Abstract
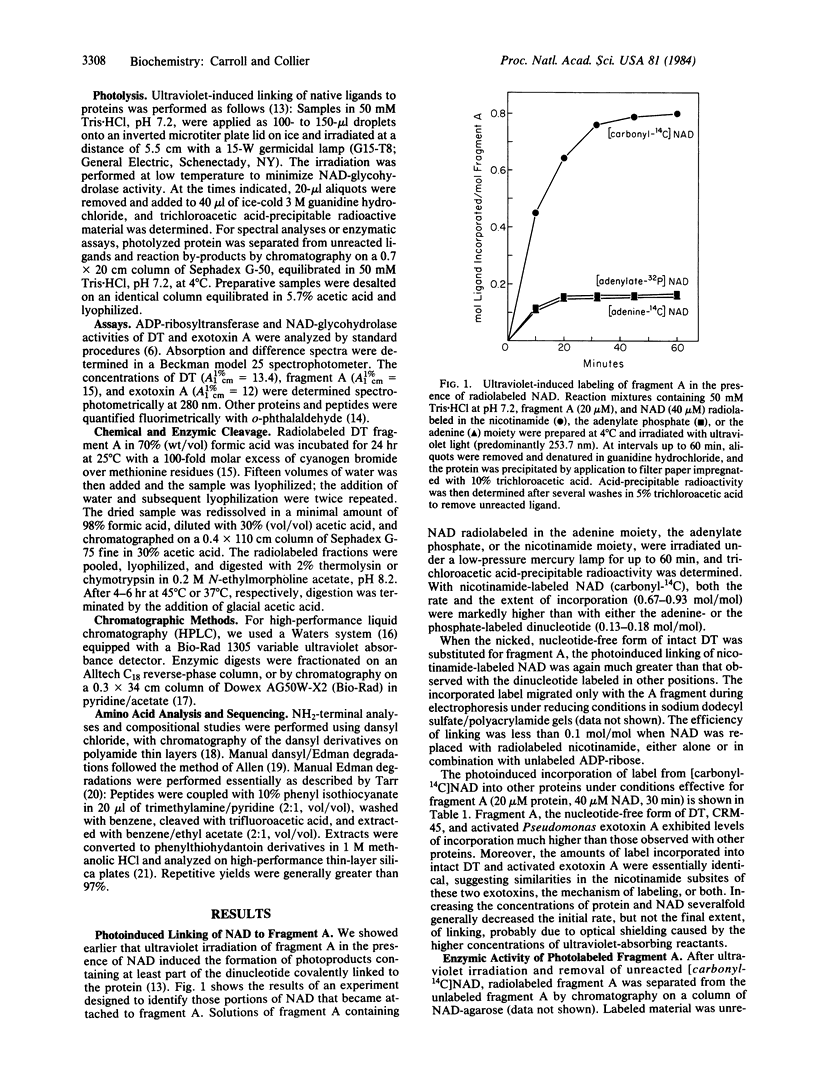
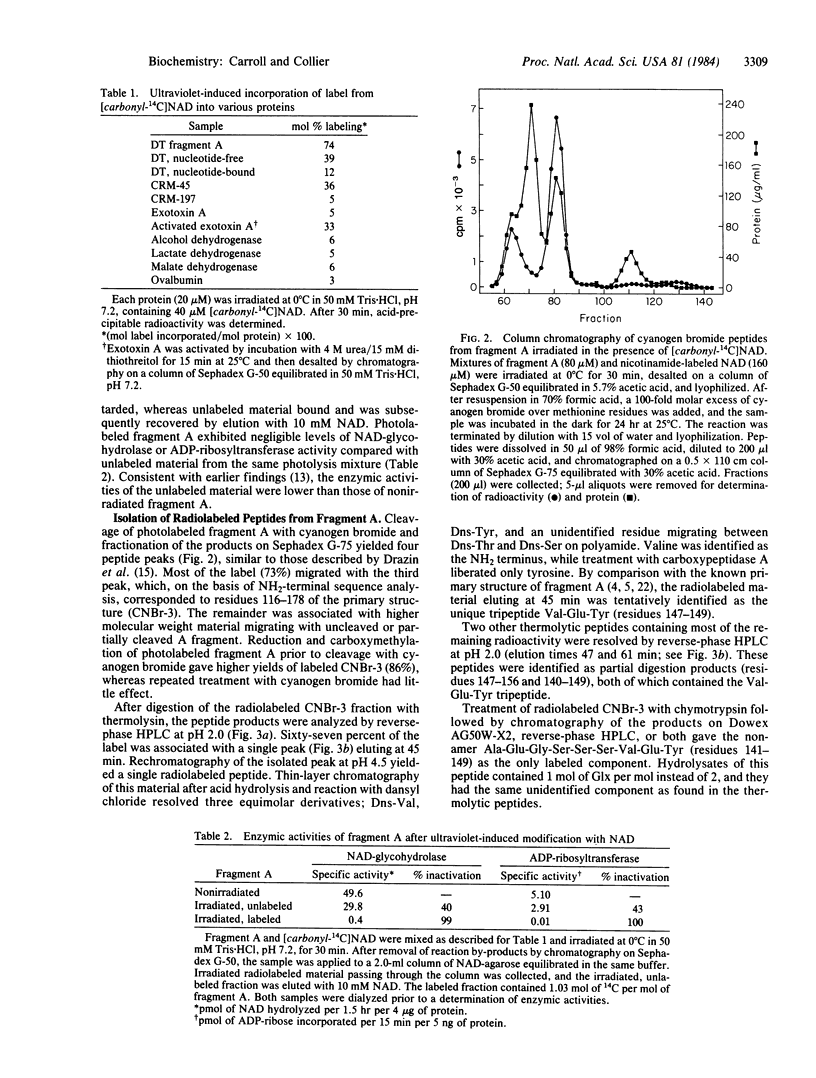
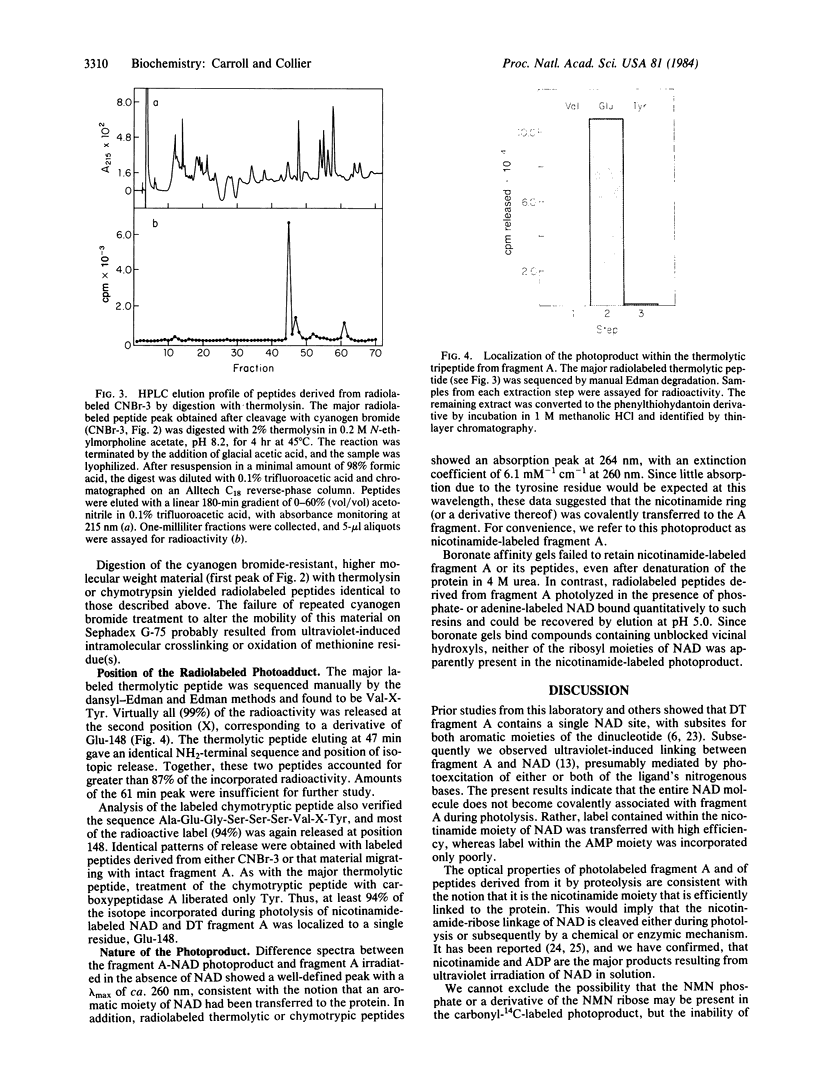
We showed earlier that exposing mixtures of NAD and diphtheria toxin fragment A to ultraviolet radiation (253.7 nm) induced the formation of covalently linked protein-ligand photoproducts. Here we report that when [carbonyl-14C]NAD was employed in such procedures, the efficiency of labeling of the protein approached 1 mol/mol, and at least 94% of the incorporated label was associated with a single residue, glutamic acid at position 148. Fragment A photolabeled in this manner was enzymically inactive. The efficiency of photolabeling was much lower (less than 0.2 mol/mol) when NAD radiolabeled in either the adenine moiety or the adenylate phosphate was used, and the label was attached to different site(s) within fragment A. Efficient photochemical transfer of label from [carbonyl-14C]NAD occurred under identical conditions with the nucleotide-free form of whole diphtheria toxin, CRM-45, or activated exotoxin A from Pseudomonas aeruginosa, but not with nucleotide-bound diphtheria toxin, CRM-197, native exotoxin A, or any of several NAD-linked dehydrogenases. On the basis of these and other results we suggest that part or all of the nicotinamide moiety of NAD is efficiently transferred to glutamate-148 of fragment A under the influence of ultraviolet irradiation and that this residue is located within the nicotinamide subsite. This location implies that glutamate-148 is at or near the catalytic center of the toxin. Our data provide direct evidence for the location of the NAD site in an ADP-ribosylating toxin and demonstrate highly efficient and specific photolabeling by [carbonyl-14C]NAD.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alouf J. E. Bacterial toxins: an outlook. Toxicon. 1982;20(1):211–216. doi: 10.1016/0041-0101(82)90203-3. [DOI] [PubMed] [Google Scholar]
- Barbieri J. T., Carroll S. F., Collier R. J., McCloskey J. A. An endogenous dinucleotide bound to diphtheria toxin. Adenylyl-(3',5')-uridine 3'-monophosphate. J Biol Chem. 1981 Dec 10;256(23):12247–12251. [PubMed] [Google Scholar]
- Carroll S. F., Lory S., Collier R. J. Ligand interactions of diphtheria toxin. III. Direct photochemical cross-linking of ATP and NAD to toxin. J Biol Chem. 1980 Dec 25;255(24):12020–12024. [PubMed] [Google Scholar]
- Carroll S. F., Martinez R. J. Antibacterial peptide from normal rabbit serum. 2. Compositional microanalysis. Biochemistry. 1981 Oct 13;20(21):5981–5987. doi: 10.1021/bi00524a009. [DOI] [PubMed] [Google Scholar]
- Carroll S. F., Martinez R. J. Antibacterial peptide from normal rat serum. 1. Isolation from whole serum, activity, and microbicidal spectrum. Biochemistry. 1981 Oct 13;20(21):5973–5981. doi: 10.1021/bi00524a008. [DOI] [PubMed] [Google Scholar]
- Chung D. W., Collier R. J. Enzymatically active peptide from the adenosine diphosphate-ribosylating toxin of Pseudomonas aeruginosa. Infect Immun. 1977 Jun;16(3):832–841. doi: 10.1128/iai.16.3.832-841.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLange R. J., Williams L. C., Drazin R. E., Collier R. J. The amino acid sequence of fragment A, an enzymically active fragment of diphtheria toxin. III. The chymotryptic peptides, the peptides derived by cleavage at tryptophan residues, and the complete sequence of the protein. J Biol Chem. 1979 Jul 10;254(13):5838–5842. [PubMed] [Google Scholar]
- Drazin R. E., Collier R. J., Williams L. C., DeLange R. J. The amino acid sequence of fragment A, an enzymically active fragment of diphtheria toxin. II. The cyanogen bromide peptides. J Biol Chem. 1979 Jul 10;254(13):5832–5837. [PubMed] [Google Scholar]
- Greenfield L., Bjorn M. J., Horn G., Fong D., Buck G. A., Collier R. J., Kaplan D. A. Nucleotide sequence of the structural gene for diphtheria toxin carried by corynebacteriophage beta. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6853–6857. doi: 10.1073/pnas.80.22.6853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel J., Collier R. J., Chung D. W. Interaction of fragment A from diphtheria toxin with nicotinamide adenine dinucleotide. J Biol Chem. 1974 Apr 10;249(7):2088–2097. [PubMed] [Google Scholar]
- Katada T., Ui M. Direct modification of the membrane adenylate cyclase system by islet-activating protein due to ADP-ribosylation of a membrane protein. Proc Natl Acad Sci U S A. 1982 May;79(10):3129–3133. doi: 10.1073/pnas.79.10.3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lory S., Carroll S. F., Collier R. J. Ligand interactions of diphtheria toxin. II. Relationships between the NAD site and the P site. J Biol Chem. 1980 Dec 25;255(24):12016–12019. [PubMed] [Google Scholar]
- Lory S., Collier R. J. Expression of enzymic activity by exotoxin A from Pseudomonas aeruginosa. Infect Immun. 1980 May;28(2):494–501. doi: 10.1128/iai.28.2.494-501.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marburg S., Tolman R. L., Callahan L. T., 3rd Pseudomonas exotoxin A: toxoid preparation by photoaffinity inactivation. Proc Natl Acad Sci U S A. 1983 May;80(10):2870–2873. doi: 10.1073/pnas.80.10.2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel A., Dirkx J. Occurrence of tryptophan in the enzymically active site of diphtheria toxin fragment A. Biochim Biophys Acta. 1977 Mar 28;491(1):286–295. doi: 10.1016/0005-2795(77)90064-2. [DOI] [PubMed] [Google Scholar]
- Modak M. J., Gillerman-Cox E. Biochemistry of terminal deoxynucleotidyl transferase. Conditions for and characterization of ultraviolet light mediated substrate cross-linking to terminal deoxynucleotidyl transferase. J Biol Chem. 1982 Dec 25;257(24):15105–15109. [PubMed] [Google Scholar]
- Montanaro L., Sperti S. Binding of nicotinamide-adenine dinucleotides to diphtheria toxin. Biochem J. 1967 Nov;105(2):635–640. doi: 10.1042/bj1050635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratti G., Rappuoli R., Giannini G. The complete nucleotide sequence of the gene coding for diphtheria toxin in the corynephage omega (tox+) genome. Nucleic Acids Res. 1983 Oct 11;11(19):6589–6595. doi: 10.1093/nar/11.19.6589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SERAYDARIAN M. W., COHEN A. I., SABLE H. Z. Inactivation of diphosphopyridine nucleotide by ultraviolet irradiation and identification of degradation products. Am J Physiol. 1954 Apr;177(1):150–156. doi: 10.1152/ajplegacy.1954.177.1.150. [DOI] [PubMed] [Google Scholar]
- Sperling J., Havron A. Photochemical cross-linking of neighboring residues in protein-nucleic acid complexes: rnase and pyrimidine nucleotide inhibitors. Biochemistry. 1976 Apr 6;15(7):1489–1495. doi: 10.1021/bi00652a020. [DOI] [PubMed] [Google Scholar]
- Tarr G. E. Improved manual sequencing methods. Methods Enzymol. 1977;47:335–357. doi: 10.1016/0076-6879(77)47036-8. [DOI] [PubMed] [Google Scholar]
- Yue V. T., Schimmel P. R. Direct and specific photochemical cross-linking of adenosine 5'-triphosphate to an aminoacyl-tRNA synthetase. Biochemistry. 1977 Oct 18;16(21):4678–4684. doi: 10.1021/bi00640a023. [DOI] [PubMed] [Google Scholar]




