precursors of higher molecular weight. J Biol Chem. 1980 May 25;255(10):4937–4945. [PubMed] [Google Scholar]
- Hoffmann H. P., Olsen B. R., Chen H. T., Prockop D. J. Segment-long-spacing aggregates and isolation of COOH-terminal peptides from type I procollagen. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4304–4308. doi: 10.1073/pnas.73.12.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J. S., Huang S. S., Tang J. Cathepsin D isozymes from porcine spleens. Large scale purification and polypeptide chain arrangements. J Biol Chem. 1979 Nov 25;254(22):11405–11417. [PubMed] [Google Scholar]
- Hulmes D. J., Bruns R. R., Gross J. On the state of aggregation of newly secreted procollagen. Proc Natl Acad Sci U S A. 1983 Jan;80(2):388–392. doi: 10.1073/pnas.80.2.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine G. B., Blumsom N. L., Elmore D. T. The kinetics of hydrolysis of some synthetic substrates containing neutral hydrophilic groups by pig pepsin and chicken liver cathepsin D. Biochem J. 1983 Apr 1;211(1):237–242. doi: 10.1042/bj2110237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler E., Goldberg B. A method for assaying the activity of the endopeptidase which excises the nonhelical carboxyterminal extensions from type I procollagen. Anal Biochem. 1978 Jun 1;86(2):463–469. doi: 10.1016/0003-2697(78)90770-4. [DOI] [PubMed] [Google Scholar]
- Knight C. G., Barrett A. J. Interaction of human cathepsin D with the inhibitor pepstatin. Biochem J. 1976 Apr 1;155(1):117–125. doi: 10.1042/bj1550117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lapiere C. M., Nusgens B. Polymerization of procollagen in vitro. Biochim Biophys Acta. 1974 Apr 11;342(2):237–246. doi: 10.1016/0005-2795(74)90078-6. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Leblond C. P., Wright G. M. Steps in the elaboration of collagen by odontoblasts and osteoblasts. Methods Cell Biol. 1981;23:167–189. doi: 10.1016/s0091-679x(08)61498-3. [DOI] [PubMed] [Google Scholar]
- Leibovich S. J., Weiss J. B. Electron microscope studies of the effects of endo- and exopeptidase digestion on tropocollagen. A novel concept of the role of terminal regions in fibrillogenesis. Biochim Biophys Acta. 1970 Sep 29;214(3):445–454. doi: 10.1016/0005-2795(70)90303-x. [DOI] [PubMed] [Google Scholar]
- Leung M. K., Fessler L. I., Greenberg D. B., Fessler J. H. Separate amino and carboxyl procollagen peptidases in chick embryo tendon. J Biol Chem. 1979 Jan 10;254(1):224–232. [PubMed] [Google Scholar]
- Miyahara M., Bruckner P., Helle O., Prockop D. J. Aggregation of a type I collagen precursor containing N-terminal propeptides. Coll Relat Res. 1983 Jul;3(4):279–293. doi: 10.1016/s0174-173x(83)80010-7. [DOI] [PubMed] [Google Scholar]
- Miyahara M., Njieha F. K., Prockop D. J. Formation of collagen fibrils in vitro by cleavage of procollagen with procollagen proteinases. J Biol Chem. 1982 Jul 25;257(14):8442–8448. [PubMed] [Google Scholar]
- Neblock D. S., Berg R. A. Lysosomotropic agents ammonium chloride and chloroquine inhibit both the synthesis and secretion of procollagen by freshly isolated embryonic chick tendon cells. Biochem Biophys Res Commun. 1982 Apr 14;105(3):902–908. doi: 10.1016/0006-291x(82)91055-5. [DOI] [PubMed] [Google Scholar]
- Njieha F. K., Morikawa T., Tuderman L., Prockop D. J. Partial purification of a procollagen C-proteinase. Inhibition by synthetic peptides and sequential cleavage of type I procollagen. Biochemistry. 1982 Feb 16;21(4):757–764. doi: 10.1021/bi00533a028. [DOI] [PubMed] [Google Scholar]
- Offermann M. K., Chlebowski J. F., Bond J. S. Action of cathepsin D on fructose-1,6-bisphosphate aldolase. Biochem J. 1983 Jun 1;211(3):529–534. doi: 10.1042/bj2110529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen B. R., Guzman N. A., Engel J., Condit C., Aase S. Purification and characterization of a peptide from the carboxy-terminal region of chick tendon procollagen type I. Biochemistry. 1977 Jun 28;16(13):3030–3036. doi: 10.1021/bi00632a034. [DOI] [PubMed] [Google Scholar]
- Prockop D. J., Tuderman L. Posttranslational enzymes in the biosynthesis of collagen: extracellular enzymes. Methods Enzymol. 1982;82(Pt A):305–319. doi: 10.1016/0076-6879(82)82068-5. [DOI] [PubMed] [Google Scholar]
- Rosenfeld M. G., Kreibich G., Popov D., Kato K., Sabatini D. D. Biosynthesis of lysosomal hydrolases: their synthesis in bound polysomes and the role of co- and post-translational processing in determining their subcellular distribution. J Cell Biol. 1982 Apr;93(1):135–143. doi: 10.1083/jcb.93.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryhänen L., Tan E. M., Rantala-Ryhänen S., Uitto J. Conversion of type II procollagen to collagen in vitro: removal of the carboxy-terminal extension is inhibited by several naturally occurring amino acids, polyamines, and structurally related compounds. Arch Biochem Biophys. 1982 Apr 15;215(1):230–236. doi: 10.1016/0003-9861(82)90299-5. [DOI] [PubMed] [Google Scholar]
- Sahagian G. G., Distler J., Jourdian G. W. Characterization of a membrane-associated receptor from bovine liver that binds phosphomannosyl residues of bovine testicular beta-galactosidase. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4289–4293. doi: 10.1073/pnas.78.7.4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salisbury J. L., Condeelis J. S., Satir P. Receptor-mediated endocytosis: machinery and regulation of the clathrin-coated vesicle pathway. Int Rev Exp Pathol. 1983;24:1–62. [PubMed] [Google Scholar]
- Scott P. G., Pearson C. H. Cathepsin D: cleavage of soluble collagen and crosslinked peptides. FEBS Lett. 1978 Apr 1;88(1):41–45. doi: 10.1016/0014-5793(78)80602-4. [DOI] [PubMed] [Google Scholar]
- Scott P. G., Pearson H. Cathepsin D: specificity of peptide-bond cleavage in type-I collagen and effects on type-III collagen and procollagen. Eur J Biochem. 1981;114(1):59–62. doi: 10.1111/j.1432-1033.1981.tb06172.x. [DOI] [PubMed] [Google Scholar]
- Skinner M. K., Griswold M. D. Fluorographic detection of radioactivity in polyacrylamide gels with 2,5-diphenyloxazole in acetic acid and its comparison with existing procedures. Biochem J. 1983 Jan 1;209(1):281–284. doi: 10.1042/bj2090281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sly W. S., Fischer H. D., Gonzalez-Noriega A., Grubb J. H., Natowicz M. Role of the 6-phosphomannosyl-enzyme receptor in intracellular transport and adsorptive pinocytosis of lysosomal enzymes. Methods Cell Biol. 1981;23:191–214. doi: 10.1016/s0091-679x(08)61499-5. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Tang J. Cathepsin D from porcine and bovine spleen. Methods Enzymol. 1981;80(Pt 100):565–581. doi: 10.1016/s0076-6879(81)80045-6. [DOI] [PubMed] [Google Scholar]
- Taubman M. B., Goldberg B. The processing of procollagen in cultures of human and mouse fibroblasts. Arch Biochem Biophys. 1976 Apr;173(2):490–494. doi: 10.1016/0003-9861(76)90286-1. [DOI] [PubMed] [Google Scholar]
- Tuderman L., Kivirikko K. I., Prockop D. J. Partial purification and characterization of a neutral protease which cleaves the N-terminal propeptides from procollagen. Biochemistry. 1978 Jul 25;17(15):2948–2954. doi: 10.1021/bi00608a002. [DOI] [PubMed] [Google Scholar]
- Uitto J., Allan R. E., Polak K. L. Conversion of type II procollagen to collagen. Extracellular removal of the amino-terminal and carboxy-terminal extensions without a preferential sequence. Eur J Biochem. 1979 Aug 15;99(1):97–103. doi: 10.1111/j.1432-1033.1979.tb13236.x. [DOI] [PubMed] [Google Scholar]
- Uitto J., Lichtenstein J. R. Removal of amino-terminal and carboxy-terminal extension peptides from procollagen during synthesis of chick embryo tendon collagen. Biochem Biophys Res Commun. 1976 Jul 12;71(1):60–67. doi: 10.1016/0006-291x(76)90249-7. [DOI] [PubMed] [Google Scholar]
- Veis A., Anesey J., Yuan L., Levy S. J. Evidence for an amino-terminal extension in high-molecular-weight collagens from mature bovine skin. Proc Natl Acad Sci U S A. 1973 May;70(5):1464–1467. doi: 10.1073/pnas.70.5.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeks J. G., Halme J., Woessner J. F., Jr Extraction of collagenase from the involuting rat uterus. Biochim Biophys Acta. 1976 Aug 12;445(1):205–214. doi: 10.1016/0005-2744(76)90173-x. [DOI] [PubMed] [Google Scholar]
- Weiss J. B. Enzymic degradation of collagen. Int Rev Connect Tissue Res. 1976;7:101–157. doi: 10.1016/b978-0-12-363707-9.50009-5. [DOI] [PubMed] [Google Scholar]
- Woessner J. F., Jr Purification of cathepsin D from cartilage and uterus and its action on the protein-polysaccharide complex of cartilage. J Biol Chem. 1973 Mar 10;248(5):1634–1642. [PubMed] [Google Scholar]