Abstract
Purpose
Prediction of IVF outcome on the first days of ovarian stimulation has focused clinical research for many years. The aim of this work is to predict the probability of pregnancy on the fourth day of ovarian stimulation for IVF cycle, using parameters usually determined in this stage—estradiol, antral follicle count—together with parameters determined previously: FSH on the third day of cycle and women age.
Materials and methods
One hundred and ten patients with primary infertility due to a tubal factor were recruited to participate in a prospective study. FSH was determined on the third day of spontaneous cycle. Antral follicles and estradiol were measured on the fourth day of ovarian stimulation. After oocyte pick-up, quality and quantity of oocytes and embryos and pregnancy rates were assessed.
Results
In stepwise multiple logistic regression the variables with better predictiveness over pregnancy are: antral follicles count, estradiol and woman age. The logistic regression analyses demonstrate that the capacity of the model that uses these variables to predict pregnancy is 75%, with a positive predictive value of 69% and a negative predictive value of 80%.
Conclusions
On the fourth day of ovarian stimulation of IVF cycles, the variables with highest predictiveness are: antral follicle count, estradiol and women age. When these variables are included in a model of prediction, the capacity to predict pregnancy is 75%.
Keywords: Estradiol, Antral follicle count, IVF outcome, Pregnancy
Introduction
In IVF cycles the main priorities are to obtain a sufficient number of mature oocytes, quality embryos and finally obtain a pregnancy. Prediction of IVF outcome has focused clinical research for many years. Many tests have been suggested to predict the probability of pregnancy in IVF cycles, such as FSH [1] and estradiol [2] on the third day of the cycle, FSH levels after treatment with clomiphene [3], levels of inhibin A and inhibin B [4], number of ovarian antral follicles [5, 6], antimullerian hormone [7] and the influence of women age [8]. These tests have been described to have an acceptable predictive power for IVF outcome, but reports with conflicting results have been described [9, 10]. The majority of these tests were conducted in an isolated manner and prior to ovarian stimulation. Some papers have shown that the power of prediction of these tests increase when a combination of them is used. Thus, combination of day 3 FSH, estradiol and age has been used to improve the prediction of live birth in IVF cycles [11]. Few works have analysed the capacity of prediction over pregnancy of these variables when they are used jointly. The aim of this study is to predict the probabilities of pregnancy in the initial stages of IVF cycle (fourth day of ovarian stimulation) using parameters usually determined in this stage—estradiol and antral follicle count—together with parameters determined previously—FSH on the third day of cycle—and women age.
Materials and methods
Patients
One hundred and ten patients under the age of 38 diagnosed of primary infertility due to a tubal factor who initiated IVF cycles between January 2005 and June 2006 were included in the study. The diagnosis of tubal obstruction was performed by hysterosalpingography and laparoscopy. All patients were in their first IVF attempt, and they could enter in the study only once. Women with history of genetic risks, pregnancy loss or with indication for preimplantation genetic diagnosis were excluded from the study. In all patients, measurements of basal FSH, LH, estradiol (E2), prolactin and TSH were made on the third day of spontaneous cycle within 6 months before undergoing an assisted reproductive technology (ART) program.
All patients met the following inclusion criteria: age <38 years, regular menstrual cycles ranging from 24 to 32 days, normal basal serum FSH (<12 mIU/ml), LH (<10 mIU/ml), E2 (<60 pg/ml), Prolactin (<21 ng/ml) and TSH (<4 μU/ml) levels, body mass index (BMI) < 30 kg/m2 and no uterine or ovarian abnormalities assessed by vaginal ultrasound. All semen specimens were within normal values for concentration, motility and normal sperm forms. Patients gave an informed consent to participate in the study.
Experimental design
Patients initiated down regulation with a GnRH analogue (GnRH-a) in luteal phase, followed, after menstruation, by stimulation with recombinant FSH (FSHr) and recombinant LH (LHr). On the fourth day of stimulation with FSHr and LHr, E2 levels were measured and the number of antral follicles was determined. Then, measurements of E2 levels and follicle number and size were repeated every 24–48 h depending on individual response until oocyte recovery.
Transvaginal ultrasonography was performed on the fourth day of ovarian stimulation to measure the number of antral follicles (AFC), defined as number of follicles <10 mm in all diameters. Round or oval echo-free structures in both ovaries were regarded as follicles and were counted and measured as such. Ultrasonography was performed using a Toshiba Capasse unit (Toshiba Corporation, Tokyo, Japan) equipped with a 7 MHz vaginal transducer. With this unit the limit of sensitivity was 2 mm. All ultrasound scans were performed by a single researcher.
The maturational status of the oocytes retrieved was assessed according to published criteria [12]. Embryos were evaluated on the third day after fertilization and classified as follows (Veeck grades): grade 1: perfectly symmetrical with no fragmentation; grade 2: perfectly symmetrical with slight fragmentation (<20% fragmentation of the total embryonic volume); grade 3: uneven blastomeres with no fragmentation; grade 4: uneven blastomeres with gross fragmentation (>20% fragments). Embryos of Veeck grades 1 or 2 were considered of high quality.
Ovarian stimulation and IVF procedure
In all patients pituitary desensitisation was performed with leuprolide acetate (Procrin; Abbot Laboratories, Madrid Spain). Down regulation was initiated after 21 days of menstruation (1 mg daily SC), and stimulation with recombinant follicle-stimulating hormone (FSHr; Puregon, NV Organon, Oss, The Netherlands) and recombinant luteinizing hormone (LHr; Luveris, Serono, Spain) started at fourth day of the cycle. When stimulation started, the dose of leuprolide acetate was reduced to 0.5 mg/day. The median starting dose was 300 units for FSHr and 75 units for LHr. The doses of both were adjusted according to individual response. When one or more follicles reached 18 mm, recombinant human chorionic gonadotropin (hCGr; Ovitrelle 250 μg, Serono, Spain) was administered. Oocyte retrieval was carried out 34–36 h after hCG administration by transvaginal ultrasound-guided puncture of follicles. Oocytes were retrieved in modified human tubal fluid (MHTF, Irvine Scientific, Irvine CA), located and placed into P1 (Irvine Scientific) with 0.5% of human serum albumin (HSA; Irvine Scientific) until insemination. All cultures were in humidified atmosphere with 5% CO2. Oocytes were inseminated in microdrops (100 μl) of HTF and HSA for 2 h. Following insemination, oocytes were moved into microdrops (30 μl) of G1.2 (Vitrolife, Gothenburg, Sweden) under oil. Oocytes were evaluated for fertilization 16–19 h post-insemination. Subsequent evaluation of embryos was performed in day 2 and day 3. Embryos were transferred on day 3 after oocyte retrieval. The number of embryos transferred was 2, except in six patients for whom only one embryo could be transferred (no more embryos were obtained in these patients) and five patients with poor embryo quality for whom only three embryos were obtained. For these five patients, the three embryos were transferred. The luteal phase was sustained by intravaginal progesterone 600 mg daily, in three separate doses, starting 1 day after oocyte retrieval and continuing for 14 days (Utrogestan, Paris, France).
In the patients included in the study, the management of cycle was not changed in function of their predicted probability of pregnancy.
Laboratory assays
The FSH, LH, E2, Prolactin and TSH assays were performed by micro particle enzyme immunoassay technology (MEIA) with the AxSYM immunoanalyzer (Abbot Laboratories, Abbot Park, IL). The interassay and intraassay coefficients of variation were 5.4 and 5% for FSH, 6 and 5.2% for LH, 5.8 and 5.1% for E2, 5.9 and 5.1% for prolactin and 5.7 and 5% for TSH, respectively.
Statistical methods
Data were analysed with SPSS (SPSS Inc. Chicago, IL). The normal distribution of variables was checked before use of parametric statistical tests. Descriptive analyses were first conducted in the total eligible cohort. Linear and non-parametric correlation (Kendall’s tau-b and Spearman) and factor analyses were used to determine the association and relationship between variables.
Univariate and multivariate logistic regression analyses were used to study the potential prognostic variables (antral follicles count, estradiol, FSH in third day of cycle and age) to determine which of the analysed variables were the better predictors of pregnancy. The model of logistic regression was obtained by stepwise method. Forward as well as backward selection of parameters was applied, using P < 0.05 for entry and P > 0.1 for removal.
Receiver operating characteristic (ROC) curves of the model and analysed parameters were constructed and area under the curves was used for the assessment and comparison of the effectiveness to predict pregnancy.
To determine the influence of variables evaluated over pregnancy rates, oocytes and embryos, we used the following variables:
Pregnancy: when there were gestational sacs seen on ultrasonography.
Global embryo quality (GEQ): the sum of scores of all embryos of each patient in the third day after fertilization. In order to calculate GEQ, embryos were scored according to their quality, thus embryos of grade 1, 2, 3 and 4 were scored 2.5, 2, 1.5 and 1, respectively, as it has been suggested by others [13].
Mean quality for embryo (MEQ): the result of GEQ divided by the number of embryos of each patient. MEQ indicates the mean quality score of the embryos.
Antral Follicle Count (AFC): number of antral follicles observed in ecography on the fourth day of ovarian stimulation.
Estradiol on the fourth day (ES4d): level of estradiol on the fourth day of ovarian stimulation.
Estradiol concentrations per follicle on the fourth day (ES4d/AFC): levels of estradiol per antral follicle on the fourth day of ovarian stimulation.
Oocytes retrieved (OR): oocytes retrieved on the day of ovary pick-up.
Number of embryos (EMB): total number of embryos obtained after IVF procedure.
Quality and quantity of embryos were assessed with the variables: EMB, GEQ and MEQ. In the case of oocytes, OR was the selected variable to study the relation with the analysed variables.
Results
Patient’s characteristics and cycle outcome are shown in Table 1.
Table 1.
Patient’s descriptive statistics
| Patient’s characteristics and cycle outcome | |
|---|---|
| Number of subjects | n = 110 |
| Age (range) | 26–37 |
| Duration of infertility (years) | 1.6 ± 0.52 |
| Duration of infertility (months) | 18.7 ± 5.02 |
| FSH 3 day (mIU/ml) | 7.11 ± 2.19 |
| E2 day of puncture (pg/ml) | 1,817 ± 954 |
| Days of stimulation | 10.7 ± 1.4 |
| Recombinant FSH dose (IU) | 2,541 ± 435 |
| Ampules of FSH | 33.8 ± 5.8 |
| Recombinant LH dose (IU) | 747 ± 47 |
| Ampules of LH | 9.96 ± 0.62 |
| Number of antral follicles | 8.2 ± 3.4 |
| Oocytes retrieved | 8.9 ± 6.2 |
| Embryos transferred | 1.99 ± 0.3 |
| Cancelled cycles | 8 (7%) |
| Pregnancy cycles | 46 (42%) |
| Non-pregnancy cycles | 56 (51%) |
Values are mean ± SD.
Table 2 offers the correlations of predictive variables in relation to pregnancy and oocyte and embryo variables. For the variables pregnancy, embryos (EMB and GEQ) and OR the highest correlations with the predictive variables were obtained for AFC and ES4, which showed a positive correlation. Negative correlations were observed for FSHt and age. The variable MEQ showed positive correlation with age. The highest level of correlation in analysed variables was between GEQ and EMB. The table shows Pearson’s correlation coefficients and significance, and the levels of significance of non-parametric correlations (Kendall’s tau-b and Spearman).
Table 2.
Correlations of predictive variables on the fourth day of ovarian stimulation, in relation to oocyte, embryo and pregnancy variables
| Pregnancy | ES4d | AFC | AGE | FSHt | ES4d/AFC | GEQ | EMB | MEQ | OR | |
|---|---|---|---|---|---|---|---|---|---|---|
| Pregnancy | 1 | 0.421b | 0.470b | −0.309b | −0.324b | 0.168d | 0.349b | 0.300b | 0.134 | 0.381b |
| . | 0.000 | 0.000 | 0.002 | 0.001 | 0.095 | 0.000 | 0.002 | 0.185 | 0.000 | |
| ES4d | 0.421b | 1 | 0.671b | −0.033 | −0.475b | 0.699b | 0.604b | 0.562b | 0.054 | 0.512b |
| 0.000 | . | 0.000 | 0.748 | 0.000 | 0.000 | 0.000 | 0.000 | 0.593 | 0.000 | |
| AFC | 0.470b | 0.671b | 1 | −0.275b | −0.572b | 0.083d | 0.614b | 0.625b | −0.031 | 0.779b |
| 0.000 | 0.000 | . | 0.006 | 0.000 | 0.409 | 0.000 | 0.000 | 0.760 | 0.000 | |
| AGE | −0.309b | −0.033 | −0.275b | 1 | 0.333b | 0.169 | −0.131 | −0.213a | 0.337b | −0.411b |
| 0.002 | 0.748 | 0.006 | . | 0.001 | 0.092 | 0.193 | 0.034 | 0.001 | 0.000 | |
| FSHt | −0.324b | −0.475b | −0.572b | 0.333b | 1 | −0.153d | −0.385b | −0.404b | 0.098 | −0.506b |
| 0.001 | 0.000 | 0.000 | 0.001 | . | 0.129 | 0.000 | 0.000 | 0.331 | 0.000 | |
| ES4d/AFC | 0.168d | 0.699b | 0.083d | 0.169 | −0.153d | 1 | 0.201a | 0.155c | 0.003 | 0.079d |
| 0.095 | 0.000 | 0.409 | 0.092 | 0.129 | . | 0.045 | 0.124 | 0.973 | 0.433 | |
| GEQ | 0.349b | 0.604b | 0.614b | −0.131 | −0.385b | 0.201a | 1 | 0.966b | 0.032 | 0.703b |
| 0.000 | 0.000 | 0.000 | 0.193 | 0.000 | 0.045 | . | 0.000 | 0.753 | 0.000 | |
| EMB | 0.300b | 0.562b | 0.625b | −0.213a | −0.404b | 0.155c | 0.966b | 1 | −0.194c | 0.753b |
| 0.002 | 0.000 | 0.000 | 0.034 | 0.000 | 0.124 | 0.000 | . | 0.053 | 0.000 | |
| MEQ | 0.134 | 0.054 | −0.031 | 0.337b | 0.098 | 0.003 | 0.032 | −0.194c | 1 | −0.206a |
| 0.185 | 0.593 | 0.760 | 0.001 | 0.331 | 0.973 | 0.753 | 0.053 | . | 0.039 | |
| OR | 0.381b | 0.512b | 0.779b | −0.411b | −0.506b | 0.079d | 0.703b | 0.753b | −0.206a | 1 |
| 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.433 | 0.000 | 0.000 | 0.039 | . |
Pearson’s correlation coefficients and significance.
ES4D Levels of estradiol on the fourth day of ovarian stimulation; AFC antral follicle count; FSHt FSH in third day of cycle; GEQ global embryo quality; EMB number of embryos; MEQ mean quality for embryo; OR oocytes retrieved.
aPearson’s correlation is significant at the 0.05 level and non-parametric correlation is significant at the 0.01 level.
bCorrelation is significant at the 0.01 level (two-tailed) in Pearson’s and in non-parametric correlations.
cNon-parametric correlations are significant at the 0.01 level.
dNon-parametric correlations are significant at the 0.05 level.
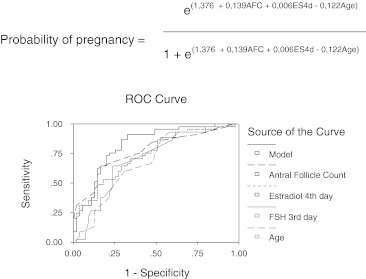
Figure 1 shows the results of logistic regression and the equation of the logistic regression model to estimate the probability of pregnancy and the receiver operating characteristic (ROC) curves of the analysed variables.
Fig. 1.
Results of logistic regression, equation of logistic regression model to estimate the probability of pregnancy and ROC curves of analyzed variables
In logistic regression analyses significant predictors on the fourth day of IVF cycle over pregnancy are: antral follicles count, estradiol, FSH in third day of cycle and age. In forward and backward stepwise multiple logistic regression the variables that are included in the predictive model to predict pregnancy are: antral follicle count, estradiol and age.
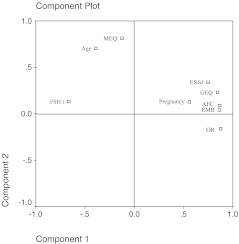
Figure 2 shows a plotter of factorial analyses of variables and the interrelation between the analysed variables. Component 1 shows the relation with ovarian responsiveness, and in the positive extreme we can observe the variables related with good responsiveness (ES4d, AFC, EMB, GEQ and OR) and pregnancy. FSHt and age show a negative relation with this component and are related with poor ovarian response. In component 2 we can observe a positive relation with MEQ and age, and a negative relation with number of oocytes retrieved. This component is mainly related with age and MEQ. This graphic shows the close positive relation existing between ES4d, AFC, GEQ, EMB, OR and pregnancy. Age also shows a positive relation with MEQ.
Fig. 2.
Plotter of factorial analyses of variables. ES4D, levels of estradiol on the fourth day of ovarian stimulation; AFC, antral follicle count; FSHt, FSH in third day of cycle; GEQ, global embryo quality; EMB, number of embryos; MEQ, mean quality for embryo; OR, oocytes retrieved
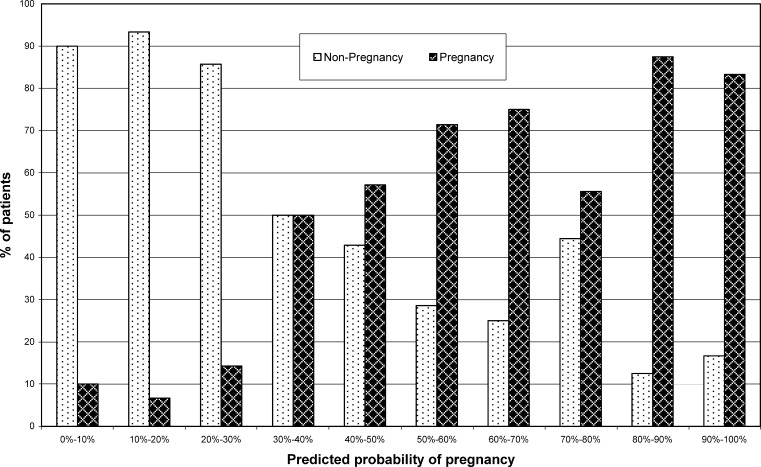
Figure 3 shows the pregnant and non-pregnant cycles related to the predicted probability of pregnancy using the logistic regression model.
Fig. 3.
The actual pregnant and non-pregnant cycles compared to the predicted probability of pregnancy using the logistic regression model
Discussion
The main aim in IVF cycles is to obtain a pregnancy. The effectiveness of IVF treatments is near 35–40% in pregnancy rates. This percentage has only slightly increased in the last years. Early identification of factors that will predict IVF outcome is, therefore, of great value for adequate medical decisions and procedures. For a long time, clinical research has been focused on the evaluation of predictor factors like FSH [1], estradiol [2], inhibin A, inhibin B [4], age and antral follicles count [5] before IVF cycle started and, in most cases, with the objective to predict patients with risk to cancel the treatment due to a low response. Few works have analysed the possibility to predict IVF outcome in initial steps of controlled ovarian stimulation cycles. The aim of this study is to predict the probabilities of pregnancy in initial steps of IVF cycle—fourth day of ovarian stimulation—using variables that seem to influence IVF outcome and that are usually determined in this stage: woman age, FSH in third day of cycle, antral follicle count and estradiol levels.
Our data shows a close positive association between pregnancy and AFC and ES4d, and a negative association between pregnancy and FSHt and age. AFC, ES4d, FSHt and age have high levels of correlation with pregnancy. Later in the IVF process, other variables present significant levels of correlation too: OR, GEQ and EMB (Table 2).
Like others works [11], the power of prediction increases when a model that includes several variables is used. Thus, with the use of logistic regression to determine the variables and their associations that are better predictors of pregnancy, we obtain that the variables that are included in the equation of the logistic regression model to estimate the probability of pregnancy are: AFC, ES4D and age (Fig. 1). With the inclusion of these variables in the model the negative predictive value over pregnancy is 80% and the positive predictive value is near 70% (Table 3).
Table 3.
Variables in the probability of pregnancy
| Model and parameters | Chi-square | Degrees of freedom | Significance | Area under ROC curve | Correct predictions (%) | Positive predictive value (%) | Negative predictive value (%) |
|---|---|---|---|---|---|---|---|
| Model | 33.34 | 3 | p < 0,001 | 0.825 | 75 | 68.9 | 80.0 |
| Antral follicle count | 24.37 | 1 | p < 0,001 | 0.771 | 75 | 62.2 | 85.5 |
| Estradiol fourth day | 19.48 | 1 | p < 0,001 | 0.741 | 67 | 48.9 | 81.8 |
| FSH third day | 11.20 | 1 | p = 0,001 | 0.705 | 63 | 46.7 | 76.4 |
| Age | 10.02 | 1 | p = 0,002 | 0.680 | 63 | 46.7 | 76.4 |
FSHt and age have significant and comparable levels of correlation with pregnancy, but only age is included in the equation of prediction. The reason for this is that in logistic regression, the inclusion of any variable in the model of prediction is related to the increase in capacity of prediction that produces that inclusion, and it’s not dependent to the level of correlation of that variable. The entrance of FSHt in the model of prediction is evaluated together with other variables—AFC, Age, ES4d and ES4d/AFC—and in this situation the inclusion of FSH did not increase the capacity of prediction.
The association, in an isolated manner, between the analysed variables and pregnancy has been described previously. Thus, the number of antral follicles has been described as a powerful predictor of number of oocytes retrieved [5, 6]. Our data suggests that AFC related to fourth day of ovarian stimulation, has a significant relation with number of oocytes retrieved, number of embryos obtained, GEQ and pregnancy. The higher the number of antral follicles in one patient, the higher the number of oocytes that will be recovered and more embryos will be obtained, and this patient has a higher probability to obtain a pregnancy. These results are in concordance with the fact that the number of antral follicles, originated from the cohort of growing follicles, reflects the size of the pool of resting follicles and ovarian reserve [14]. The number of antral follicles is, among the analysed variables, the one with the strongest prediction power over pregnancy.
Estradiol presents a high correlation with number and quality of embryos obtained. Patients with high levels of ES4d are more likely to obtain more and better embryos than patients with lower levels of ES4d. These results are in concordance with the critical role of estradiol in follicular growth. Thus, the early appearance of estrogen within the follicle allows the follicle to respond to relatively low concentrations of FSH. The dominance of estradiol and FSH in follicular fluid is essential for sustained accumulation of granulosa cells, continued follicular growth and estradiol production [15].
Due to the capacity of prediction over pregnancy of ES4d and AFC, the relation between estradiol concentrations per follicle (ES4d/AFC) and pregnancy was analysed. In our results this variable trends to have a positive correlation with pregnancy (Table 2) with significant levels of correlation in non-parametric tests, but it doesn’t reach significative level in parametric tests. This fact could be explained for many reasons. One is the number of patients studied. It is possible that with a major number of patients the levels of correlation obtained would be significative. Another reason is that ES4d/AFC presents a complex relation with pregnancy. Thus, it seems necessary that certain level of estradiol per follicle exits for to obtain a pregnancy. Patients with very high or very low levels of estradiol per follicle have diminished their probabilities to get pregnant. This fact could be explained by the critical role of follicular estradiol concentration over oocyte cytoplasmic maturation. Thus, elevated or diminished levels of estradiol could produced impaired maturation of oocyte cytoplasm [16] and decrease the probabilities to obtain a pregnancy [17].
The value of patient age in predicting performance in assisted reproductive technologies is well established. Thus, fecundity in natural and stimulated cycles declines with maternal age. This decline is initiated in the late 20s and is more abrupt in the late 30s [8]. With increasing age, ovarian reserve diminishes and spontaneous fecundity rate as well as success rates in IVF programs decline. Older women produce less oocytes and have lower implantation rates. In this work, maternal age is an important factor for the prediction of number and quality of oocytes and embryos, especially over the number of oocytes retrieved. But as in others works, its predictive value is limited if considered as an isolated factor [18].
In our patients the levels of FSH on the third day are related to quantity of oocytes and embryos (OR, EMB and GEQ). These results are in concordance with other authors that suggest that FSH has less predictive power than AFC and ES4d on pregnancy rates and should be interpreted in the light of other variables [19]. Different reasons can explain this fact. One is that day 3 FSH levels can be affected by many variables like time of testing, irregular cycles and genetic polymorphism in the FSH receptor [20]. Another reason is that elevated basal FSH levels are indicative of diminished ovarian reserve in the number of oocytes, but the quality of the remaining follicle pool will be not diminished. Therefore, younger women (<35 years old) with elevated basal FSH can still have a favourable IVF outcome reflected by a good ongoing pregnancy rate despite poorer IVF performances. That is to say that basal FSH is a good predictor of the size of the remaining follicle, rather than their quality [21].
One surprising finding is the positive association obtained between MEQ and age. Thus, in this work, patients with increased age have better score for embryo quality than younger patients. The explanation for this fact could be the greater variability in the quality and number of embryos obtained depending on women age. Thus, younger women (<35 years old) have a higher number of embryos, and these embryos have different grades of quality, and in the same patient we can obtain embryos with the best and with the worst score of quality. In this group of patients, the quality of embryos is not related to their number. When age increases (>35 years old), the number of embryos is lower, but, in some patients the score for embryo quality is better than in the younger group. This improvement in embryo quality is not necessarily related to the number of embryos, and could be explained by the increase of embryos with chromosomal abnormalities in this group. An increase in embryo chromosomal abnormalities in women with advanced age has been reported [22]. This increase in abnormal embryos is related with the increase of chromosomal abnormalities in the oocytes of these patients [23]. In the first stages of embryo development, the morphology of the embryo does not correlate with chromosome normality, thus the morphology or quality of abnormal embryos is similar or better than normal embryos.
When we estimate the probability of pregnancy with the predictive model (Fig. 1), we obtain an estimation of the probability for a woman to get pregnant in that IVF cycle.
As we can observe in Fig. 3 the estimated probability for the equation has a good power of prediction in the extremes of the graphic. Thus, when the result obtained is a low probability to get pregnant, this patient has a very low probability to get pregnant in that cycle of IVF, and when the result is a high probability this patient has a high probability of getting pregnant. For the cases that are located in the middle of the graphic, the model is less able to discriminate between pregnancy and non-pregnancy. This situation could be explained by the fact that the patients with this intermediate probability levels have inconclusive values of AFC, ES4d and age or a combination of them.
In the cases that have a high probability to get pregnant, it is possible that the result of cycle will be no pregnancy. The explanation for this could be that to obtain a pregnancy in an IVF cycle it is important that three events occur. The first event needed is to have good quality embryos. Secondly, the moment of transfer of these embryos to the uterus is very important. for the result of the cycle. Thus, in patients with good embryos but with difficult or bad transfer, the result will probably be no pregnancy [24, 25]. The third necessary event is a good interaction between the embryos and the endometrium in order to favour the implantation process [26, 27]. When we estimate the probability of pregnancy in this work, it is not possible to know what will happen in the moment of embryo transfer or if good interaction between embryo and endometrium will exist.
Conclusions
The present study shows that it is possible, in patients with primary infertility due to a tubal factor, to obtain a prediction of the probability of pregnancy in the first stages of IVF cycle, using variables that are usually assessed in this stage. This data provides valuable information for a better understanding of the relation between parameters related to IVF cycles, and may help clinicians in counselling patients regarding cycle response and expected pregnancy rates. Further studies that included patients with other causes of infertility are needed to confirm this model of prediction.
Acknowledgements
We are grateful to the Research Unit of Hospital Universitari de Tarragona Joan XXIII. We also would like to thank Dr. Xavier Allué for valuable discussion and Núria Estrada for a careful reading of the manuscript.
Footnotes
On the fourth day of ovarian stimulation for IVF cycles, antral follicles count, estradiol and woman age predict IVF outcome as reflected by quantity and quality of oocytes, embryos obtained and pregnancy rates.
Contributor Information
J. Carrera-Rotllan, Phone: +34-93-8816090, Email: 4398jcr@comg.es
L. Estrada-García, Email: luis.estrada@tele2.es
J. Sarquella-Ventura, Email: labfiv@girofiv.com
References
- 1.Scott RT, Toner JP, Muasher SJ, Oehninger S, Robinson S, Rosenwaks Z. Follicle-stimulating hormone levels on cycle day 3 are predictive of in vitro fertilization outcome. Fertil Steril. 1989;4:651–654. doi: 10.1016/s0015-0282(16)60615-5. [DOI] [PubMed] [Google Scholar]
- 2.Licciardi FL, Liu HC, Rosemwaks Z. Day 3 estradiol serum concentrations as prognosticators of ovarian stimulation response and pregnancy outcome in patients undergoing in vitro fertilization. Fertil Steril. 1995;64(5):991–994. doi: 10.1016/s0015-0282(16)57916-3. [DOI] [PubMed] [Google Scholar]
- 3.Yanushpolsky EH, Hurwitz S, Tikh E, Racowsky C. Predictive usefulness of cycle day 10 follicle-stimulating hormone level in a clomiphene citrate challenge test for in vitro fertilization in women younger than 40 years of age. Fertil Steril. 2003;80(1):111–115. doi: 10.1016/S0015-0282(03)00499-0. [DOI] [PubMed] [Google Scholar]
- 4.Hofmann GE, Danforth DR, Seifer DB. Inhibin-B: the physiologic basis of the clomiphene citrate challenge test for ovarian reserve screening. Fertil Steril. 1998;69(3):474–477. doi: 10.1016/S0015-0282(97)00531-1. [DOI] [PubMed] [Google Scholar]
- 5.Tomas C, Nuojua-Huttunen S, Martinaken H. Pretreatment transvaginal ultrasound examination predicts ovarian responsiveness to gonadotrophins in in-vitro fertilization. Hum Reprod. 1997;12(2):220–223. doi: 10.1093/humrep/12.2.220. [DOI] [PubMed] [Google Scholar]
- 6.Hendriks DJ, Mol BW, Bancsi LF, Te Velde ER, Broekmans FJ. Antral follicle count in the prediction of poor ovarian response and pregnancy after in vitro fertilization: a meta-analysis and comparison with basal follicle-stimulating hormone level. Fertil Steril. 2005;83(2):291–301. doi: 10.1016/j.fertnstert.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 7.Ficicioglu C, Kutlu T, Baglam E, Bakacak Z. Early follicular antimullerian hormone as an indicator of ovarian reserve. Fertil Steril. 2006;85(3):592–596. doi: 10.1016/j.fertnstert.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 8.Hull MG, Fleming CF, Hughes AO, McDermott A. The age-related decline in female fecundity: a quantitative controlled study of implanting capacity and survical of individual embryos after in vitro fertilization. Fertil Steril. 1996;65(4):783–790. doi: 10.1016/s0015-0282(16)58214-4. [DOI] [PubMed] [Google Scholar]
- 9.Scott RT, Hofmann GE, Oehninger S, Muasher SJ. Intercycle variability of day 3 follicle-stimulating hormone levels and its effect on stimulation quality in in vitro fertilization. Fertil Steril. 1990;54(2):297–302. doi: 10.1016/s0015-0282(16)53707-8. [DOI] [PubMed] [Google Scholar]
- 10.Ocal P, Aydin S, Cepni J, Idil S, Idil M, Uzun H, Benian A. Follicular fluid concentrations of vascular endothelial growth factor, inhibin A, Inhibin B in IVF cycles: are they markers for ovarian response and pregnancy outcome. Eur J Obstet Gynecol Reprod Biol. 2004;115(2):194–199. doi: 10.1016/j.ejogrb.2004.01.034. [DOI] [PubMed] [Google Scholar]
- 11.Srouji SS, Mark A, Levine Z, Betensky RA, Horstein MD, Ginsburg ES. Predicting in vitro fertilization live birth using stimulation day 6 estradiol, age, and follicle-stimulating hormone. Fertil Steril. 2005;84(3):795–797. doi: 10.1016/j.fertnstert.2005.02.042. [DOI] [PubMed] [Google Scholar]
- 12.Veeck L. In: The morphological assessment of human oocytes and early concepti. Handbook of the laboratory diagnosis and treatment of infertility. Keel BA, Webster BW, editors. Boca Raton, FL: CRC; 1990. pp. 353–369. [Google Scholar]
- 13.Schwartz LB, Chiu AS, Courtney M, Krey L, Schmidt-Sarosi C. The embryo versus endometrium controversy revisited as it relates to predicting pregnancy outcome in in-vitro fertilization-embryo transfer cycles. Hum Reprod. 1997;12(1):45–50. doi: 10.1093/humrep/12.1.45. [DOI] [PubMed] [Google Scholar]
- 14.László F, Bancsi MMJ, Broekmans JM, Ejikemans MJC, Jong FH, Habbema JDF, et al. Predictors of poor ovarian response in in vitro fertilization: a prospective study comparing basal markers of ovarian reserve. Fertil Steril. 2002;77(2):328–336. doi: 10.1016/S0015-0282(01)02983-1. [DOI] [PubMed] [Google Scholar]
- 15.Speroff L, Glass RH, Kase AG. Regulation of menstrual cycle. Clinical gynecologic endocrinology and infertility. Maryland: Williams & Wilkins; 1994. pp. 141–183. [Google Scholar]
- 16.Tesarik J, Mendoza C. Nongenomic effects of 17 beta- estradiol on maturing human oocytes: relationship to oocyte developmental potential. J Clin Endocrinol Metab. 1995;80:1438–1443. doi: 10.1210/jc.80.4.1438. [DOI] [PubMed] [Google Scholar]
- 17.Rabinovici J, Blankstein J, Goldman B, Rudak E, Dor Y, Pariente C. In vitro fertilization and primary embryonic cleavage are possible in 17 alpha-hydroxylase deficiency despite extremely low intrafollicular 17 beta-estradiol. J Clin Endocrinol Metab. 1989;68:693–697. doi: 10.1210/jcem-68-3-693. [DOI] [PubMed] [Google Scholar]
- 18.Weghofer A, Margreiter M, Fauster Y, Schaetz T, Brandstetter A, Boehm D, et al. Age-specific FSH levels as a tool for appropriate patient counselling in assisted reproduction. Hum Reprod. 2005;20(9):2448–2452. doi: 10.1093/humrep/dei076. [DOI] [PubMed] [Google Scholar]
- 19.Abdalla H, Thum MY. An elevated basal FSH reflects a quantitative rather than qualitative decline of the ovarian reserve. Hum Reprod. 2004;19(4):893–898. doi: 10.1093/humrep/deh141. [DOI] [PubMed] [Google Scholar]
- 20.Greb RR, Grieshaber K, Gromoll J, Sonntag, Nieschlag E, Kiesel L, et al. A common single nucleotide polymorphism in exon 10 of the human follicle stimulating hormone receptor is a major determinant of length and hormonal dynamics of the menstrual cycle. J Clin Endocrinol Metab. 2005;90(8):4866–4872. doi: 10.1210/jc.2004-2268. [DOI] [PubMed] [Google Scholar]
- 21.Chuang CC, Chen CD, Chao KH, Chen SU, Ho HN, Yang YS. Age is better predictor of pregnancy potential than basal follicle-stimulating hormone levels in women undergoing in vitro fertilization. Fertil Steril. 2003;79(1):63–68. doi: 10.1016/S0015-0282(02)04562-4. [DOI] [PubMed] [Google Scholar]
- 22.Marquez C, Sandalinas M, Bahce M, Alikani M, Munne S. Chromosome abnormalities in 1255 cleavage-stage embryos. Reprod Biomed Online. 2000;1(1):17–26. doi: 10.1016/S1472-6483(10)61988-8. [DOI] [PubMed] [Google Scholar]
- 23.Kuliev A, Cieslak J, Ilkevitch Y, Verlinsky Y. Chromosomal abnormalities in a series of 6733 human oocytes preimplantation diagnosis for age-related aneuploidies. Reprod Biomed Online. 2003;6(1):54–59. doi: 10.1016/S1472-6483(10)62055-X. [DOI] [PubMed] [Google Scholar]
- 24.Coroleu B, Barri PN, Carreras O, Martinez F, Parriego M, Hereter L, et al. The influence of the depth of embryo replacement into the uterine cavity on implantation rates after IVF: a controlled, ultrasound-guided study. Hum Reprod. 2002;17(2):341–346. doi: 10.1093/humrep/17.2.341. [DOI] [PubMed] [Google Scholar]
- 25.Tomas C, Tikkinen K, Tuomivaara L, Tapanainen JS, Martikainen H. The degree of difficulty of embryo transfer is an independent factor for predicting pregnancy. Hum Reprod. 2002;17(10):2632–2635. doi: 10.1093/humrep/17.10.2632. [DOI] [PubMed] [Google Scholar]
- 26.Achache H, Revel A. Endometrial receptivity markers, the journey to successful embryo implantation. Hum Reprod Update. 2006;12(6):731–746. doi: 10.1093/humupd/dml004. [DOI] [PubMed] [Google Scholar]
- 27.Makker A, Singh MM. Endometrial receptivity: clinical assessment in relation to fertility, infertility, and antifertility. Med Res Rev. 2006;26(6):699–746. doi: 10.1002/med.20061. [DOI] [PubMed] [Google Scholar]