Abstract
Objective
We assessed the role of DHEA supplementation on pregnancy rates in women with diminished ovarian function.
Design
This is a case control study of 190 women with diminished ovarian function. The study group includes 89 patients who used supplementation with 75 mg daily of oral, micronized DHEA for up to 4 months prior to entry into in vitro fertilization (IVF). The control group is composed of 101 couples who received infertility treatment, but did not use DHEA. The primary outcome was clinical pregnancy after the patient’s initial visit. We developed a Cox proportional hazards model to compare the proportional hazards of pregnancy among women using DHEA with the controls group.
Results
Cumulative clinical pregnancy rates were significantly higher in the study group (25 pregnancies; 28.4% vs. 11 pregnancies; 11.9%; relative hazard of pregnancy in study group (HR 3.8; 95% CI 1.2–11.8; p < 0.05).
Conclusions
DHEA treatment resulted in significantly higher cumulative pregnancy rates. These data support a beneficial effect of DHEA supplementation among women with diminished ovarian function.
Keywords: Dehydroepiandrosterone; Diminished ovarian reserve; Infertility; In vitro fertilization, Pregnancy rates; Age; Life table analysis
Introduction
A beneficial effect of dehydroepiandrosterone (DHEA) on ovarian function among patients with diminished ovarian reserve was reported by Casson et al. [1]. That study reported only small improvement in ovarian function and DHEA treatment of diminished ovarian reserve. Three years ago, one of our patients, unbeknown to us, started supplementation with DHEA and demonstrated a very significant improvement in oocyte and embryo yield during repeated cycles of in vitro fertilization (IVF) [2].
Subsequently, we have investigated the potential benefits of DHEA supplementation on diminished ovarian reserve through other study designs. In a prior study, where patients served as their own pre- and post DHEA treatment controls, we were able to demonstrate that DHEA, indeed, appears to increase egg and embryo numbers. That study suggested that DHEA may also improve egg and embryo quality [3].
The present study was designed to test the hypothesis that DHEA supplementation might be associated with increased pregnancy rates and a shorter interval to pregnancy among women with evidence of decreased ovarian function entering evaluation and treatment for infertility.
Materials and methods
This is study involved a retrospective analysis of a cohort of 190 women with diminished ovarian function above 30 years old, who were treated at our New York City center between 1999 and December 2005.
Patients were identified from our center’s IVF data base, which is being maintained in accordance with federal US law and annually reported to the Centers for Disease Control [4].
Once identified via the data base, the diagnosis of diminished ovarian function was confirmed by detailed chart review by two of the authors. The diagnosis was considered confirmed if patients either qualified for a sub-diagnosis of premature ovarian aging (POA) or diminished ovarian reserve (DOR). POA was, in turn, defined as baseline follicle stimulating hormone (b-FSH), on cycle 2/3 of cycle as <12 mIU/ml, but exceeding the 95% CI of the mean value for the patient’s age group, as previously reported [5].
Specifically, this meant b-FSH ≥7.4 mIU/ml at age 30–34 years and ≥8.6 mIU/ml at age ≥35 years. DOR, in turn, was defined as b-FSH ≥12 mIU/ml and or a baseline estradiol level ≥75 pg/ml. Chart review confirmed 49 POA and 52 DOR patients who fulfilled the criteria, creating a control group of 101 women. Because of our concern for their impending loss of ovarian function, these women were treated with IVF as soon as possible.
In June 2004, we began exploring the use of DHEA to improve ovulatory response [2]. During the time studied, 89 consecutive patients, with diminished ovarian function (POA 24, DOR 65), were placed on DHEA supplementation. They represent the study group. In contrast to the controls, women in the study group did not enter IVF right away. Instead, since our previous experience suggested that the positive effects of DHEA supplementation upon ovarian function peak after two to 4 months of usage [2], they were placed on supplementation with 25 mg of (pharmaceutical grade) micronized DHEA, three times daily, for up to 4 months (mean 3.8 ± 0.3 months). This delay of IVF treatment, allowed the possibility of spontaneously conceived pregnancies. Those patients, who did not conceive spontaneously within 4 months of beginning DHEA, entered IVF.
Ovarian stimulation was identical for study and control groups and consisted of microdose agonist flare, followed by maximal dosage gonadotropin stimulation, using 300–450 IU of FSH and 150 IU of HMG [6].
Study patients received DHEA continuously until a positive pregnancy test was obtained or until the patient dropped out of treatment. DHEA and DHEAS levels were monitored monthly, and patients were interviewed at each visit about adverse reactions to DHEA supplementation.
Because of the dynamics of the DHEA treatment algorhythm, at the time of this data analysis, 16 women in the study group were at various stages of DHEA supplementation, prior to any intervention, nine received ovarian stimulation while on DHEA, and 64 have undergone an IVF cycle.
In order to assess the impact of DHEA supplementation on time interval to the establishment of pregnancy, this study was designed as a life-table analysis, measuring not only total pregnancy rates, but also the time between initial presentation at our center and end of last treatment intervention. Each recorded clinical pregnancy, defined as positive fetal cardiac activity on ultrasound examination after 6 weeks, was recorded as a positive outcome. Patients, who continued treatments beyond the study period, or stopped treatments, were considered right censored data at the end of the study period, or at treatment cessation, respectively.
The following was compared between study and control groups: female age, months of infertility prior to initial visit, length of treatment from first presentation, gravidity, race, IVF treatments, maximal baseline FSH levels, maximal baseline estradiol levels, IVF cycle cancellation rates, oocyte numbers, number of embryos transferred, implantation rates, cumulative clinical pregnancy rates and miscarriage rates.
A Cox regression analysis was used to evaluate time-to-event. The model that we used stratified for level of ovarian reserve (POA and DOR) and adjusted for age, day 3 FSH, fertility treatments (none, Intrauterine Insemination and controlled ovarian hyperstimulation (IUI/COH), or IVF) and race/ethnicity. We tested for trend in pregnancy rates over months of DHEA exposure with an interaction term for time and DHEA months of exposure.
SPSS for Windows, Standard version 10.0.7 (SPSS Co. Chicago, IL) was utilized for data analysis. Continuous outcomes are presented as mean ± 1 standard error. Univariate comparisons were made with analysis of variance, or by using Fisher’s exact test.
Patients in the DHEA arm of the study all signed informed consent approved by our institutional review board. Patients in the control group were evaluated retrospectively and were not asked to sign consent.
Results
Table 1 summarizes patient characteristics. Study patients were slightly older than the controls at 41.6 ± 0.4 and 40.0 ± 0.4 years (p < 0.05) respectively. Pregnancy histories, duration of infertility and of treatment (in months) were similar between the two groups. Controls represented a non significant larger proportion of minorities, received more treatment cycles overall (1.6 ± 0.9 versus 1.3 ± 1.0; p < 0.05) and differed significantly in the various treatments they received (p < 0.001). Study patients demonstrated a non-significantly higher b-FSH 16.0 ± 1.2 13.6 ± 1.0 mIU/ml) and a significantly higher baseline estradiol level (366 ± 53 versus 188 ± 24 pml/ml; p < 0.05). More women in the study group had b-FSH ≥10 mIU/ml that amongst controls (73% versus 51.5%; p < 0.05). In addition, greater proportion of women in the study group had DOR (p < 0.005).
Table 1.
Characteristics of DHEA treated and controls
| DHEA | Control | p | |
|---|---|---|---|
| N | 89 | 101 | |
| Age | 41.6 ± 0.4 | 40.0 ± 0.4 | <0.05 |
| Months infertility | 44.5 ± 4.8 | 41.9 ± 5.9 | Ns |
| Months from first visit | 8.1 ± 0.7 | 7.8 ± 1.0 | Ns |
| Race | Ns | ||
| White | 62 (70.5%) | 57 (56.4%) | – |
| Hispanic | 7 (7.9%) | 12 (11.9%) | – |
| Black | 9 (10.2%) | 14 (13.9%) | – |
| Asian | 11 (12.5%) | 18 (17.8%) | – |
| Cycles of treatment | 1.3 ± 1 | 1.6 ± 0.9 | <0.05 |
| Treatment | <0.01 | ||
| No treatment | 16 (18.2%) | 0 (0%) | – |
| IUI/COH | 9 (10.2%) | 0 (0%) | – |
| IVF | 64 (71.6%) | 101 (100%) | – |
| Day 3 FSH (mIU/ml) | 16.0 ± 1.2 | 13.6 ± 1.0 | Ns |
| Day 3 E2 (pmol/ml) | 366 ± 53 | 188 ± 24 | <0.05 |
| Ovarian function | <0.005 | ||
| POA | 24 (27%) | 49 (48.5%) | – |
| DOR | 65 (73%) | 52 (51.5%) | – |
Table 2 lists the results of univariate comparisons of treatment outcomes. Confirming a more severe degree of diminished ovarian function, the study group produced significantly fewer oocytes, normal day-3 embryos (2.4 ± 0.03 versus 3.5 ± 0.2; p < 0.05) and transferred embryos (2.1 ± 0.2 versus 2.7 ± 0.2; p < 0.05). Cycle cancellations were, however, nominally higher among the controls (25.7 versus 14.3%).
Table 2.
Univariate comparison of results between control and DHEA treated patients
| DHEA | Control | p | |
|---|---|---|---|
| N total; (IVF) | 89; (64) | 101 | |
| Months of DHEA | 3.8 ± 0.3 | – | – |
| Cancellation (IVF) | 9/63 (14.3%) | 26/101 (25.7%) | Ns |
| Oocytes | 3.9 ± 0.4 | 5.8 ± 0.5 | <0.01 |
| Normal day 3 embryos | 2.4 ± 0.3 | 3.5 ± 0.2 | <0.05 |
| Transferred embryos | 2.1 ± 0.2 | 2.7 ± 0.2 | <0.05 |
| Positive hCG (>25 mIU/ml) | 26/88 (30%) | 18/101 (18%) | Ns |
| Implantation (FH/Embryos trans) | 13/101 (11.4%) | 11/148 (6.9%) | Ns |
| Clinical pregnancy | 25/89 (28.1%) | 11/101 (10.9%) | <0.01 |
| No treatment | 6/16 (35.3%) | – | – |
| IUI/COH | 6/9 (66.7%) | – | – |
| IVF | 13/64 (20.6%) | 11/101 (11.9%) | Ns |
| Miscarriage (per clinical pregnancy) | 5/25 (20%) | 4/11 (36%) | Ns |
Overall clinical pregnancy rates were significantly higher in study patients (28.1 versus 10.9%; p < 0.01). Positive first pregnancy tests and implantation rates showed similar trends but failed to reach statistical significance. Remarkably, almost half of all pregnancies in the study group were established spontaneously before IVF start; however, even within the patients reaching IVF, there was a strong trend towards higher pregnancy rates (20.6 versus 11.9%). A beneficial effect of DHEA was also suggested by lower miscarriage rates in study patients (20 versus 36%), even though this difference did not reach statistical significance.
Two months after initiation of treatment the mean DHEA and DHEAS levels at cycle day 2 blood drawing were in the low normal ranges. Few patients reported side effects from DHEA use. These included mild transient acne on the face, chest or back, oily skin and mild hair loss. No facial or body hair growth was reported, nor was there any deepening of voice. Some patients reported an increased sense of well-being or increased libido.
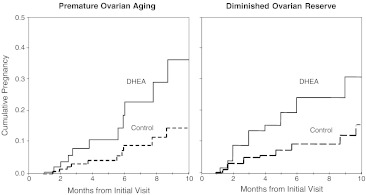
Cox regression of months from initial visit until clinical pregnancy, adjusted for age, race/ethnicity, fertility treatment, and stratified for level of ovarian reserve (POA and DOR), revealed that DHEA treated patients had a significantly increased proportional hazards ratio for clinical pregnancy relative to controls (HR 3.8; 95% CI 1.2–11.8; p < 0.05). Proportional Hazard curves (Fig. 1), depicting clinical pregnancy by months from the initial visit to our center, reveal a rapidly separating increase in cumulative clinical pregnancies between study and control groups from the first month on.
Fig. 1.
Cumulative pregnancy rate based on Cox regression of time from initial visit to clinical pregnancy or censor by DHEA use stratified by ovarian reserve and adjusted for ART treatment, race/ethnicity, age and baseline FSH (HR 3.8; 95% CI 1.2–11.8; p < 0.05)
Extended Cox models with correction for time dependent variables “months of DHEA use” and “Treatment” did not decrease the proportional hazards estimation of pregnancy associated with DHEA treatment (HR 4.8; 95% CI 1.6–14.2; p = 0.005).
Discussion
In this study we noted a significantly increased pregnancy rate in a group of women with a very poor prognosis for pregnancy. We recently liberalized our criteria for entry into fertility treatment, and are accepting women who only a few years ago would have been directed towards oocyte donation. Thus it is not surprising that the control patients had evidence of better ovarian reserve and produced more eggs and embryos than the DHEA treatment group. Indeed, a majority of patients in the study group commenced DHEA treatment at our center after having been refused further IVF cycles elsewhere. Spontaneous background pregnancy rates in average infertile women occur at an approximate rate of one to two percent per month [7, 8]. Spontaneous pregnancies in women with clear evidence of diminished ovarian function are obviously an even rarer occurrence. Given the degree of loss of ovarian reserve in this group, a 28.1% cumulative pregnancy rate in a patient population, previously largely referred into oocyte donation, has to be considered as quite remarkable.
Recently we, and others, have presented evidence that DHEA supplementation can improve ovarian function in women with diminished ovarian reserve. A first such suggestion was made by Casson et al. [1]. This was followed by a longitudinal study of one of our patients [2]. We also recently reported a case control study where patients served as their own pre- and post-DHEA controls [3]. This study represents the fourth study of DHEA supplementation.
Study and control patients received identical ovarian stimulation protocols during IVF cycles. IVF protocols during the study years 1999–2005 have not significantly changed at our center, in that we have utilized microdose agonist/gonadotropin stimulations in women with diminished ovarian reserve for many years [9], most of the key clinical and embryology personnel have been the same and the program’s clinical pregnancy rates with IVF among women with normal ovarian reserve have not changed significantly.
One strength of this study is its rather large sample size. A potential weakness of this study is that DHEA treated patients experienced 2–4 months of less aggressive infertility treatment before entering IVF. During this same time interval, more control patients were undergoing IVF. Their more aggressive treatments would, therefore, be expected to bias against the study group. This is a potential violation of the assumption of proportional hazards in the Cox model. We, however, repeated our model with a time dependent covariate, controlling for the different treatments that the groups experienced over time. The effect of DHEA remained significant even after this added level of control.
The mechanism of DHEA’s action on the ovary remains speculative. DHEA declines with age [10] and DHEA supplementation may simply improve the substrate pool for steroidogenesis, since DHEA is, a precursor hormone for estradiol and testosterone [11].
Androgens may, however, influence ovarian follicular growth not only by acting as metabolic precursors for steroid production [12], but also by serving as ligands for androgen receptors 11 or by other, non-classical mechanisms. During ovulation induction with exogenous gonadotropins, DHEA is the prehormone for up to 48% of follicular fluid testosterone [13], which is, in turn, the prehormone for estradiol. There is evidence that androgens act, together with FSH, to stimulate follicular differentiation [14–16]. Androgens are also known to promote steroidogenesis [17], follicular recruitment and to increase insulin like growth factor (IGF-1) in the primate ovary [18]. DHEA-treated rat ovaries express elevated levels of IGF-1 in pre-antral and early antral follicles [19].
Casson et al. demonstrated a transient increase in IGF-1 in patients undergoing exogenous gonadotropin ovulation induction after pretreatment for only 8 weeks of DHEA [20]. They later hypothesized that the effect of DHEA on ovulation induction might have been mediated by increased IGF-1 [1, 20].
Higher baseline testosterone levels have been associated with improved IVF outcomes [21], and higher serum testosterone has been correlated with higher oocyte numbers retrieved at IVF [22]. Some authors have suggested that improved outcomes in women with diminished ovarian reserve after co-treatment with aromatase inhibitors may be the consequence of induction of FSH receptors on granulosa cell by androgens [23, 24]. The resultant ovarian response may then lead to improved follicular survival, increased follicle numbers and higher estradiol levels during stimulation, as classically also observed in polycystic ovarian disease [25].
Human polycystic ovaries have been described as representing a “stock-piling” of primary follicles, secondary to an alteration at the transition from primordial to primary follicle [26]. It is possible that DHEA treatment may create PCO like characteristics in the aging ovary. Long term exogenous androgen exposure can induce PCO like histological and sonographic changes [27, 28]. Androgens have been reported to suppress apoptosis [29, 30].
Women using DHEA may experience possible androgenic effects including acne, deepening of the voice and facial hair growth. These effects appear to be minimal with a dose of 75 mg/day [31]. Long-term effects of DHEA supplementation remain unknown. Since DHEA is a precursor of sex steroids its use could increase the risk of estrogen- or androgen-dependent malignancies [32]. Our current protocol allows for DHEA exposure during the first two weeks of embryo development. Pregnancy, in itself, is a high androgen/DHEA state [33], and women with polycystic ovarian disease, also a high androgen/DHEA state [34], do not deliver daughters with masculinized external genitalia.
In summary, in this case control study we have noted a significant increase in the odds of pregnancy among DHEA treated women. This increase appears to be rapid in onset and to continue progressively within 8 months of initial observation. We recognize the potential bias inherent in the present study design and look forward to completing enrollment in our randomized trial to shed further light DHEA treatment of women with diminished ovarian function.
Footnotes
DB and NG are signatories on a pending patent application which claims a beneficial benefit for dehydroepiandrosterone supplementation on ovarian function.
References
- 1.Casson PR, Lindsay MS, Pisarska MD, Carson SA, Buster JE. Dehydroepiandrosterone supplementation augments ovarian stimulation in poor responders: a case series. Hum Reprod. 2000;15(10):2129–32. doi: 10.1093/humrep/15.10.2129. [DOI] [PubMed] [Google Scholar]
- 2.Barad D, Gleicher N. Increased oocyte production after treatment with dehydroepiandrosterone. Fertil Steril. 2005;84(3):756. doi: 10.1016/j.fertnstert.2005.02.049. [DOI] [PubMed] [Google Scholar]
- 3.Barad D, Gleicher N. Effect of dehydroepiandrosterone on oocyte and embryo yields, embryo grade and cell number in IVF. Hum Reprod. 2006;21(11):2845–9. doi: 10.1093/humrep/del254. [DOI] [PubMed] [Google Scholar]
- 4.ASRM/SART-Registry Assisted reproductive technology in the United States: 2000 results generated from the American Society for Reproductive Medicine/Society for Assisted Reproductive Technology Registry. Fertil Steril. 2004;81(5):1207–20. doi: 10.1016/j.fertnstert.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 5.Barad D, Weghofer A, Gleicher N. O-291: evidence for an empiric definition of diminished ovarian reserve (DOR), ovarian resistance to stimulation and diagnosis of premature ovarian aging (POA), based on age-specific baseline FSH levels. Fertil Steril. 2006;86(3, Supplement 1):S125–S6. doi: 10.1016/j.fertnstert.2006.07.331. [DOI] [Google Scholar]
- 6.Schoolcraft W, Schlenker T, Gee M, Stevens J, Wagley L. Improved controlled ovarian hyperstimulation in poor responder in vitro fertilization patients with a microdose follicle-stimulating hormone flare, growth hormone protocol. Fertil Steril. 1997;67(1):93–7. doi: 10.1016/S0015-0282(97)81862-6. [DOI] [PubMed] [Google Scholar]
- 7.Gleicher N, VanderLaan B, Pratt D, Karande V. Background pregnancy rates in an infertile population. Hum Reprod. 1996;11(5):1011–2. doi: 10.1093/oxfordjournals.humrep.a019286. [DOI] [PubMed] [Google Scholar]
- 8.Collins JA, Wrixon W, Janes LB, Wilson EH. Treatment-independent pregnancy among infertile couples. N Engl J Med. 1983;309(20):1201–6. doi: 10.1056/NEJM198311173092001. [DOI] [PubMed] [Google Scholar]
- 9.Karande V, Gleicher N. A rational approach to the management of low responders in in-vitro fertilization. Hum Reprod. 1999;14(7):1744–8. doi: 10.1093/humrep/14.7.1744. [DOI] [PubMed] [Google Scholar]
- 10.Harper AJ, Buster JE, Casson PR. Changes in adrenocortical function with aging and therapeutic implications. Semin Reprod Endocrinol. 1999;17(4):327–38. doi: 10.1055/s-1999-7718. [DOI] [PubMed] [Google Scholar]
- 11.Hillier SG, Whitelaw PF, Smyth CD. Follicular oestrogen synthesis: The ‘two-cell, two-gonadotrophin’ model revisited. Mol Cell Endocrinol. 1994;100(1–2):51–4. doi: 10.1016/0303-7207(94)90278-x. [DOI] [PubMed] [Google Scholar]
- 12.Dorrington JH, Moon YS, Armstrong DT. Estradiol 17[beta] biosynthesis in cultured granulosa cells from hypophysectomized immature rats; stimulation by follicle stimulating hormone. Endocrinology. 1975;97(5):1328–31. doi: 10.1210/endo-97-5-1328. [DOI] [PubMed] [Google Scholar]
- 13.Haning R, Jr, Hackett R, Flood C, Loughlin J, Zhao Q, Longcope C. Plasma dehydroepiandrosterone sulfate serves as a prehormone for 48% of follicular fluid testosterone during treatment with menotropins. J Clin Endocrinol Metab. 1993;76(5):1301–7. doi: 10.1210/jc.76.5.1301. [DOI] [PubMed] [Google Scholar]
- 14.Hillier SG. Sex steroid metabolism and follicular development in the ovary. Oxf Rev Reprod Biol. 1985;7:168–222. [PubMed] [Google Scholar]
- 15.Daniel SAJ, Armstrong DT. Androgens in the ovarian microenvironment. Sem Reprod Endocrinol. 1986;4(2):89–100. doi: 10.1055/s-2007-1022489. [DOI] [Google Scholar]
- 16.Gore-Langton R, Armstrong D. Follicular steroidogenesis and its control. In: Knobil E, Neill J, editors. The physiology of reproduction. New York: Raven Press; 1988. pp. p. 331–85. [Google Scholar]
- 17.Harlow CR, Hillier SG, Hodges JK. Androgen modulation of follicle-stimulation hormone-induced granulosa cell steroidogenesis in the primate ovary. Endocrinology. 1986;119(3):1403–5. doi: 10.1210/endo-119-3-1403. [DOI] [PubMed] [Google Scholar]
- 18.Vendola K, Zhou J, Wang J, Famuyiwa OA, Bievre M, Bondy CA. Androgens promote oocyte insulin-like growth factor I expression and initiation of follicle development in the primate ovary. Biol Reprod. 1999;61(2):353–7. doi: 10.1095/biolreprod61.2.353. [DOI] [PubMed] [Google Scholar]
- 19.Yan Z, Lee GY, Anderson E. Influence of dehydroepiandrosterone on the expression of insulin-like growth factor-1 during cystogenesis in polycystic rat ovaries and in cultured rat granulosa cells. Biol Reprod. 1997;57(6):1509–16. doi: 10.1095/biolreprod57.6.1509. [DOI] [PubMed] [Google Scholar]
- 20.Casson PR, Santoro N, Elkind-Hirsch K, et al. Postmenopausal dehydroepiandrosterone administration increases free insulin-like growth factor-I and decreases high-density lipoprotein: a six-month trial. Fertil Steril. 1998;70(1):107–10. doi: 10.1016/S0015-0282(98)00121-6. [DOI] [PubMed] [Google Scholar]
- 21.Frattarelli JL, Peterson EH. Effect of androgen levels on in vitro fertilization cycles. Fertil Steril. 2004;81(6):1713–4. doi: 10.1016/j.fertnstert.2003.11.032. [DOI] [PubMed] [Google Scholar]
- 22.Barbieri RL, Sluss PM, Powers RD, et al. Association of body mass index, age, and cigarette smoking with serum testosterone levels in cycling women undergoing in vitro fertilization. Fertil Steril. 2005;83(2):302–8. doi: 10.1016/j.fertnstert.2004.07.956. [DOI] [PubMed] [Google Scholar]
- 23.Goswami SK, Das T, Chattopadhyay R, et al. A randomized single-blind controlled trial of letrozole as a low-cost IVF protocol in women with poor ovarian response: a preliminary report. Hum Reprod. 2004;19(9):2031–5. doi: 10.1093/humrep/deh359. [DOI] [PubMed] [Google Scholar]
- 24.Mitwally MF, Casper RF. Aromatase inhibition improves ovarian response to follicle-stimulating hormone in poor responders. Fertil Steril. 2002;77(4):776–80. doi: 10.1016/S0015-0282(01)03280-0. [DOI] [PubMed] [Google Scholar]
- 25.MacDougall M, Tan S, Balen A, Jacobs H. A controlled study comparing patients with and without polycystic ovaries undergoing in-vitro fertilization. Hum Reprod. 1993;8(2):233–7. doi: 10.1093/oxfordjournals.humrep.a138029. [DOI] [PubMed] [Google Scholar]
- 26.Maciel GAR, Baracat EC, Benda JA, et al. Stockpiling of transitional and classic primary follicles in ovaries of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2004;89(11):5321–7. doi: 10.1210/jc.2004-0643. [DOI] [PubMed] [Google Scholar]
- 27.Amirikia H, Savoy-Moore RT, Sundareson AS, Moghissi KS. The effects of long-term androgen treatment on the ovary. Fertil Steril. 1986;45(2):202–8. doi: 10.1016/s0015-0282(16)49155-7. [DOI] [PubMed] [Google Scholar]
- 28.Pache TD, Chadha S, Gooren LJ, et al. Ovarian morphology in long-term androgen-treated female to male transsexuals. A human model for the study of polycystic ovarian syndrome? Histopathology. 1991;19(5):445–52. doi: 10.1111/j.1365-2559.1991.tb00235.x. [DOI] [PubMed] [Google Scholar]
- 29.Kaipia A, Hsueh AJ. Regulation of ovarian follicle atresia. Annu Rev Physiol. 1997;59:349–63. doi: 10.1146/annurev.physiol.59.1.349. [DOI] [PubMed] [Google Scholar]
- 30.Billig H, Furuta I, Hsueh AJ. Estrogens inhibit and androgens enhance ovarian granulosa cell apoptosis. Endocrinology. 1993;133(5):2204–12. doi: 10.1210/en.133.5.2204. [DOI] [PubMed] [Google Scholar]
- 31.Kroboth PD, Salek FS, Pittenger AL, Fabian TJ, Frye RF. DHEA and DHEA-S: a review. J Clin Pharmacol. 1999;39(4):327–48. doi: 10.1177/00912709922007903. [DOI] [PubMed] [Google Scholar]
- 32.Kaaks R, Berrino F, Key T, et al. Serum sex steroids in premenopausal women and breast cancer risk within the European Prospective Investigation into Cancer and Nutrition (EPIC) J Natl Cancer Inst. 2005;97(10):755–65. doi: 10.1093/jnci/dji132. [DOI] [PubMed] [Google Scholar]
- 33.McClamrock HD, Adashi EY. Gestational hyperandrogenism. Fertil Steril. 1992;57(2):257–74. doi: 10.1016/s0015-0282(16)54828-6. [DOI] [PubMed] [Google Scholar]
- 34.Sir-Petermann T, Maliqueo M, Angel B, Lara HE, Perez-Bravo F, Recabarren SE. Maternal serum androgens in pregnant women with polycystic ovarian syndrome: possible implications in prenatal androgenization. Hum Reprod. 2002;17(10):2573–9. doi: 10.1093/humrep/17.10.2573. [DOI] [PubMed] [Google Scholar]