Abstract
Purpose
This study was designed to examine the effect of bacterial contamination on in vitro fertilization treatment outcomes.
Method
In a prospective clinical trial, 152 patients aged 23–38 years, mean 33.3 ± 4.6, undergoing IVF treatment were selected for this study. During embryo transfer, separate samples were collected for microbial examination from the following sites: the fundus of the vagina, the cervix, the embryo culture medium prior and post-embryo transfer, the tip of the catheter, and the external sheet. All the samples were separately cultured to identify any bacteria or yeast present.
Results
Pregnancy rates in patients testing positive for Entrobacteriaceae (22.2% versus 51%) and Staphylococcus species (17.6% versus 44%) were significantly lower than those in the negative culture group (p < 0.001). The pregnancy rates do not seem to be affected by the other isolated microorganisms.
Conclusion
This study shows that the presence of vaginal–cervical microbial contamination at the time of embryo transfer is associated with significantly decreased pregnancy rates.
Keywords: Bacterial population, Embryo transfer catheter, Implantation, In vitro fertilization, Vaginal–cervical contamination
Introduction
Despite considerable progress in the assisted reproduction field, the implantation rate of replaced embryos remains low. This has largely been attributed to variables such as the patient’s age, endometrial receptivity, embryo quality [1, 2] and embryo transfer technique per se [3–5]. As in vitro fertilization treatment (IVF) involves the placement of embryos into the uterine cavity using a catheter that passes through the cervix, the possibility of bacterial contamination during the embryo transfer procedure clearly exists, but has been poorly explored. In fact, there is growing evidence that bacterial contamination of the uterine cavity following transcervical embryo transfer can negatively affect the implantation rates and the pregnancy outcome. Such contamination can occur during embryo replacement through the tip of the embryo transfer catheter from various vaginal–cervical microorganisms [6–9]. Antimicrobials in the culture media probably provide little inhibition to the potentially large number of bacteria that could contaminate the embryo transfer catheter when it traverses the cervix. It is of note that genital infections, particularly those caused by sexually transmitted microorganisms, are among the leading causes of infertility [10, 11]. In fact, detection of the Chlamydia species in the endocervices of women undergoing IVF treatment has been associated with decreased implantation and decreased ongoing pregnancy rates [12, 13].
In addition, studies have shown that subjects who test negative for bacterial contamination have approximately 50% higher pregnancy rates than subjects who test positive for bacterial contamination resulting from the embryo transfer catheter tip. An increased risk of pregnancy loss prior to the sixth week of gestation was also reported in women with bacterial vaginosis (BV) undergoing in vitro fertilization treatment [14, 15]. The decrease in live birth rate following IVF in women with catheter tip contamination as well as in women with BV, may be caused by decreased conception rates, increased pregnancy loss, or both. Furthermore, studies have shown that pathogenic bacteria cultured from the tip of the catheter produce a detrimental effect on live birth rates [6–9]. Conversely, recovery of hydrogen peroxide (H2O2)-producing lactobacilli from the catheter tip appears to be associated with an increased live birth rate [9]. The understanding of whether genital bacteria have a negative impact on conception could lead to the adoption of an efficacious intervention that can improve pregnancy and delivery outcomes.
This study was designed to assess the impact of individual bacteria isolated from the vagina, the cervix, and the tip of the embryo-transfer catheter on the IVF treatment outcome.
Materials and methods
Patient selection
In a prospective clinical study, 152 patients aged 23–38 year, mean 33.3 ± 4.6, undergoing IVF treatment were enrolled as subjects. The clinical indications for IVF treatment were sperm abnormalities, tubal factor or idiopathic infertility. In order to limit additional factors (e.g., embryo quality and endometrium receptivity) that might affect the results, we selected only patients aged ≤38 years with a morphologically normal uterus and at least two good quality embryos available for embryo transfer. Embryo transfer was performed by the same physician. All the women were negative for Chlamydia trachomatis infection and none of them exhibited clinical evidence of vaginitis or cervicitis. Semen cultures were performed 2 months prior to IVF treatment. The men with positive microbial growth were given antibiotic treatment; thus all the men had negative semen cultures at the time of the IVF treatment. All patients were counselled about the nature of the study and gave their written informed consent. The study was approved by our local research ethics committee.
All the participating women underwent a microbiological examination of vaginal-cervical and urethral pathogenic microorganisms two months prior to IVF treatment. The patients who were positive for any pathogenic microorganism underwent a specific therapy and thereafter a post-therapeutic control was performed. All patients underwent a standard down-regulation protocol for ovarian stimulation induction. Oocyte recovery was carried out 36 h after HCG administration and embryo transfer took place 48 hours following insemination.
Culture collection and bacterial isolation
Just before the embryo transfer, six separate samples were collected for microbial examination: a swab from the fundus of the vagina and from the cervix was performed after washing and speculum insertion; a sample of embryo culture medium, used for embryo transfer, was collected prior to embryo loading, as well as a sample of the same medium after flushing of the embryo-transfer catheter post embryo transfer; a sample from the tip of the embryo transfer internal catheter, and also one from the tip of the external sheet. All the samples were then cultured to isolate any bacteria present. The samples were considered positive when 50 colonies forming units per sample were recovered. Specifically, aerobic and anaerobic microorganisms physiologically present in the genital tract, and pathogenic microorganisms such as urogenital Mycoplasma, Neisseria gonorrhoeae and Gardnerella vaginalis were considered in this study. The patients were categorized on the basis of the presence of each single microorganism in the examined samples. Patients were considered positive to one microorganism when a positive culture of the vaginal and/or cervical samples was confirmed in the tip of the internal catheter, external sheet and the medium used for flushing of the catheter post transfer. The resulting data were statistically correlated to the pregnancy rates in both positive-testing and negative-testing patients.
For statistical analysis χ2 and t test were used where appropriate. The differences were considered statistically significant when p ≤ 0.05.
Results
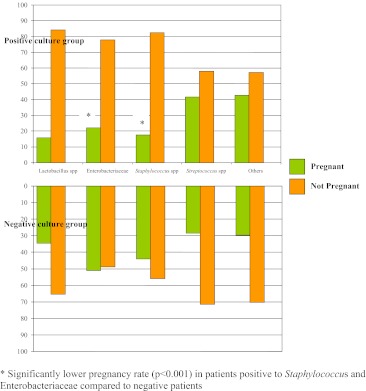
Data on patients’ characteristics, stimulation protocol, embryology, and number and quality of embryos transferred did not vary significantly between the positive and negative culture groups (Table 1). The overall pregnancy rate of the 152 studied women was 32.2 %. Of the 152 studied patients, 133 (87.5%) tested positive to one or more microorganisms and the remaining 19 patients (12,5%) tested completely negative to bacterial contamination. Although pregnancy rates were similar in both groups, implantation rates significantly differed (p ≤ 0.001) between patients positive to one or more bacteria and those completely negative (12.4 vs 14%). Higher miscarriage rates, though not statistically significant, were also found in positive patients compared to those negative for the presence of any bacteria (Table 1). The microorganisms identified in the positive culture group were as follows: Lactobacillus species in 19 patients (12.5%), Streptococcus species in 43 patients (28.2%), Enterobacteriaceae in 99 patients (65.1%), Staphylococcus species in 68 patients (45%), and other microorganisms such as S. agalactiae, G. vaginalis, Ureaplasma urealyticun and yeasts in 28 patients (18.3%). Among the Entrobacteriaceae observed, Escherichia coli was the species most frequently recovered, followed by Klebsiella, and to a lesser extent, Proteus. A considerable number of patients tested positive to more than one microorganism. The cultures of all the samples from the embryo-transfer culture medium prior to embryo transfer were negative for any microbial contamination. As depicted in Table 2, the patients (no = 152) were distributed on the basis of a single type of isolated bacteria. Comparing the positive and negative culture groups for each isolated microorganism in terms of clinical pregnancy rates, we found that pregnancy rates in patients testing positive for Entrobacteriaceae (22.2 versus 51%) and Staphylococcus species (17.6 versus 44%) were significantly lower than those of the negative culture group (p ≤ 0.001). The pregnancy rates were apparently not affected by the other isolated microorganisms (Fig. 1).
Table 1.
Global data of patients’ culture groups
| Characteristics | Culture groups | P | |
|---|---|---|---|
| Positive | Negative | ||
| No. of patients | 133 | 19 | |
| Mean age ± SD | 34.7 ± 3.9 | 33.2 ± 5.6 | ns |
| Male factor (%) | 40 | 43.2 | ns |
| Tubal factor (%) | 44.7 | 41.3 | ns |
| Unexplained (%) | 3.8 | 2.9 | ns |
| Other factors (%) | 11.5 | 12.6 | ns |
| No. of embryo transfer | 133 | 19 | |
| Mean number of embryos per patients ± SD | 2.9 ± 0.3 | 3 ± 0.0 | ns |
| Clinical pregnancies (%) | 41 (30.8) | 6 (31.6) | ns |
| Multiple pregnancies (%) | 7 (17) | 2 (16.7) | ns |
| Miscarriage rate (%) | 5 (12.2) | 0 (0) | ns |
| Implantation rate (%)a | 12.4 | 14.0 | ≤0.001 |
ns Not significant
aImplantation rate significantly higher in favour of negative group
Table 2.
Distribution of patients based on each isolated bacterium and its correlation with pregnancy outcome
| Isolated microbial organisms | Number of positive patients | Number of negative patients | P | ||
|---|---|---|---|---|---|
| Pregnant (%) | Not pregnant (%) | Pregnant (%) | Not pregnant (%) | ||
| Lactobacillus | 3 (15.8) | 16 (84.2) | 46 (34.6) | 87 (65.4) | ns |
| Entrobacteriaceae | 22 (22.2) | 77 (77.8) | 27 (51) | 26 (49) | ≤0.001 |
| Staphylococcus spp. | 12 (17.6) | 56 (82.4) | 37 (44) | 47 (56) | ≤0.001 |
| Streptococcus spp. | 18 (41.8) | 25 (58.1) | 31 (28.4) | 78 (71.6) | ns |
| Othersa | 12 (42.8) | 16 (57.2) | 37 (29.8) | 87 (70.2) | ns |
ns Not significant
aOther microorganisms include: S agalactiae, G. vaginalis, Mycoplasma and yeast
Fig. 1.
Correlation between single isolated microorganism and pregnancy rate
Discussion
Although few reports on bacterial contamination at embryo transfer and its implication in infertility treatment have been published, evidence implicating bacterial infection as detrimental to in vitro fertilization outcomes is steadily increasing. A decreased live birth rate has been reported in those patients in whom pathogenic bacteria were isolated from the embryo transfer catheter [6–9].
In our study, we assessed the possible consequences of bacterial contamination of the tip of the catheter at embryo transfer on the IVF treatment outcome. Microorganisms were assessed through bacteriologic analysis of the cervical and vaginal swab and of the catheter tip prior to and after embryo transfer. Although the pregnancy rate did not differ significantly between the positive and negative groups, significant differences in terms of pregnancy rates were observed when considering the correlation of single isolated microorganisms. A decreased pregnancy rate was observed in patients who tested positive to Entrobacteriaceae and Staphylococcus species compared to those who tested negative to the same microorganisms, while other isolated microorganisms did not seem to affect the pregnancy rate. Since the patients’ characteristics, stimulation protocol, embryology, and the number and quality of embryos transferred did not vary significantly between the positive and negative culture groups, we can speculate that the presence of such microorganisms on the cervix at embryo transfer could be implicated in the decreased embryo implantation rate.
The lower pregnancy rates among patients with positive cultures could be explained by at least three mechanisms: (1) the embryo transfer procedure may inoculate endocervical microorganisms into the uterine cavity, thus altering the biochemical [16, 17] or ultrastructural [17, 18] characteristics of the endometrium; (2) the high concentration of microorganisms on the cervix may be associated with subclinical chronic endometritis [19] which subsequently causes poor uterine receptivity; (3) possible direct contamination of the embryos during transcervical embryo transfer may inhibit their ability to implant. In a previous study, a 50% reduction in pregnancy rate occurred when bacteria were recovered from the tip of the embryo transfer catheter [6–8, 20]. Of interest is the fact that the dominant microorganisms studied were D. streptococcus, E. coli, S. viridians, Entrococcus, S. epidermidis and H2O2-producing lactobacilli [6–8].
In our study, we found that the presence of endocervical Entrobacteriaceae and staphylococci species in women undergoing IVF treatment is clearly associated with both decreased pregnancy rates and increased abortion rates. This could support the increasing evidence that the presence of some pathogenic microorganisms detected at embryo transfer have a negative effect on clinical pregnancy rate. Additionally, it is important to remember that success following in vitro fertilization is related to a variety of factors. Our results are consistent with previous reports [6–9] which suggest that the presence of bacterial contamination at embryo transfer could be one of the factors influencing the IVF outcome.
However, a better understanding of the effects of the cervicovaginal flora and catheter contamination on the IVF outcome may allow specific interventions to be potentially targeted to decrease the pro-inflammatory cytokine response and to establish the normal vaginal bacterial flora. Concerning this issue, it seems particularly relevant that endometrial cells express Toll-like receptor 4, a molecule involved in Gram negative endotoxin and that E. coli binding to these cells produces prostaglandin F and E2, thus modulating inflammatory response and endocrine functions [21].
Recently, it has been reported that ceftriaxone and metronidazole given at oocyte recovery in IVF-embryo transfer cycles may reduce bacteria on the transfer catheter and therefore improve the pregnancy rate [7], while administering doxycycline appears to have no effect either on vaginal bacterial flora or live birth rates [9]. Indeed, further study is needed to determine which specific antibiotics can actually reduce the virulent bacteria and, more important, preserve the vaginal protective bacteria. Finally, evidence of inflammation following embryo transfer needs to be documented in a larger sample of patients to further examine the hypothesis that inflammation may be the mechanism through which bacteria may negatively affect pregnancy.
Footnotes
The presence of vaginal-cervical microbial contamination, examined at embryo transfer, has revealed to negatively affect in-vitro fertilization treatment outcome
References
- 1.Roseboom TJ, Vermeiden JPW, Schoute E, Lens JW, Schats R. The probability of pregnancy after embryo transfer is affected by the age of the patient, cause of infertility, number of embryos transferred and the average morphology score, as revealed by multiple logistic regression analysis. Hum Reprod. 1995;10:3035–3041. doi: 10.1093/oxfordjournals.humrep.a135842. [DOI] [PubMed] [Google Scholar]
- 2.Munne S, Alikani M, Tomkin G, Grifo J, Cohen J. Embryo morphology, developmental rate, and maternal age are correlated with chromosome abnormalities. Fertil Steril. 1995;64:382–391. [PubMed] [Google Scholar]
- 3.Karande VC, Morris R, Chapman C, Rinehart J, Gleicher N. Impact of the “physician factor” on pregnancy rates in a large assisted reproductive technology program: Do too many cooks spoil the broth? Fertil Steril. 1999;71:1001–1009. doi: 10.1016/S0015-0282(99)00139-9. [DOI] [PubMed] [Google Scholar]
- 4.Hearns-Stokes RM, Miller BT, Scott L, Creuss D, Chakraborty PK, Segars JH. Pregnancy rates after embryo transfer depend on the provider at embryo transfer. Fertil Steril. 2000;74:80–86. doi: 10.1016/S0015-0282(00)00582-3. [DOI] [PubMed] [Google Scholar]
- 5.Angelini A, Brusco GF, Barnocchi N, El-Danasouri I, Pacchiarotti A, Selman H. Impact of physician performing embryo transfer on pregnancy rates in an assisted reproductive program. J Assist Reprod Genet. 2006;23:329–332. doi: 10.1007/s10815-006-9032-6. [DOI] [PubMed] [Google Scholar]
- 6.Egbase PE, Al-Sharhan M, Al-Othman S, Al-Mutawa M, Udo EE, Grudzinskas JG. Incidence of microbial growth from the tip of embryo transfer catheter after embryo transfer in relation to clinical pregnancy rate following in-vitro fertilization and embryo transfer. Hum Reprod. 1996;11:1687–1689. doi: 10.1093/oxfordjournals.humrep.a019470. [DOI] [PubMed] [Google Scholar]
- 7.Egbase PE, Udo EE, Al-Sharhan M, et al. Prophylactic antibiotics and endocervical microbial inoculation of the endometrium at embryo transfer (letter) Lancet. 1999;354:651–652. doi: 10.1016/S0140-6736(99)02415-0. [DOI] [PubMed] [Google Scholar]
- 8.Fanchin R, Harmas A, Benaoudia F, Lundkvist U, Olivennes F, Frydman R. Microbial flora of the cervix assessed at the time of embryo transfer adversely affects in vitro fertilization outcome. Fertil Steril. 1998;70:866–870. doi: 10.1016/S0015-0282(98)00277-5. [DOI] [PubMed] [Google Scholar]
- 9.Moore DE, Soules MR, Klein NA, Fujimoto VY, Agnew KJ, Eschenobach DA. Bacteria in the transfer catheter tip influence the live-birth rate after in vitro fertilization. Fertil Steril. 2000;74:1118–1124. doi: 10.1016/S0015-0282(00)01624-1. [DOI] [PubMed] [Google Scholar]
- 10.Westrom L. Effect of acute pelvic inflammatory disease on fertility. Am J Obstet Gynecol. 1975;121:707–713. doi: 10.1016/0002-9378(75)90477-9. [DOI] [PubMed] [Google Scholar]
- 11.Faro S. Chlamydia trachomatis: female pelvic infection. Am J Obstet Gynecol. 1991;164:1767–1770. doi: 10.1016/0002-9378(91)90558-9. [DOI] [PubMed] [Google Scholar]
- 12.Witkin SS, Sultan KM, Neal GS, Jeremias J, Grifo JA, Rosenwaks Z. Unsuspected Chlamydia trachomatis infection and in vitro fertilization outcome. Am J Obstet Gynecol. 1994;171:1208–1214. doi: 10.1016/0002-9378(94)90134-1. [DOI] [PubMed] [Google Scholar]
- 13.Witkin SS, Kligman II, Grifo JA, Rosenwaks Z. Chlamydia trachomatis detected by polymerase chain reaction in cervices of culture-negative women correlates with adverse in vitro fertilization outcome. J Infect Dis. 1995;17:1657–1659. doi: 10.1093/infdis/171.6.1657. [DOI] [PubMed] [Google Scholar]
- 14.Ralph SG, Rutherford AJ, Wilson JD. Influence of bacterial vaginosis on conception and miscarriage in the first trimester: cohort study. BMJ. 1999;319:220–223. doi: 10.1136/bmj.319.7204.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liversedge NH, Turner A, Horner PJ, Keay SD, Jenkins JM, Hull MG. The influence of bacterial vaginosis on in-vitro fertilization and embryo implantation during assisted reproduction treatment. Hum Reprod. 1999;14:2411–2415. doi: 10.1093/humrep/14.9.2411. [DOI] [PubMed] [Google Scholar]
- 16.Tatbibzadeh S, Babaknia A. The signals and molecular pathway involved in implantation: a symbiotic interaction between blastocyst and endometrium involving adhesion and tissue invasion. Hum Reprod. 1995;10:1579–1602. doi: 10.1093/humrep/10.6.1579. [DOI] [PubMed] [Google Scholar]
- 17.Paulson RJ, Sauer MV, Lobo RA. Factors affecting embryo implantation after human in vitro fertilization: a hypothesis. Am J Obstet Gynecol. 1990;163:2020–2023. doi: 10.1016/0002-9378(90)90790-e. [DOI] [PubMed] [Google Scholar]
- 18.Navot D, Anderson TL, Drosech K, Scott RT, Kreiner D, Rosenwaks Z. Hormonal manipulation of endometrial maturation. J Clin Endocrinol Metab. 1989;68:801–807. doi: 10.1210/jcem-68-4-801. [DOI] [PubMed] [Google Scholar]
- 19.Czernobilisky B. Endometritis and infertility. Fertil Steril. 1978;30:119–130. doi: 10.1016/s0015-0282(16)43448-5. [DOI] [PubMed] [Google Scholar]
- 20.Hillier SL, Krohn MA, Rabe LK, Klebanoff SJ, Eschenbach DA. The normal vaginal flora, H2O2-producing lactobacilli, and bacterial vaginosis in pregnant women. Clin Infect Dis. 1993;16(Suppl 4):S273–S281. doi: 10.1093/clinids/16.supplement_4.s273. [DOI] [PubMed] [Google Scholar]
- 21.Herath S, Fischer DP, Werling D, Williams EJ, Lilly ST, Dobson H, et al. Expression and function of Toll-like receptor 4 in the endometrial cells of the uterus. Endocrinology. 2006;147:562–570. doi: 10.1210/en.2005-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]