Abstract
Purpose: To develop an in vitro method for tagging embryos and to compare the development of the embryos after nanoparticles injection versus externally-applied nanoparticles derived from either polystyrene or polyacrylonitrile.
Methods: Each mouse 1-cell embryo (the selected test-model) was either: (a) injected by intracytoplasmic injection or (b) co-incubated with different nanoparticles at 37°C, 5% CO2 in air. The embryos were assessed after 2 and 6 days of culture.
Results: Embryo development was similar for externally-applied polystyrene nanoparticles and control (97.6 ± 2.7 versus 100.0 ± 0%) but different for polyacrylonitrile nanoparticles (90.0 ± 2.8 %) on day 2. However, the results were similar on Day 6. Injected embryos were linked to lower percent development on Day 2. Few injected embryos reached blastocyst stage on Day 6 after a brief UV-fluorescence exposure.
Conclusions: Tagging embryos by external polystyrene-based nanoparticles was the better method when compared with injected nanoparticles. Larger nanoparticles in microsphere range were easier to qualitate. Inhibited hatching limited their use beyond the blastocyst stage.
Keywords: Mouse preimplantation embryos, ID tagging, ICSI ART, Nanoparticles, Microspheres
Introduction
The tagging of cells using fluorescent fusion proteins has been carried out for the purpose of tracking the intracellular movement of proteins, signaling dynamics or nuclear activity [1–3]. The need to tag and identify embryos during assisted reproductive technologies (ART) is important and the availability of synthetic nanoparticles may address such a need. A nanoparticle or ultrafine particle has a particle mass of less than 100 nm in diameter. Nanoparticles are made from materials such as cadmium selenide [4,5], gold [6], silver [7], perylene [8], polystyrene [9], carbon [10,11], iron oxide [12], silica [13], titanium dioxide [14]. Some types of nanoparticles are organic-based such as latex [15], polylactic acid [16], polyglycolic acid [17] and polyalkylcyanoacrylate [18] while others are based on carbon, sulfates, nitrates [19,20], aluminum, silicon, or titanium [21].
In applied medicine, nanoparticles are used to tag antibodies to identify molecules, organisms, or nucleic acid sequences. In addition, they are used as nanoshells to deliver drugs for cancer or gene therapy [22], tissue implants and cellular imaging [23,24]. Nanoparticles are commercially available in specific sizes and identifiable colors. A preliminary step would be to ascertain the feasibility of placement of the nanoparticles in the embryo either injected internally or externally in the culture medium. Hence, the hypothesis was that embryos could be tagged internally or externally using nanoparticles. The objectives were: (a) to assess the development of embryos after nanoparticles injection versus externally-applied nanoparticles and (b) to compare development in 2 different types of nanoparticles, an inorganic-based polystyrene and an organic-based polyacrylonitrile nanoparticles.
Materials and methods
Preparation of mouse embryos and nanoparticles
Cryopreserved one-cell mouse embryos in plastic straws (Embryotech Laboratories, Wilmington, MA) were thawed at room temperature (22°C) for 2 mins, warmed at 37°C for 2 mins, and cooled at room temperature for another 2 mins before being expelled into G1.3 culture medium (Vitrolife, Inc. Englewood, CO) and washed by passage through medium droplets. The embryos were pooled according to a randomization protocol and were incubated at 37°C, 6% CO2 in air mixture in double-welled Falcon culture dish (3037, Becton Dickinson and Co., Oxnard, CA) with sterile-filtered water in the outer moat for humidity. The pooled embryos were divided into 2 groups for the internal versus external nanoparticles experiment. The experiment was repeated 3 times and the results analyzed as described below.
Two different types of nanoparticles were utilized. The first type was from a kit (Constellation kit, Molecular Probes Inc., Eugene, OR) containing mixed-size ultrafine polystyrene particles ranging from 40 nm to over 120 nm microsphere-size particles. The different fluorescent colors corresponded to the particle size. The smallest particles fluoresced green or red and were barely visible under high magnification (500×) UV epi-fluorescence microscopy. The larger nanoparticles were clearly visible and the colors easily distinguished.
The second type of nanoparticles was 40 nm carboxylated polyacrylonitrile nanobeads (Fluka Chemie AG GmbH, Buch, Switzerland). These particles appeared as an orange-red fluorescent hazy cloud under high magnification UV epi-flourescence microscopy. All nanoparticles were washed by high-speed centrifugation, resuspended in G1.3 medium and tested at either 0 (nanoparticles-absent control) or at 14–16 million/mL.
Intracytoplasmic injection of nanoparticles
Ideally, the nanoparticles could be injected together with the sperm into the ooplasm during the ICSI procedure but for the purposes here, injection of fertilized one-cell embryos was performed. The embryos were pipetted into microdrops (100 uL) of G1.3 culture medium maintained under paraffin oil (Day 0). Using micromanipulation techniques, each embryo was removed and held in place by a holding pipet and ‘tagged’ using a small volume (equal to the volume used in the ICSI procedure) of either polystyrene or polyacrylonitrile nanoparticles injected into the cytoplasm of the embryo. Care was taken to avoid injecting in the area proximal to the 2 polar bodies to avoid disrupting the chromosomes. The negative control consisted of sham injection of the embryos. The injected embryos were placed into culture medium (1 mL) at the center well of the double-well marked dishes and incubated at 37°C, 6 % CO2 in air mixture.
After 2 days of incubation, the developmental stages of the embryos were assessed by phase contrast microscopy. Additionally, the embryos in the culture dishes were exposed to UV light on the stage of a UV fluorescent microscope for an arbitrary 5 s to simulate the identification process for the embryos. The embryos were further incubated until day 6 and embryo development assessed.
Externally-applied nanoparticles
One-cell mouse embryos were cultured in G1.3 medium in double-well petri dishes at 37°C, 6 % CO2 in air mixture. The embryos were externally ‘tagged’ with specific nanoparticles (14–16 million/mL polystyrene or polyacrylonitrile) pipetted into the medium containing the embryos (Day 0). The control embryos received G1.3 culture medium. All the embryos were incubated for 2 days and the embryonic stage of development assessed. Aliquots of culture medium from the dishes with embryos were pipetted out and examined for type and color of nanoparticles under UV-epifluorescence microscopy in a simulated identification procedure. The embryos were further incubated until day 6 and embryo development assessed.
Statistical analysis
The embryos were assessed for development from the one-cell stage to the 2 to 8-cell stages after 2 days of incubation. Assessment after 48 h of culture was carried out to rule out inhibited embryo development due to the 2-cell block noted for in vitro cultures. The number of blastomeres were counted for each embryo and fragmentation or degeneration noted. After 6 days of incubation, the embryos were assessed for blastocyst development. Advanced development of blastocysts were characterized by hatching or hatched with evidence of empty zona pellucida shells. The results were presented as mean ± standard error of the mean (S.E.M.). Significant difference was tested using the Chi-square test statistic (Epistat Services, Richardson, TX). A difference with P < 0.05 was considered significant.
Results
After 2 days of incubation, the percentages of developed embryos were significantly lower in the groups injected with both types of nanoparticles when compared with the sham-injected control group (Table 1). Although the group of embryos injected with polystyrene-based nanoparticles had a numerically higher percentage of development when compared with the polyacrylonitrile group, this difference was not statistically significant. Very few embryos developed to the blastocyst stage following the brief UV light exposure required for the identification process to characterize the nanoparticles.
Table 1.
Application of 2 different types of nanoparticles for the in vitro tagging of embryos
| Percentage 2–8 cells on | Percentage blastocysts on | Percentage hatching blastocysts | ||
|---|---|---|---|---|
| Parameter | N | Day 2 | Day 6 | on Day 6 |
| Internal injected and UV-pulsed nanoparticles | ||||
| Sham-injected control | 23 | 95.7±6.8 | 4.5±4.1 | 0±0 |
| Polyacrylonitrile-based | 23 | 47.8±20.1a | 0±0 | 0±0 |
| Polystyrene-based | 22 | 72.7±18.0a | 6.3±8.2 | 0±0 |
| Externally-applied nanoparticles | ||||
| Nanoparticles-absent control | 45 | 100.0±0.0 | 86.7±9.8 | 56.4±37.7 |
| Polyacrylonitrile-based | 40 | 90.0±2.8a | 0±0a | 0±0a |
| Polystyrene-based | 42 | 97.6±2.7 | 73.2±1.3 | 3.3±3.4a |
Note. The development of embryos were analyzed after the nanoparticles were either injected into fertilized 1-cell mouse oocytes or added into culture media containing the 1-cell mouse embryos and cultured for 6 days. Internal-injected embryos also received a 5 s UV exposure from an epifluorescent microscope simulating the identification procedure. Results are presented as mean±S.E.M.
aDifferent from control. P < 0.05.
The group of embryos co-incubated with external polystyrene nanoparticles had a similar percentage of development as the control on Day 2. Furthermore, on Day 6, development to the blastocyst stage was similar for embryos in the polystyrene nanoparticles and control groups. In contrast, embryos co-incubated with the polyacrylonitrile nanoparticles had a significantly lower percentage of development on Day 2 and further development ceased. On Day 6, embryos co-incubated with either type of nanoparticles had lower percentages of hatching blastocysts when compared with the control.
Larger nanoparticles injected into the one-cell embryos could be observed in the blastomeres of the developing embryo as fluorescent colored light dots. Polyacrylonitrile-based nanoparticles inside the embryo imparted a faint fluorescent orange-red glow to the blastomeres (Fig. 1B). The number of injected nanoparticles appeared to be equally divided in the blastomeres of each embryo. The intracellular location of the nanoparticles was random and did not aggregate around any particular organelle or structure. Interestingly, larger nanoparticles were frequently observed in the intercellular space outside the blastomeres but still remaining inside the zona pellucida enclosed embryo.
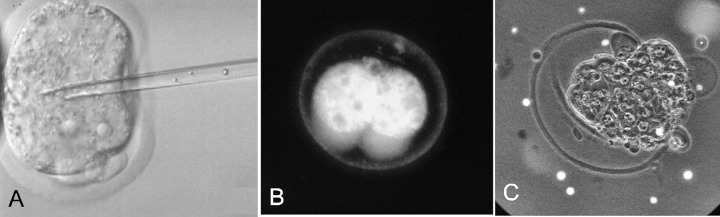
Fig. 1.
Comparison of two methods for the in vitro tagging of embryos using nanoparticles. (A). Nanoparticles being injected into one-cell mouse embryo using ICSI techniques (magnification 500×). (B). Fluorescent image of 4-cell mouse embryo 2 days after an injection of polyacrylonitrile nanoparticles (magnification 400×). (C). Fluorescent-phase contrast image of hatching blastocyst with external polystyrene nanoparticles (tiny spots outside the zona pellucida) on Day 6 (magnification 400×)
Discussion
The present study demonstrated that for the purpose of tagging and identifying embryos, externally-applied nanoparticles co-incubated with the embryos had the advantage over injected nanoparticles in terms of embryo development. One-cell embryos developed to blastocysts when co-incubated with the mixed-size polystyrene-based nanoparticles. The result was consistent with a recent study using 2-cell mouse embryos [25]. Removing an aliquot of medium surrounding each embryo and determining the color of the nanoparticles should assist in identifying the embryo origin. Not surprisingly the larger nanoparticles at microsphere sizes were easier to identify using the fluorescence microscope and were preferable for tagging procedures. The advantages of tagging with nanoparticles over separate embryo cultures included: (a) the one-time only tagging at the beginning of culture, (b) reduced mistakes from incorrect culture dish labeling during transfer into fresh culture medium, (c) inexpensive compared with radio frequency identification (RFID)-based labels, (d) reduced requirement for multiple dishes and incubators and (e) as an additional back-up identification marker for embryos.
In contrast to the external nanoparticles group, poly- styrene nanoparticles injected into the embryo reduced embryo development. Results from the sham-injected embryos suggested that the inhibited embryo development did not involve mechanical disruption resulting from the intracytoplasmic injection procedure. In contrast to mammalian embryos, a study of amphibian Xenopus embryos injected with cadmium selenide based quantum dot nanoparticles did not show any evidence of cellular disruption [5].
Note that different nanomaterials would yield different results as evidenced by the inhibitory effect of both externally-applied and injected polyacrylonitrile-based nanoparticles on mouse embryo development. The results from the polyacrylonitrile nanoparticles suggested that this nanomaterial mainly had an effect on the 8-cell embryo, a cell stage physically characterized by compaction, blastomere membrane fusion and intercellular gap junction communication [26]. The nanoparticles were localized in all blastomeres without translocating to any specific organelles. However, in amphibian Xenopus embryos, the nanoparticles were initially in the cytoplasm but with rapid cell division, the nanoparticles translocated to the nuclei by the mid-blastula stage [2].
An interesting observation was the inhibition of the blastocyst hatching process by the polystyrene-based nanoparticles while no blastocysts developed in the polyacrylonitrile group. The mechanisms for the lack of continued embryo development remained unknown. Studies in other cell types have shown that internalized nanoparticles [7] derived from materials other than that tested here disrupted fibroblast and cancer cells after adhering to the cell membranes, impaling and/or releasing toxic ions [27–32]. Moreover, nanoparticles internalized into cells increased the reactive oxygen species (ROS), elevated intracellular calcium, activated transcription factors [10] and initiated apoptosis or cell death [14]. Although these events have not been reported for embryos, the possibility exists that specific nanomaterials may interfere with the further development of the blastocysts. This suggested the need to assess and identify nontoxic nanomaterials such cadmium selenide for tagging embryos in future studies. Notably, the importance of the finding is tempered by the fact that embryos are transferred back before Day 6 and before the hatching stage in ART programs.
In summary, the present study showed that externally-applied nanoparticles co-incubated with the embryos were more suitable for tagging embryos in comparison with injected nanoparticles. Polystyrene-based nanoparticles supported limited embryo development up to the blastocyst stage while polyacrylonitrile-based nanoparticles exhibited embryotoxicity. More studies are needed to investigate the effects of different types, concentrations and sizes of nanoparticles suitable for tagging and identifying embryos.
Footnotes
Financial disclosure: The authors have no connection to any companies or products mentioned in this article.
References
- 1.Aguirre-Ghiso JA, Ossowski L, Rosenbaum SK. Green fluorescent protein tagging of extracellular signal-regulated kinase and p38 pathways reveals novel dynamics of pathway activation during primary and metastatic growth. Cancer Res. 2004;64:7336–45. doi: 10.1158/0008-5472.CAN-04-0113. [DOI] [PubMed] [Google Scholar]
- 2.Chapman S, Oparka KJ, Roberts AG. New tools for in vivo fluorescence tagging. Curr Opin Plant Biol. 2005;8:565–73. doi: 10.1016/j.pbi.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 3.Fraser ST, Hadjantonakis AK, Sahr KE, Willey S, Kelly OG, Jones EA, Dickinson ME, Baron MH. Using a histone yellow fluorescent protein fusion for tagging and tracking endothelial cells in ES cells and mice. Genesis. 2005;42:162–71. doi: 10.1002/gene.20139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Empedocles SA, Bawendi MG. Quantum-confined stark effect in single CdSe nanocrystallite quantum dots. Science. 1997;278:2114–17. doi: 10.1126/science.278.5346.2114. [DOI] [PubMed] [Google Scholar]
- 5.Dubertret B, Skourides P, Norris DJ, Noireaux V, Brivanlou AH, Libchaber A. In vivo imaging of quantum dots encapsulated in phospholipid micelles. Science. 2002;298:1759–62. doi: 10.1126/science.1077194. [DOI] [PubMed] [Google Scholar]
- 6.Stevenson KA, Muralidharan G, Maya L, Wells JC, Barhen J, Thundat T. Covalent attachment of gold nanoparticles to DNA templates. J Nanosci Nanotechnol. 2002;2:397–404. doi: 10.1166/jnn.2002.110. [DOI] [PubMed] [Google Scholar]
- 7.Kyriacou SV, Brownlow WJ, Xu XH. Using nanoparticle optics assay for direct observation of the function of antimicrobial agents in single live bacterial cells. Biochemistry. 2004;43:140–47. doi: 10.1021/bi0351110. [DOI] [PubMed] [Google Scholar]
- 8.Jinshui L, Lun W, Feng G, Yongxing L, Yun W. Novel fluorescent colloids as a DNA fluorescence probe. Anal Bioanal Chem. 2003;377:346–49. doi: 10.1007/s00216-003-2098-4. [DOI] [PubMed] [Google Scholar]
- 9.Brown DM, Wilson MR, MacNee W, Stone V, Donaldson K. Size-dependent proinflammatory effects of ultrafine polystyrene particles: a role for surface area and oxidative stress in the enhanced activity of ultrafines. Toxicol Appl Pharmacol. 2001;175:191–99. doi: 10.1006/taap.2001.9240. [DOI] [PubMed] [Google Scholar]
- 10.Brown DM, Donaldson K, Borm PJ, Schins RP, Dehnhardt M, Gilmour P, Jimenez LA, Stone V. Calcium and ROS-mediated activation of transcription factors and TNF-alpha cytokine gene expression in macrophages exposed to ultrafine particles. Am J Physiol Lung Cell Mol Physiol. 2004;286:L344–53. doi: 10.1152/ajplung.00139.2003. [DOI] [PubMed] [Google Scholar]
- 11.Oberdorster G, Sharp Z, Atudorei V, Elder A, Gelein R, Kreyling W, Cox C. Translocation of inhaled ultrafine particles to the brain. Inhal Toxicol. 2004;16:437–45. doi: 10.1080/08958370490439597. [DOI] [PubMed] [Google Scholar]
- 12.Jendelova P, Herynek V, Urdzikova L, Glogarova K, Kroupova J, Andersson B, Bryja V, Burian M, Hajek M, Sykova E. Magnetic resonance tracking of transplanted bone marrow and embryonic stem cells labeled by iron oxide nanoparticles in rat brain and spinal cord. J Neurosci Res. 2004;76:232–43. doi: 10.1002/jnr.20041. [DOI] [PubMed] [Google Scholar]
- 13.Luo D, Han E, Belcheva N, Saltzman WM. A self-assembled, modular DNA delivery system mediated by silica nanoparticles. J Control Release. 2004;95:333–41. doi: 10.1016/j.jconrel.2003.11.019. [DOI] [PubMed] [Google Scholar]
- 14.Rahman Q, Lohani M, Dopp E, Pemsel H, Jonas L, Weiss DG, Schiffmann D. Evidence that ultrafine titanium dioxide induces micronuclei and apoptosis in Syrian hamster embryo fibroblasts. Environ Health Perspect. 2002;110:797–800. doi: 10.1289/ehp.02110797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gesler RM, Garvin PJ, Klamer B, Robinson RU, Thompson CR, Gibson WR, Wheeler FC, Carlson RG. The biologic effects of polystyrene latex particles administered intravenously to rats–a collaborative study. Bull Parenter Drug Assoc. 1973;27:101–13. [PubMed] [Google Scholar]
- 16.Bazile DV, Ropert C, Huve P, Verrecchia T, Marlard M, Frydman A, Veillard M, Spenlehauer G. Body distribution of fully biodegradable [14C]-poly(lactic acid) nanoparticles coated with albumin after parenteral administration to rats. Biomaterials. 1992;13:1093–102. doi: 10.1016/0142-9612(92)90142-B. [DOI] [PubMed] [Google Scholar]
- 17.Venier-Julienne MC, Benoit JP. Preparation, purification and morphology of polymeric nanoparticles as drug carriers. Pharm Acta Helv. 1996;71:121–8. doi: 10.1016/0031-6865(95)00059-3. [DOI] [PubMed] [Google Scholar]
- 18.Fattal E, Vauthier C, Aynie I, Nakada Y, Lambert G, Malvy C, Couvreur P. Biodegradable polyalkylcyanoacrylate nanoparticles for the delivery of oligonucleotides. J Control Release. 1998;53:137–43. doi: 10.1016/S0168-3659(97)00246-0. [DOI] [PubMed] [Google Scholar]
- 19.Pope CA. Epidemiology of fine particulate air pollution and human health: biologic mechanisms and Who’s at risk? Environ Health Perspect. 2000;108:713–23. doi: 10.2307/3454408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perera FP, Weinstein IB. Molecular epidemiology: recent advances and future directions. Carcinogenesis. 2000;21:517–24. doi: 10.1093/carcin/21.3.517. [DOI] [PubMed] [Google Scholar]
- 21.Powell JJ, Harvey RS, Ashwood P, Wolstencroft R, Gershwin ME, Thompson RPH. Immune potentiation of ultrafine dietary particles in normal subjects and patients with inflammatory bowel disease. J Autoimmun. 2000;14:99–105. doi: 10.1006/jaut.1999.0342. [DOI] [PubMed] [Google Scholar]
- 22.Michaelis M, Matousek J, Vogel JU, Slavik T, Langer K, Cinatl J, Kreuter J, Schwabe D, Cinatl J. Bovine seminal ribonuclease attached to nanoparticles made of polylactic acid kills leukemia and lymphoma cell lines in vitro. Anticancer Drug. 2000;11:369–76. doi: 10.1097/00001813-200006000-00007. [DOI] [PubMed] [Google Scholar]
- 23.Curtis A, Wilkinson C. Nantotechniques and approaches in biotechnology. Trends Biotechnol. 2001;19:97–101. doi: 10.1016/S0167-7799(00)01536-5. [DOI] [PubMed] [Google Scholar]
- 24.Kaul Z, Yaguchi T, Kaul SC, Hirano T, Wadhwa R, Taira K. Mortalin imaging in normal and cancer cells with quantum dot immuno-conjugates. Cell Res. 2003;13:503–7. doi: 10.1038/sj.cr.7290194. [DOI] [PubMed] [Google Scholar]
- 25.Bosman SJ, Nieto SP, Patton WC, Jacobson JD, Corselli JU, Chan PJ. Development of mammalian embryos exposed to mixed-size nanoparticles. Clin Exp Obstet Gynecol. 2005;32:222–24. [PubMed] [Google Scholar]
- 26.McLachlin JR, Caveney S, Kidder GM. Control of gap junction formation in early mouse embryos. Dev Biol. 1983;98:155–64. doi: 10.1016/0012-1606(83)90344-5. [DOI] [PubMed] [Google Scholar]
- 27.Jordan A, Wust P, Scholz R, Tesche B, Fahling H, Mitrovics T, Vogl T, Cervos-Navarro J, Felix R. Cellular uptake of magnetic fluid particles and their effects on human adenocarcinoma cells exposed to AC magnetic fields in vitro. Int J Hyperthermia. 1996;12:705–22. doi: 10.3109/02656739609027678. [DOI] [PubMed] [Google Scholar]
- 28.Lam CW, James JT, McCluskey R, Hunter RL. Pulmonary toxicity of single-wall carbon nanotubes in mice 7 and 90 days after intratracheal instillation. Toxicol Sci. 2004;77:126–34. doi: 10.1093/toxsci/kfg243. [DOI] [PubMed] [Google Scholar]
- 29.Warheit DB, Laurence BR, Reed KL, Roach DH, Reynolds GA, Webb TR. Comparative pulmonary toxicity assessment of single-wall carbon nanotubes in rats. Toxicol Sci. 2004;77:117–25. doi: 10.1093/toxsci/kfg228. [DOI] [PubMed] [Google Scholar]
- 30.Shiohara A, Hoshino A, Hanaki K, Suzuki K, Yamamoto K. On the cyto-toxicity caused by quantum dots. Microbiol Immunol. 2004;48:669–75. doi: 10.1111/j.1348-0421.2004.tb03478.x. [DOI] [PubMed] [Google Scholar]
- 31.Kirchner C, Liedl T, Kudera S, Pellegrino T, Munoz Javier A, Gaub HE, Stolzle S, Fertig N, Parak WJ. Cytotoxicity of colloidal CdSe and CdSe/ZnS nanoparticles. Nano Lett. 2005;5:331–38. doi: 10.1021/nl047996m. [DOI] [PubMed] [Google Scholar]
- 32.Ye HQ, Gan L, Yang XL, Xu HB. Membrane toxicity accounts for apoptosis induced by realgar nanoparticles in promyelocytic leukemia HL-60 cells. Biol Trace Elem Res. 2005;103:117–32. doi: 10.1385/BTER:103:2:117. [DOI] [PubMed] [Google Scholar]