Abstract
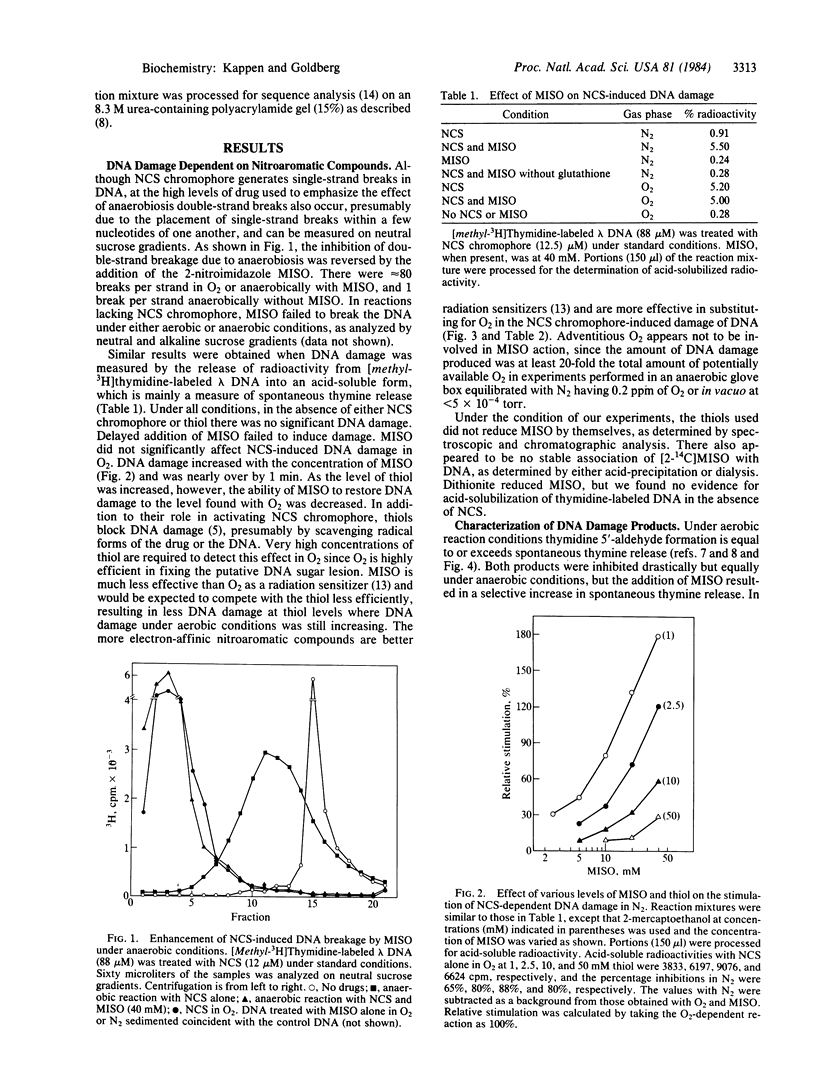
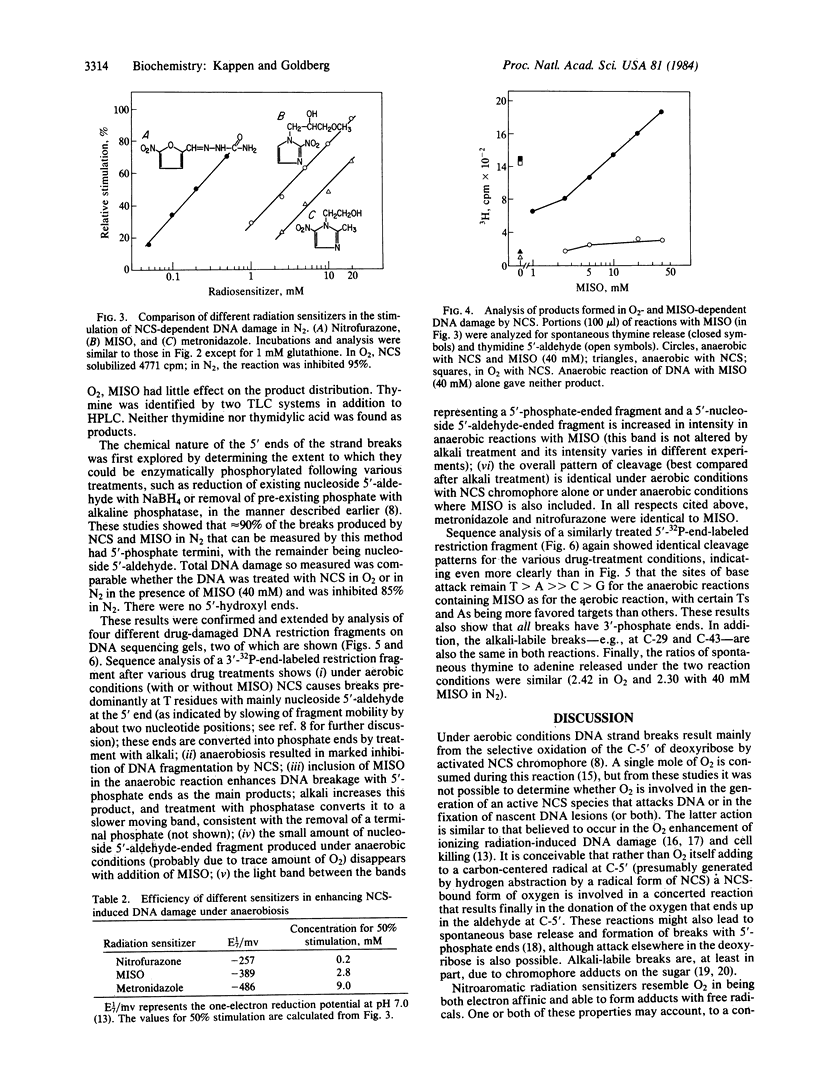
The ability of neocarzinostatin (NCS) chromophore to damage DNA, as manifested by strand breaks and base release, is markedly decreased under anaerobic conditions but can be restored by nitroaromatic radiation sensitizers, which by themselves have no effect. The effectiveness of these compounds is correlated with their electron affinity as measured by their one-electron reduction potentials and is inversely related to the concentration of thiol used to activate the NCS. Whereas strand breaks with thymidine 5'-aldehyde at the 5' end and released thymine are the main DNA damage products in O2, under anaerobic conditions misonidazole causes a marked increase in the release of thymine and in the formation of breaks with 5'- phosphate ends. In both cases the 3' end of the break carries a phosphate group, and the attack-site specificity of spontaneous and alkali-labile DNA strand breakage and base release are identical. In O2, misonidazole does not affect the extent of DNA damage or alter the distribution of DNA damage products found with NCS alone. The data do not distinguish whether the nitroaromatic compounds function by interacting with NCS-induced nascent damage on the DNA, by being converted by activated NCS into a DNA-damaging species, or by participating in the activation of NCS to a DNA-damaging species. The implications of these results for the treatment of hypoxic tumor cells with the combined use of radiomimetic drugs and radiation sensitizers are discussed.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burger R. M., Peisach J., Horwitz S. B. Effect of light and oxygen on neocarzinostatin stability and DNA-cleaving activity. J Biol Chem. 1978 Jul 25;253(14):4830–4832. [PubMed] [Google Scholar]
- Favaudon V. Gamma-radiolysis study of the reductive activation of neocarzinostatin by the carboxyl radical. Biochimie. 1983 Nov-Dec;65(11-12):593–607. doi: 10.1016/s0300-9084(84)80023-1. [DOI] [PubMed] [Google Scholar]
- Hatayama T., Goldberg I. H. Deoxyribonucleic acid sugar damage in the action of neocarzinostatin. Biochemistry. 1980 Dec 9;19(25):5890–5898. doi: 10.1021/bi00566a035. [DOI] [PubMed] [Google Scholar]
- Hensens O. D., Dewey R. S., Liesch J. M., Napier M. A., Reamer R. A., Smith J. L., Albers-Schönberg G., Goldberg I. H. Neocarzinostatin chromophore: presence of a highly strained ether ring and its reaction with mercaptan and sodium borohydride. Biochem Biophys Res Commun. 1983 Jun 15;113(2):538–547. doi: 10.1016/0006-291x(83)91759-x. [DOI] [PubMed] [Google Scholar]
- Kappen L. S., Goldberg I. H. Activation and inactivation of neocarzinostatin-induced cleavage of DNA. Nucleic Acids Res. 1978 Aug;5(8):2959–2967. doi: 10.1093/nar/5.8.2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappen L. S., Goldberg I. H. Deoxyribonucleic acid damage by neocarzinostatin chromophore: strand breaks generated by selective oxidation of C-5' of deoxyribose. Biochemistry. 1983 Oct 11;22(21):4872–4878. doi: 10.1021/bi00290a002. [DOI] [PubMed] [Google Scholar]
- Kappen L. S., Goldberg I. H., Liesch J. M. Identification of thymidine-5'-aldehyde at DNA strand breaks induced by neocarzinostatin chromophore. Proc Natl Acad Sci U S A. 1982 Feb;79(3):744–748. doi: 10.1073/pnas.79.3.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappen L. S., Napier M. A., Goldberg I. H. Roles of chromophore and apo-protein in neocarzinostatin action. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1970–1974. doi: 10.1073/pnas.77.4.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox R. J., Knight R. C., Edwards D. I. Misonidazole-induced thymidine release from DNA. Biochem Pharmacol. 1981 Jul 15;30(14):1925–1929. doi: 10.1016/0006-2952(81)90201-x. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Mousstacchi E., Favaudon V. Cytotoxic and mutagenic effects of neocarzinostatin in wild-type and repair-deficient yeasts. Mutat Res. 1982 Apr;104(1-3):87–94. doi: 10.1016/0165-7992(82)90125-7. [DOI] [PubMed] [Google Scholar]
- Povirk L. F., Dattagupta N., Warf B. C., Goldberg I. H. Neocarzinostatin chromophore binds to deoxyribonucleic acid by intercalation. Biochemistry. 1981 Jul 7;20(14):4007–4014. doi: 10.1021/bi00517a009. [DOI] [PubMed] [Google Scholar]
- Povirk L. F., Goldberg I. H. Binding of the nonprotein chromophore of neocarzinostatin to deoxyribonucleic acid. Biochemistry. 1980 Oct 14;19(21):4773–4780. doi: 10.1021/bi00562a009. [DOI] [PubMed] [Google Scholar]
- Povirk L. F., Goldberg I. H. Covalent adducts of DNA and the nonprotein chromophore of neocarzinostatin contain a modified deoxyribose. Proc Natl Acad Sci U S A. 1982 Jan;79(2):369–373. doi: 10.1073/pnas.79.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Povirk L. F., Goldberg I. H. Inhibition of mammalian deoxyribonucleic acid synthesis by neocarzinostatin: selective effect on replicon initiation in CHO cells and resistant synthesis in ataxia telangiectasia fibroblasts. Biochemistry. 1982 Nov 9;21(23):5857–5862. doi: 10.1021/bi00266a020. [DOI] [PubMed] [Google Scholar]
- Povirk L. F., Goldberg I. H. Neocarzinostatin chromophore-DNA adducts: evidence for a covalent linkage to the oxidized C-5' of deoxyribose. Nucleic Acids Res. 1982 Oct 25;10(20):6255–6264. doi: 10.1093/nar/10.20.6255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Povirk L. F., Goldberg I. H. Stoichiometric uptake of molecular oxygen and consumption of sulfhydryl groups by neocarzinostatin chromophore bound to DNA. J Biol Chem. 1983 Oct 10;258(19):11763–11767. [PubMed] [Google Scholar]
- Raleigh J. A., Kremers W., Whitehouse R. Effect of oxygen and nitroaromatic cell radiosensitizers on radiation-induced cleavage of internucleotide bonds: ApA, dApA, and poly(A). Radiat Res. 1975 Jul;63(1):53–63. [PubMed] [Google Scholar]
- Shiloh Y., Becker Y. Reduced inhibition of replicon initiation and chain elongation by neocarzinostatin in skin fibroblasts from patients with ataxia telangiectasia. Biochim Biophys Acta. 1982 Dec 30;721(4):485–488. doi: 10.1016/0167-4889(82)90105-7. [DOI] [PubMed] [Google Scholar]
- Shiloh Y., Tabor E., Becker Y. Repair of potentially lethal and sublethal damage induced by neocarzinostatin in normal and ataxia-telangiectasia skin fibroblasts. Biochem Biophys Res Commun. 1983 Jan 27;110(2):483–490. doi: 10.1016/0006-291x(83)91175-0. [DOI] [PubMed] [Google Scholar]
- Wardman P. The use of nitroaromatic compounds as hypoxic cell radiosensitizers. Curr Top Radiat Res Q. 1977 Aug;11(4):347–398. [PubMed] [Google Scholar]