Abstract
Purpose: The objective of this study was to evaluate the effects of growth factor supplementation and Vero cell co-culture on apoptosis and development of frozen thawed one-cell mouse embryos.
Methods: The following treatment regimens were assessed: (a) control medium (b) Vero cell co-culture and (c) growth factor supplemented medium. The individual growth factors tested were: GM-CSF, IGF-I, IGF-II, TNF-α, FGF-4, LIF, TGF-α, TGF-β, IL-6, PDGF and EGF. Blastocyst development and differentiation were monitored. At termination of the experiments, overall blastomere number and apoptosis were assessed using the TUNEL assay.
Results: No differences were observed in blastulation and hatching rates. ICM differentiation in thawed embryos was notably improved with either co-culture or growth factor supplementation. The only growth factor significantly modulating apoptosis in thawed embryos was granulocyte-macrophage colony stimulating factor (GM-CSF). GM-CSF enhanced continued cell survival and prevented apoptosis but did not influence overall cell number in developing blastocysts. Vero cell co-culture significantly increased cell number in blastocysts (124±42 vs 100±44 in control; P<0.05). Embryonic apoptosis was higher in the co-cultured embryos. The increased presence of apoptotic cells in blastocysts of high cell number may reflect the regulatory role of apoptosis in balancing ICM: TE ratios.
Conclusion: These data indicate that culture conditions can modulate post-thaw embryonic development and apoptosis.
Keywords: Apoptosis, Blastocyst, Cryopreservation, Co-culture, Granulocyte-macrophage colony-stimulating factor
Introduction
Programmed cell death or apoptosis is a mechanism that is vital for embryonic development and homeostasis [1–7]. The balance between the pro-survival factors and the pro-apoptotic signals control the apoptotic rate in any given cell [6]. The increase in embryonic apoptosis observed during in-vitro growth could potentially impede blastulation, interfere with normal cell distribution between ICM and TE and result in poor quality blastocysts with reduced implantation potential. Amongst factors potentially capable of modulating apoptosis, growth factors may be major contributors. Considerable evidence suggests that growth factors have profound effects on embryonic metabolism, blastocyst development and differentiation [reviewed in [8, 9]].
Over the past decade, researchers have focused on trying to optimize in-vitro culture systems by adjusting media formulations to more closely mimic the female reproductive tract. Understanding apoptosis and its modulation by culture environment may aid in designing an optimal culture media for embryonic growth. The concept behind commercially available sequential media systems developed for blastocyst culture reflect this new awareness of environmental stress and how it may ultimately affect embryonic development. Designing culture media to meet the changing metabolic needs of the fertilized zygote as it divides, activates its genome and eventually blastulates should theoretically reduce environmental stress.
Various growth factors and/or cytokines have also been explored as potential augmenting factors. Supplementation with growth factors like TGF-α [2], IGF-I and II [4, 10–12] and GM-CSF [13] have been shown to increase embryonic cell number, stimulate ICM development and decrease the percentage of apoptotic cells. Paracrine/autocrine interaction amongst group cultured embryos also enhances embryonic parameters. Platelet activating factor (PAF) secreted by embryos can modulate embryo metabolism, cell number and apoptosis [14]. Another approach for improving embryonic growth in vitro has been co-culture of embryos on feeder cell layers with embryotrophic properties [15–18].
Little information is available on apoptosis and modulation of embryonic stress in frozen-thawed embryos. The present study addresses this issue by systematically evaluating post-thaw development of mouse one-cell zygotes and the effect of growth factors, cytokines and co-culture on subsequent embryonic development and apoptosis. Study of different factors modulating embryonic apoptosis in vitro could potentially be used to derive optimal culture systems for embryo development.
Materials and methods
Embryo culture
Frozen one-cell embryos (B6D2F1 males X B6C3F1 females) were purchased from Conception Technologies (San Diego, CA, USA). Thawed embryos were pooled and cultured overnight at 37°C with 5% CO2 in air in a humidified incubator. Embryos cleaving to the two-cell stage were randomly allocated to different treatment regimens and cultured for an additional 72 hours. The treatment regimens tested were: (a) MEM-α (GIBCO BRL, Grand Island, NY, USA) alone (control), (b) co-culture with Vero cells and (c) addition of growth factors namely GM-CSF, IGF-I, IGF-II, FGF-4, TNF-α, LIF, TGF-α, TGF-β, IL6, PDGF and EGF (R & D Systems, Minneapolis, MN). Table 1 shows the concentration of growth factors used in this study. Embryos were cultured in 50 μl drops of basal medium supplemented with the test factor additives, under an oil overlay (5 embryos/drop).
Table 1.
Growth factor and cytokine treatment groups
| Growth factor | Concentration (ng/mL) |
|---|---|
| GM-CSF | 2.0 |
| LIF | 1.0 |
| TGF-α | 2.0 |
| IGF-I | 30.0 |
| IGF-II | 1.0 |
| FGF-4 | 10.0 |
| TNF-α | 5.0 |
| TGF-β | 2.0 |
| IL6 | 1.0 |
| PDGF | 1.0 |
| EGF | 4.0 |
The basal medium for all treatments was α-modified Minimum Essential Medium (α-MEM) with 10% Synthetic Serum Substitute (SSS; Irvine Scientific, Santa Ana,CA). Alpha-MEM is an Eagle's-based medium containing essential and nonessential amino acids, ascorbic acid, lipoic acid, biotin and vitamin B12. Synthetic Serum Substitute is a preparation of human serum albumin (HA) and α- and β-globulins derived from pooled human sera.
Co-culture methodology
Vero cell seed stocks were purchased from the ATCC (Manassas, VA, USA). The flask was cultured to confluence and subsequently passaged once a week by trypsinization. For co-culture experiments, four well dishes (Falcon Plastics, Oxnard, CA) were seeded with 35,000 cells/well. Cultures were incubated at 37°C in a humidified incubator with 5% CO2. On day 2, monolayers were rinsed with α-MEM supplemented with 10% SSS and allowed to equilibrate for at least one hour in the incubator before initiating co-culture.
Embryo assessment
On day 5, all embryos were assessed for developmental stage. Blastocyst formation, hatching and morphology were noted. Blastocysts were graded for maturity, ICM and trophectoderm development [19]. Maturity was graded as follows: Early blastocysts: cavity just starting or less than 1/2 of embryo volume, Late blastocysts: expanding or fully expanded with cavity >1/2 embryo volume. The inner cell mass was graded as: 0—absent, 1—scanty disorganized, 2—well developed. Trophectoderm was graded as: 1—Poorly developed, low cell number, 2—adequate cell number, well differentiated.
TUNEL assay
The TUNEL assay for DNA fragmentation was then performed on the embryos from each group. Localization of TUNEL positive cells was visualized by fluorescence microscopy using epiflourescence. Embryos were assessed for apoptosis by the TdT-mediated dUTP nick end labeling (TUNEL) assay using In-Situ Cell Death Detection kit-TMR-Red (Boehringer Mannheim, Minneapolis, MN, USA) followed by counterstaining with 4′,6-diamidino-2-phenylindole, dihydrochloride (DAPI; Molecular Probes, Eugene, OR, USA) for nuclear identification. Embryos in the culture medium were washed 3 times in PBS and fixed in freshly prepared 4% (wt/vol) paraformaldehyde in PBS (PH 7.3) for 1 hour at room temperature. After fixation, embryos were washed again 2 times in PBS followed by storage in micro-droplets of PBS under oil at 4°C until TUNEL assay. Prior to the assay, embryos were permeabilized with 0.1% Triton-X-100 in PBS on ice for 2 minutes followed by washing in PBS 2 times. Micro-droplets of TUNEL reaction mixture containing TMR-Red-conjugated dUTP and terminal deoxyribonucleotidyl transferase (TdT) were prepared under the oil overlay. Embryos were incubated in this reaction mixture for 1 hour at 37°C in the dark. Negative controls were incubated without the TdT enzyme. Embryos were washed with PBS and mounted under cover slips in DAPI and visualized by fluorescence microscopy using epiflourescence.
Data analysis
Data from three replicate experiments were combined. Percent blastulation, hatching and average cell number were calculated for each treatment group. The number of TUNEL positive cells per embryo was calculated. The apoptotic index for each embryo was calculated by dividing the number of TUNEL labeled cells by the total number of blastomeres and multiplying by 100. The average apoptotic index for each treatment group was then calculated. In this study design, all groups were compared to the control untreated embryos. Statistical analysis was done using the chi square test and ANOVA one way analysis of variance followed by Dunnett multiple comparison post-hoc test. P values less than 0.05 were considered significant.
Results
Effect of growth factors and co-culture on embryonic development
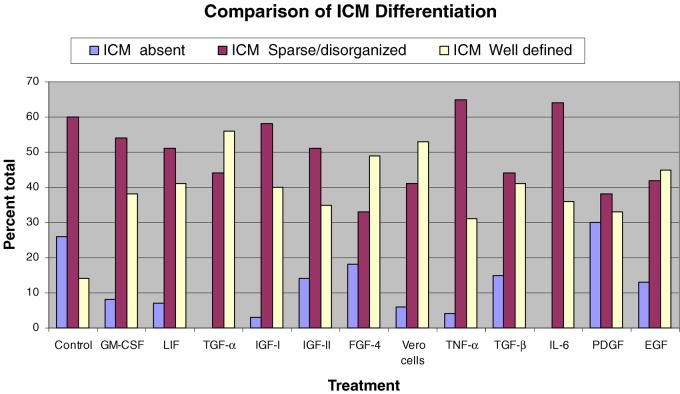
The concentrations selected for testing of growth factor additives were based on a survey of the literature and relevant studies with embryos from different species [20–23]. Post-thaw development and apoptosis was studied in 540 frozen one cell zygotes. The blastulation rate was high in all treatment groups including the medium alone control and ranged from 79–93% (Table 2). No significant difference in blastocyst formation or hatching was noted with growth factor supplementation or co-culture. Nor was there a difference in blastocyst maturity; over 95% of blastocysts were expanding or fully expanded late blastocysts. Qualitative grading of the inner cell masse (ICM) of blastocysts did however indicate better ICM differentiation in co-culture and growth factor treated embryos as compared to the control group (Fig. 1). In the control group, an ICM was not visualized in 26% of the blastocysts. Moreover only 14% of blastocysts exhibited a large, well defined ICM (+2 grade). In contrast, with co-culture, an ICM was present in 94% of the blastocysts (P<0.001) and in 53% of co-cultured blastocysts the ICMs were graded as +2. A significant improvement in ICM differentiation was observed with all growth factor additives except PDGF (P<0.001).
Table 2.
Modulation of culture conditions and their impact on in vitro embryonic growth parameters and apoptosis
| Blastocyst | Hatching | Cell count | Apoptotic index | |
|---|---|---|---|---|
| Treatment | rate (%) | rate (%) | (Mean) | (Mean) |
| Control | 89 | 59 | 100±44 | 0.50±0.68 |
| Vero cells | 87 | 78 | 124±42a | 1.70±1.29b |
| GM-CSF | 80 | 69 | 100±46 | 0.00±00c |
| LIF | 87 | 68 | 99±41 | 0.67±0.81 |
| TGF-α | 87 | 84 | 108±40 | 0.69±0.87 |
| IGF-I | 87 | 76 | 105±39 | 0.23±0.43 |
| IGF-II | 93 | 72 | 102±39 | 0.28±0.50 |
| FGF-4 | 85 | 65 | 106±40 | 0.49±0.72 |
| TNF-α | 79 | 67 | 119±38 | 0.18±0.30 |
| TGF-β | 79 | 65 | 100±41 | 0.69±0.78 |
| IL-6 | 80 | 74 | 113±39 | 0.68±0.88 |
| PDGF | 88 | 82 | 103±43 | 0.45±0.85 |
| EGF | 91 | 56 | 84±47 | 1.23±1.49 |
a,b,cSignificantly different from control (P<0.05).
cNo apoptotic cells observed with GM-CSF-treated embryos.
Fig. 1.
Qualitative grading of ICM with different treatments
Quantitative evaluation of blastocyst quality was also undertaken by blastomere enumeration. The average cell number per blastocyst was significantly higher in the co-culture group (124±42) as compared to the control group (100±44; P<0.05). Individual growth factor treatment did not enhance post-thaw cleavage rate or overall blastomere number by day 5 of culture (Table 2).
Effect of growth factors and co-culture on apoptosis
The apoptotic index for embryos from the different treatment groups is shown in Table 2. The percentage of apoptotic cells in thawed embryos cultivated to Day 5 under varying in-vitro conditions was compared using TUNEL labeling. Necrotic embryos were not evaluated. Apoptotic index for each treated embryo was calculated by dividing the total number of apoptotic cells counted in the blastocyst by the total blastomere number. GM-CSF treatment was particularly striking. In the presence of this factor, apoptotic cells were not observed in any of the analyzed embryos (apoptotic index=0). The apoptotic index in cultures supplemented with growth factors IGF-I, IGF-II, and TNF-α was also low when compared to the untreated control embryos but the data did not reach statistical significance. Interestingly, the apoptotic index was significantly higher with co-culture (1.7±1.3; P<0.05) as compared to the untreated control. Morphologically the co-cultured blastocysts were well differentiated with no outward sign of necrotic cells. Yet 75% of embryos had 1–4 apoptotic cells. In the untreated controls only 30% of the blastocysts derived from thawed embryos had an apoptotic cell.
Discussion
A careful balance between intrinsic pro-survival and pro-apoptotic factors is maintained during in-vivo embryonic development. During in vitro development, extrinsic factors may trigger apoptosis. Embryos cultivated in the laboratory under potentially suboptimal developmental conditions and in an environment devoid of growth factors may be extremely vulnerable to in-vitro stress. Brison et al noted a three-fold increase in rate of apoptosis in embryos derived from in-vitro versus in-vivo fertilized oocytes [2].
Embryo cryopreservation, thaw and subsequent in-vitro culture may subject embryos to even more stress. Recently, investigators studying the gene expression profile of frozen-thawed zygotes noted an up-regulation of six stress regulated genes [24]. Reducing embryonic stress by optimizing post-thaw culture conditions through growth factor supplementation has not been extensively explored [22, 25–27]. Only the study by Desai et al. systematically compared individual growth factor treatment to a co-culture model for improving post-thaw embryonic development [22]. Embryonic apoptosis was not however evaluated with the different treatment regimens.
The present study focused on the effects of growth factors and Vero cell co-culture on both embryonic development and apoptosis in thawed mouse zygotes cultured in-vitro for a 5 day interval. Our goal was to determine if any growth factor added singly would enhance cell number and/or reduce embryonic apoptosis. We included co-culture as a treatment group, since numerous data suggest that suboptimal development in-vitro can be improved by culture of embryos on a monolayer of somatic cells [15–17, 28, 29]. The Vero cell line was used in this investigation. Originally derived from monkey kidney cells, this cell line has been utilized for co-culture of mouse embryos [18, 25, 26, 30–32] and also human embryos in the IVF setting [31, 33, 34]. The mechanism of action of co-culture cells still remains controversial. Co-culture cells are postulated to exert their embryotrophic effect through secretion of growth factors or by possibly reducing the effects of deleterious factors in the culture system [30, 35]. Vero cell secretions include the growth factors PDGF, IL-6 and LIF [31], all of which are also found in the reproductive tract [36–39]. An interesting study by Carnegie et al suggests that in vitro production of blastocysts with high cryotolerance can be modulated by levels of LIF secretion by Vero cells [40].
Post-thaw embryonic development was excellent in control medium, co-culture wells, as well as microdrops supplemented with single growth factors. α-MEM was specifically selected for these experiments as it supports high rates of embryonic blastulation both in the presence and absence of co-culture cells [34, 41, 42]. This allowed us to test the impact of each treatment in a culture system that already supported high embryonic development with untreated controls.
The benefit of embryo co-culture on thaw was clearly evident even in this optimized culture system. Blastocysts derived from thawed zygotes cultured on Vero cell monolayers showed a statistically significant increase in proliferation, as evidenced by the high blastomere number. These data are consistent with other published studies examining the possible benefit of co-culture during thawing. The embryotrophic effect of Vero cell monolayers on post-thaw development and cell proliferation has been observed with cryopreserved mouse morula [22] and also mouse embryos frozen at the two cell stage [25, 26]. The current data corroborate this finding using earlier growth stage i.e. frozen-thawed zygotes.
None of the individual growth factor additives significantly altered blastocyst formation, hatching rate or total blastomere number by Day 5 of culture. Autocrine secretion of growth factors can exert an embryotrophic effect on embryos grown together in microdrops [20], but this effect is abolished when embryos are cultured at a lower density (one embryo per ten microliters). We were careful to control for this type of autocrine/paracrine effect by culturing no more than 5 embryos in the fifty microliter culture drops.
Our findings were contradictory to other published data suggesting that the growth factors IGF-I, IGF-II, EGF, TGF-α, TGF-β, LIF and GMCSF can positively modulate in-vitro embryonic growth parameters and blastomere number [2, 10, 23, 43–46]. These observed differences may be related to mouse strain, growth factor concentration and the state of the embryo i.e. freshly isolated versus frozen, as in the current work. An equally important consideration may be the type of basal culture media used in the study. It has been argued that LIF and GM-CSF supplementation provide no benefit unless embryos were being cultured in simple medium [23, 46]. This was not the case with thawed human embryos frozen at the 2–4 cell stage [13]. These investigators found that addition of GMCSF to culture media rescued poor quality human embryos and increased blastocyst development, hatching and attachment even in a complex medium with serum [13].
The stage of development when particular growth factors are introduced may also affect embryonic response to exogenous supplementation. Embryos pre and post genomic activation have been shown to express different receptors [8, 47]. Progression of thawed mouse morula to expanded blastocyst has been shown to be accelerated with exposure to growth factors [22]. Blastomere number by Day 6 of culture was significantly increased by media supplementation with growth factors IGF I and II, EGF, PDGF, IL6 and TGF β. Similarly, EGF has been shown to improve post-thaw survival of vitrified bovine blastocysts from in vitro matured oocytes [27, 48]. Growth factors may help “jump start” embryos cryopreserved at more advanced stages, when specific receptors are available, and thereby promote continued proliferation of ICM and trophectodermal cells.
The pattern of apoptosis in thawed embryos and its modulation through growth factor additives has proved to be extremely interesting. The number of TUNEL positive cells was surprisingly quite low. The apoptotic index which describes the percentage of apoptotic cells in an embryo was (0.5±0.6) in the control vs. (1.7±1.3) in the co-culture treated embryos. The overall low levels of apoptosis reflect positively on the quality of embryos and the basal medium used in this study. Apoptotic indices of freshly isolated embryos in an un-supplemented control medium has ranged from (1.5 to 32%) in other published studies, depending on the animal species, culture medium and embryo stage [4, 5, 10, 12, 28, 29, 44, 49].
A recent study reported the apoptotic index with mouse uterine co-culture to be 1.5%, very similar to our own results. Unlike the present study, in the absence of co-culture, these investigators observed a higher apoptotic index (5.6%) [50]. Our selection of α-MEM as a basal medium may be a factor in the low incidence of apoptosis measured in this study. In-vitro stress related to freezing and thawing of embryos and extended culture may have been attenuated by anti-oxidants such as ascorbic acid present in this medium. The anti-apoptotic effect of ascorbic acid supplementation has been documented with mouse [51] and porcine embryos [52].
The apoptotic index of embryos co-cultured with Vero cells, although relatively low was still statistically higher than that of control embryos cultured in basal medium alone (1.7 vs 0.5, respectively; P<0.05). Morphologically these blastocysts were differentiated with well developed ICM's and no evidence of necrosis. Hardy et al examining the relationship between early fragmentation and cell allocation in the human blastocyst similarly observed higher levels of apoptosis in embryos of excellent morphology [53]. The incidence of apoptosis was observed to be higher in the ICM compared to the trophectoderm [5, 54, 55]. Also, cell death was correlated to total blastomere number in both mouse and human blastocysts [53].
Apoptosis is believed to play a role in regulation of cell number in the developing blastocyst, by allowing it to control the ICM lineage and to remove damaged cells [56] or those still retaining trophectodermal properties [57]. Aberrations in overall blastomere number and specifically the ICM: TE ratio has been hypothesized to be linked to large offspring syndrome [53]. One explanation for the observed increase in apoptosis with co-culture may be that the rapid rate of blastomere proliferation combined with excellent ICM differentiation, triggered a compensatory increase in programmed cell death to retain appropriate balance between ICM and trophectodermal compartments. This may not have been observed with other co-culture systems such as primary human oviduct [28, 29] and mouse uterine cells [50], because the mean cell number in co-cultured blastocysts was far lower (i.e. 48, 63 and 62, respectively).
Of the growth factors tested, GM-CSF had the most striking anti-apoptotic effect on thawed zygotes. TUNEL positive cells were not observed in any of the analyzed embryos by Day 5 of culture. GMCSF appeared to enhance continued cell survival, prevent apoptosis but did not influence overall cell number in developing blastocysts. The anti-apoptotic effect of this growth factor has also been reported with frozen-thawed human embryos grown to blastocyst. The apoptotic index was 2.1% for embryos thawed and cultured in GMCSF versus 4.5% in control medium [13]. This type of anti-apoptotic effect was also observed with fresh mouse embryos by Behr et al. However, total cell number in blastocysts derived from control vs GM-CSF supplemented medium in that study was quite low (33 and 38 respectively) [49]. GMCSF supplementation may have simply “rescued” embryos. In contrast, our test system gave high cell counts even in un-supplemented control medium (100±44) and even on this background the effect of GMCSF supplementation was clearly evident. In vivo GMCSF is found in both oviductal and uterine fluids [58]. GM-CSF may play a vital role in maintaining cell viability.
The role of IGF-I [10, 59], IGF-II [11], EGF [4] and TGF-α [2, 44] as survival and anti-apoptotic factors has been well-documented with fresh embryos. Our data with frozen embryos suggested that culture medium supplementation with the growth factors IGF-I and IGF-II may decrease embryonic apoptosis. The apoptotic index of 0.2 was lower than that in control medium but did not reach statistical significance.
TNF-α has generally been associated with negative effects on embryonic development including high levels of apoptosis [4, 21, 60]. These negative effects, however, were not obvious under conditions of serum and growth factor deprivation [61]. TNF-α concentration in the aforementioned studies ranged from 10 to 1000 ng/ml and fresh embryos were used for testing. The negative effects of TNF-α on embryos were also found to be dose dependent [4, 21]. At high concentrations, blastocyst development and overall cell number was severely impaired. The lower concentration of TNF-α (2 ng/ml) used in the present investigation and the optimized basal culture media may explain our contradictory findings.
From these data we conclude that growth factors and co-culture can modulate the post-thaw development of cryopreserved embryos by regulating embryonic apoptosis and increasing cell proliferation and ICM differentiation. Behavior of frozen-thawed embryos in response to growth factors may be quite different from fresh embryos and needs further examination. Apoptosis in thawed embryos has not been widely studied. The extensive panel of growth factors assessed and the comparison to co-culture makes this study unique. Successful modulation of an embryo's in-vitro environment to maximize mitosis and reduce stress related apoptosis may play a major role in improving pregnancy outcomes with frozen-thawed embryos.
Acknowledgments
Financial support: Research grant from the Cleveland Clinic Foundation, Cleveland, Ohio, USA.
Footnotes
Presented in part at: the 60th annual meeting of the American Society for Reproductive Medicine, 2004, October 16–20, Philadelphia, PA, USA.
References
- 1.Hardy K, Handyside AH, Winston RM. The human blastocyst: cell number, death and allocation during late preimplantation development in vitro. Development. 1989;107:597–604. doi: 10.1242/dev.107.3.597. [DOI] [PubMed] [Google Scholar]
- 2.Brison DR, Schultz RM. Apoptosis during mouse blastocyst formation: evidence for a role for survival factors including transforming growth factor alpha. Biol Reprod. 1997;56:1088–1096. doi: 10.1095/biolreprod56.5.1088. [DOI] [PubMed] [Google Scholar]
- 3.Exley GE, Tang C, McElhinny AS, Warner CM. Expression of caspase and BCL-2 apoptotic family members in mouse preimplantation embryos. Biol Reprod. 1999;61:231–239. doi: 10.1095/biolreprod61.1.231. [DOI] [PubMed] [Google Scholar]
- 4.Kurzawa R, Glabowski W, Wenda-Rozewicka L. Evaluation of mouse preimplantation embryos cultured in media enriched with insulin-like growth factors I and II, epidermal growth factor and tumor necrosis factor alpha. Folia Histochem Cytobiol. 2001;39:245–251. [PubMed] [Google Scholar]
- 5.Kamjoo M, Brison DR, Kimber SJ. Apoptosis in the preimplantation mouse embryo: effect of strain difference and in vitro culture. Mol Reprod Dev. 2002;61:67–77. doi: 10.1002/mrd.1132. [DOI] [PubMed] [Google Scholar]
- 6.Smith S, Francis R, Guilbert L, Baker PN. Growth factor rescue of cytokine mediated trophoblast apoptosis. Placenta. 2002;23:322–330. doi: 10.1053/plac.2001.0783. [DOI] [PubMed] [Google Scholar]
- 7.Spanos S, Rice S, Karagiannis P, Taylor D, Becker DL, Winston RM, Hardy K. Caspase activity and expression of cell death genes during development of human preimplantation embryos. Reproduction. 2002;124:353–363. doi: 10.1530/rep.0.1240353. [DOI] [PubMed] [Google Scholar]
- 8.Hardy K, Spanos S. Growth factor expression and function in the human and mouse preimplantation embryo. J Endocrinol. 2002;172:221–236. doi: 10.1677/joe.0.1720221. [DOI] [PubMed] [Google Scholar]
- 9.Huppertz B, Herrler A. Regulation of proliferation and apoptosis during development of the preimplantation embryo and the placenta. Birth Defects Res C Embryo Today. 2005;75:249–261. doi: 10.1002/bdrc.20056. [DOI] [PubMed] [Google Scholar]
- 10.Spanos S, Becker DL, Winston RM, Hardy K. Anti-apoptotic action of insulin-like growth factor-I during human preimplantation embryo development. Biol Reprod. 2000;63:1413–1420. doi: 10.1095/biolreprod63.5.1413. [DOI] [PubMed] [Google Scholar]
- 11.Byrne AT, Southgate J, Brison DR, Leese HJ. Effects of insulin-like growth factors I and II on tumour-necrosis-factor-alpha-induced apoptosis in early murine embryos. Reprod Fertil Dev. 2002;14:79–83. doi: 10.1071/RD01015. [DOI] [PubMed] [Google Scholar]
- 12.Byrne AT, Southgate J, Brison DR, Leese HJ. Regulation of apoptosis in the bovine blastocyst by insulin and the insulin-like growth factor (IGF) superfamily. Mol Reprod Dev. 2002;62:489–495. doi: 10.1002/mrd.10153. [DOI] [PubMed] [Google Scholar]
- 13.Sjoblom C, Wikland M, Robertson SA. Granulocyte-macrophage colony-stimulating factor (GM-CSF) acts independently of the beta common subunit of the GM-CSF receptor to prevent inner cell mass apoptosis in human embryos. Biol Reprod. 2002;67:1817–1823. doi: 10.1095/biolreprod.101.001503. [DOI] [PubMed] [Google Scholar]
- 14.Gopichandran N, Leese HJ. The effect of paracrine/autocrine interactions on the in vitro culture of bovine preimplantation embryos. Reproduction. 2006;131:269–277. doi: 10.1530/rep.1.00677. [DOI] [PubMed] [Google Scholar]
- 15.Bongso A, Soon-Chye N, Sathananthan H, Lian NP, Rauff M, Ratnam S. Improved quality of human embryos when co-cultured with human ampullary cells. Hum Reprod. 1989;4:706–713. doi: 10.1093/oxfordjournals.humrep.a136971. [DOI] [PubMed] [Google Scholar]
- 16.Menezo YJ, Guerin JF, Czyba JC. Improvement of human early embryo development in vitro by coculture on monolayers of Vero cells. Biol Reprod. 1990;42:301–306. doi: 10.1095/biolreprod42.2.301. [DOI] [PubMed] [Google Scholar]
- 17.Wiemer KE, Hoffman DI, Maxson WS, Eager S, Muhlberger B, Fiore I, Cuervo M. Embryonic morphology and rate of implantation of human embryos following co-culture on bovine oviductal epithelial cells. Hum Reprod. 1993;8:97–101. doi: 10.1093/oxfordjournals.humrep.a137884. [DOI] [PubMed] [Google Scholar]
- 18.Desai NN, Kennard EA, Kniss DA, Friedman CI. Novel human endometrial cell line promotes blastocyst development. Fertil Steril. 1994;61:760–766. [PubMed] [Google Scholar]
- 19.Desai N, F A-H, Goldfarb J. Sequential assesment of embryonic morphology in D5 transfer cycles and relationship to pregnancy outcome. Fertil Steril. 2006;86:171. doi: 10.1016/j.fertnstert.2006.07.456. [DOI] [Google Scholar]
- 20.O’Neill C. Evidence for the requirement of autocrine growth factors for development of mouse preimplantation embryos in vitro. Biol Reprod. 1997;56:229–237. doi: 10.1095/biolreprod56.1.229. [DOI] [PubMed] [Google Scholar]
- 21.Wuu YD, Pampfer S, Becquet P, Vanderheyden I, Lee KH, De Hertogh R. Tumor necrosis factor alpha decreases the viability of mouse blastocysts in vitro and in vivo. Biol Reprod. 1999;60:479–483. doi: 10.1095/biolreprod60.2.479. [DOI] [PubMed] [Google Scholar]
- 22.Desai N, Lawson J, Goldfarb J. Assessment of growth factor effects on post-thaw development of cryopreserved mouse morulae to the blastocyst stage. Hum Reprod. 2000;15:410–418. doi: 10.1093/humrep/15.2.410. [DOI] [PubMed] [Google Scholar]
- 23.Karagenc L, Lane M, Gardner DK. Granulocyte-macrophage colony-stimulating factor stimulates mouse blastocyst inner cell mass development only when media lack human serum albumin. Reprod Biomed Online. 2005;10:511–518. doi: 10.1016/S1472-6483(10)60829-2. [DOI] [PubMed] [Google Scholar]
- 24.Boonkusol D, Gal AB, Bodo S, Gorhony B, Kitiyanant Y, Dinnyes A. Gene expression profiles and in vitro development following vitrification of pronuclear and 8-cell stage mouse embryos. Mol Reprod Dev. 2006;73:700–708. doi: 10.1002/mrd.20450. [DOI] [PubMed] [Google Scholar]
- 25.Nematollahi N, Valojerdi MR. Effect of Vero cell coculture on the development of frozen-thawed two-cell mouse embryos. J Assist Reprod Genet. 1999;16:380–384. doi: 10.1023/A:1020598031275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Valojerdi MR, Movahedin M, Hosseini A. Improvement of development of vitrified two-cell mouse embryos by vero cell coculture. J Assist Reprod Genet. 2002;19:31–38. doi: 10.1023/A:1014010706767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mtango NR, Varisanga MD, Dong YJ, Rajamahendran R, Suzuki T. Growth factors and growth hormone enhance in vitro embryo production and post-thaw survival of vitrified bovine blastocysts. Theriogenology. 2003;59:1393–1402. doi: 10.1016/S0093-691X(02)01163-9. [DOI] [PubMed] [Google Scholar]
- 28.Xu J, Cheung TM, Chan ST, Ho PC, Yeung WS. Human oviductal cells reduce the incidence of apoptosis in cocultured mouse embryos. Fertil Steril. 2000;74:1215–1219. doi: 10.1016/S0015-0282(00)01618-6. [DOI] [PubMed] [Google Scholar]
- 29.Xu JS, Lee YL, Lee KF, Kwok KL, Lee WM, Luk JM, Yeung WS. Embryotrophic factor-3 from human oviductal cells enhances proliferation, suppresses apoptosis and stimulates the expression of the beta1 subunit of sodium-potassium ATPase in mouse embryos. Hum Reprod. 2004;19:2919–2926. doi: 10.1093/humrep/deh497. [DOI] [PubMed] [Google Scholar]
- 30.Ouhibi N, Hamidi J, Guillaud J, Menezo Y. Co-culture of 1-cell mouse embryos on different cell supports. Hum Reprod. 1990;5:737–743. doi: 10.1093/oxfordjournals.humrep.a137178. [DOI] [PubMed] [Google Scholar]
- 31.Desai N, Goldfarb J. Co-cultured human embryos may be subjected to widely different microenvironments: pattern of growth factor/cytokine release by Vero cells during the co-culture interval. Hum Reprod. 1998;13:1600–1605. doi: 10.1093/humrep/13.6.1600. [DOI] [PubMed] [Google Scholar]
- 32.Desai N, Lawson J, Goldfarb J. Effect of culture regimen on blastocyst development, cell number and differentiation: Comparison of commercial blastocyst culture media to co-culture on Vero cell monolayers. Fertil Steril. 2000;74:S40-S41. [Google Scholar]
- 33.Menezo YJ, Sakkas D, Janny L. Co-culture of the early human embryo: factors affecting human blastocyst formation in vitro. Microsc Res Tech. 1995;32:50–56. doi: 10.1002/jemt.1070320105. [DOI] [PubMed] [Google Scholar]
- 34.Desai N, Kinzer D, Loeb A, Goldfarb J. Use of Synthetic Serum Substitute and alpha-minimum essential medium for the extended culture of human embryos to the blastocyst stage. Hum Reprod. 1997;12:328–335. doi: 10.1093/humrep/12.2.328. [DOI] [PubMed] [Google Scholar]
- 35.Bongso A, Fong C, Ng S. Coculture techniques for blastocyst transfer and embryonic stem cell production. Assist Reprod Rev. 1995;5:106–114. [Google Scholar]
- 36.Chegini N. Oviductal-derived growth factors and cytokines: implication in preimplantation. Semin Reprod Endocrinol. 1996;14:219–229. doi: 10.1055/s-2007-1016332. [DOI] [PubMed] [Google Scholar]
- 37.Srivastava MD, Lippes J, Srivastava BI. Cytokines of the human reproductive tract. Am J Reprod Immunol. 1996;36:157–166. doi: 10.1111/j.1600-0897.1996.tb00157.x. [DOI] [PubMed] [Google Scholar]
- 38.Tsai YJ, Lee RK, Lin SP, Chen YH. Identification of a novel platelet-derived growth factor-like gene, fallotein, in the human reproductive tract. Biochim Biophys Acta. 2000;1492:196–202. doi: 10.1016/s0167-4781(00)00066-x. [DOI] [PubMed] [Google Scholar]
- 39.Kimber SJ. Leukaemia inhibitory factor in implantation and uterine biology. Reproduction. 2005;130:131–145. doi: 10.1530/rep.1.00304. [DOI] [PubMed] [Google Scholar]
- 40.Carnegie JA, Morgan JJ, McDiarmid N, Durnford R. Influence of protein supplements on the secretion of leukaemia inhibitory factor by mitomycin-pretreated Vero cells: possible application to the in vitro production of bovine blastocysts with high cryotolerance. J Reprod Fertil. 1999;117:41–48. doi: 10.1530/jrf.0.1170041. [DOI] [PubMed] [Google Scholar]
- 41.Roudebush WE, Often NL, Butler WJ. Alpha-minimum essential medium (MEM) enhances in vitro hatched blastocyst development and cell number per embryo over Ham's F-10. J Assist Reprod Genet. 1994;11:203–207. doi: 10.1007/BF02211809. [DOI] [PubMed] [Google Scholar]
- 42.Frasor J, Sherbahn R, Soltes B, Molo MW, Binor Z, Radwanska E, Rawlins RG. Optimizing tubal epithelial cell growth promotes mouse embryo hatching in coculture. J Assist Reprod Genet. 1996;13:423–430. doi: 10.1007/BF02066176. [DOI] [PubMed] [Google Scholar]
- 43.Paria BC, Dey SK. Preimplantation embryo development in vitro: cooperative interactions among embryos and role of growth factors. Proc Natl Acad Sci USA. 1990;87:4756–4760. doi: 10.1073/pnas.87.12.4756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brison DR, Schultz RM. Increased incidence of apoptosis in transforming growth factor alpha-deficient mouse blastocysts. Biol Reprod. 1998;59:136–144. doi: 10.1095/biolreprod59.1.136. [DOI] [PubMed] [Google Scholar]
- 45.Robertson SA, Sjoblom C, Jasper MJ, Norman RJ, Seamark RF. Granulocyte-macrophage colony-stimulating factor promotes glucose transport and blastomere viability in murine preimplantation embryos. Biol Reprod. 2001;64:1206–1215. doi: 10.1095/biolreprod64.4.1206. [DOI] [PubMed] [Google Scholar]
- 46.Cheung LP, Leung HY, Bongso A. Effect of supplementation of leukemia inhibitory factor and epidermal growth factor on murine embryonic development in vitro, implantation, and outcome of offspring. Fertil Steril. 2003;80(Suppl 2):727–735. doi: 10.1016/S0015-0282(03)00772-6. [DOI] [PubMed] [Google Scholar]
- 47.Diaz-Cueto L, Gerton GL. The influence of growth factors on the development of preimplantation mammalian embryos. Arch Med Res. 2001;32:619–626. doi: 10.1016/S0188-4409(01)00326-5. [DOI] [PubMed] [Google Scholar]
- 48.Nandi S, Ravindranatha BM, Gupta PS, Raghu HM, Sarma PV. Developmental competence and post-thaw survivability of buffalo embryos produced in vitro: effect of growth factors in oocyte maturation medium and of embryo culture system. Theriogenology. 2003;60:1621–1631. doi: 10.1016/S0093-691X(03)00148-1. [DOI] [PubMed] [Google Scholar]
- 49.Behr B, Mooney S, Wen Y, Polan ML, Wang H. Preliminary experience with low concentration of granulocyte-macrophage colony-stimulating factor: a potential regulator in preimplantation mouse embryo development and apoptosis. J Assist Reprod Genet. 2005;22:25–32. doi: 10.1007/s10815-005-0817-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Azadbakht M, Valojerdi MR, Mowla SJ. Development of mouse embryos co-cultured with polarized or non-polarized uterine epithelial cells using sequential culture media. Anim Reprod Sci 2006. [DOI] [PubMed]
- 51.Lane M, Maybach JM, Gardner DK. Addition of ascorbate during cryopreservation stimulates subsequent embryo development. Hum Reprod. 2002;17:2686–2693. doi: 10.1093/humrep/17.10.2686. [DOI] [PubMed] [Google Scholar]
- 52.Tatemoto H, Ootaki K, Shigeta K, Muto N. Enhancement of developmental competence after in vitro fertilization of porcine oocytes by treatment with ascorbic acid 2-O-alpha-glucoside during in vitro maturation. Biol Reprod. 2001;65:1800–1806. doi: 10.1095/biolreprod65.6.1800. [DOI] [PubMed] [Google Scholar]
- 53.Hardy K, Stark J, Winston RM. Maintenance of the inner cell mass in human blastocysts from fragmented embryos. Biol Reprod. 2003;68:1165–1169. doi: 10.1095/biolreprod.102.010090. [DOI] [PubMed] [Google Scholar]
- 54.Hardy K, Warner A, Winston RM, Becker DL. Expression of intercellular junctions during preimplantation development of the human embryo. Mol Hum Reprod. 1996;2:621–632. doi: 10.1093/molehr/2.8.621. [DOI] [PubMed] [Google Scholar]
- 55.Hardy K. Cell death in the mammalian blastocyst. Mol Hum Reprod. 1997;3:919–925. doi: 10.1093/molehr/3.10.919. [DOI] [PubMed] [Google Scholar]
- 56.Erickson GF. Defining apoptosis: players and systems. J Soc Gynecol Investig. 1997;4:219–228. doi: 10.1016/S1071-5576(97)00044-0. [DOI] [PubMed] [Google Scholar]
- 57.Handyside A, Hunter S. Cell division and death in the mouse blastocyst before implantation. Roux’s Archives of Developmental Biology. 1986;195:519–526. doi: 10.1007/BF00375893. [DOI] [PubMed] [Google Scholar]
- 58.Giacomini G, Tabibzadeh SS, Satyaswaroop PG, Bonsi L, Vitale L, Bagnara GP, Strippoli P, et al. Epithelial cells are the major source of biologically active granulocyte macrophage colony-stimulating factor in human endometrium. Hum Reprod. 1995;10:3259–3263. doi: 10.1093/oxfordjournals.humrep.a135899. [DOI] [PubMed] [Google Scholar]
- 59.Fabian D, Il'kova G, Rehak P, Czikkova S, Baran V, Koppel J. Inhibitory effect of IGF-I on induced apoptosis in mouse preimplantation embryos cultured in vitro. Theriogenology. 2004;61:745–755. doi: 10.1016/S0093-691X(03)00254-1. [DOI] [PubMed] [Google Scholar]
- 60.Glabowski W, Kurzawa R, Wiszniewska B, Baczkowski T, Marchlewicz M, Brelik P. Growth factors effects on preimplantation development of mouse embryos exposed to tumor necrosis factor alpha. Reprod Biol. 2005;5:83–99. [PubMed] [Google Scholar]
- 61.Madge LA, Pober JS. A phosphatidylinositol 3-kinase/Akt pathway, activated by tumor necrosis factor or interleukin-1, inhibits apoptosis but does not activate NFkappaB in human endothelial cells. J Biol Chem. 2000;275:15458–15465. doi: 10.1074/jbc.M001237200. [DOI] [PubMed] [Google Scholar]