Abstract
In vitro fertilization (IVF) in women of advanced age (>42 years) represents only 5%, a comparatively minute part, of the national IVF experience in the United States (US). In view of evolving population dynamics, it, however, also represents proportionally a rather quickly expanding patient need. Because of access restrictions at many IVF programs, this market does not live up to its potential. As best demonstrated by the 2004 US National Summary and Fertility Clinic Report, which for the first time reported pregnancies and births above age 45 year, IVF in women of advanced reproductive age represents a cutting edge area of interest for improving current IVF outcomes. Access to IVF should, therefore, not be withheld based on female age and/or baseline FSH levels. Instead, a definition of acceptable minimal pregnancy and life birth rates could be used to define the limits of offered access to IVF, independent of age and/or baseline FSH levels.
Keywords: In vitro fertilization (IVF), Age, Follicle stimulating hormone (FSH), Diminished ovarian reserve, Premature ovarian aging, Oocytes, Embryos
According to the latest National Summary and Fertility Clinic Reports, 94,242 fresh (non-donor) IVF cycles were conducted in 2004. Amongst those, 4,709, or 5%, were in women above age 42 (Table 1) [1]. This relatively small number of cycles may be one reason why this age group of women has attracted only limited attention in the literature. Some recent developments suggest, however, that this should change.
Table 1.
Fresh (non-donor) IVF cycles 1999–2004
| Year | Number of IVF cycles at ages | Total cycles | ||||
|---|---|---|---|---|---|---|
| <35 | 35–37 | 38–40 | 41–42 | >42 | ||
| 1999 | 29,682 | 15,291 | 12,848 | 5,302 | 2,628 | 65,751 |
| 2000 | 33,453 | 17,284 | 14,701 | 6,118 | 3,401 | 74,957 |
| 2001 | 35,984 | 17,791 | 16,283 | 7,044 | 3,762 | 80,864 |
| 2002 | 37,591 | 19,110 | 17,454 | 7,733 | 3,938 | 85,826 |
| 2003 | 39,852 | 20,056 | 18,660 | 8,185 | 4,279 | 91,032 |
| 2004 | 40,853 | 21,019 | 19,174 | 8,487 | 4,709 | 94,242 |
| % Change | +37.6 | +37.5 | +49.2 | +60.1 | +79.2 | +43.3 |
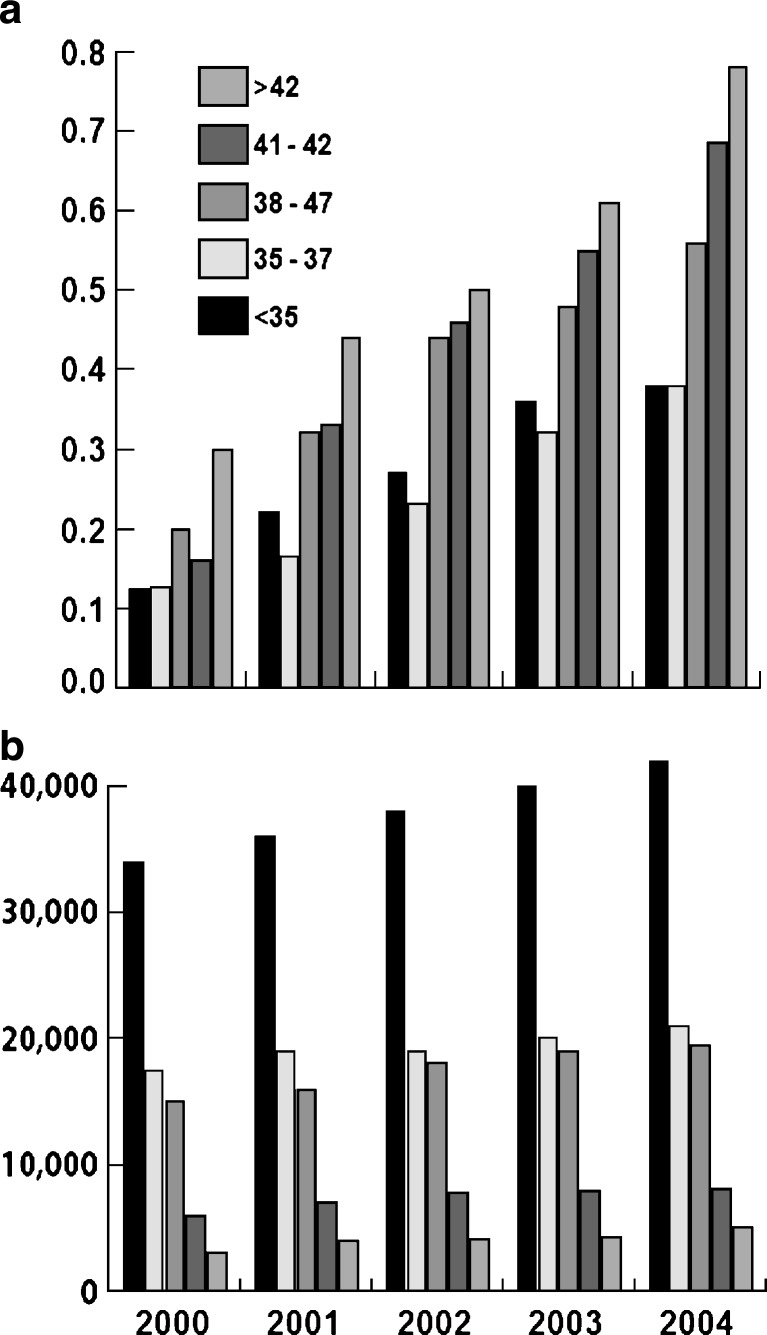
Women at ages 40–45 years in the US represent the most rapidly growing age group going through pregnancy [2, 3]. Together with the demographic fact that the last vestiges of the large baby boomer generation are now aging beyond their reproductive life spans (with use of autologous oocytes), this observation points towards increasing demand for in vitro fertilization (IVF) at advanced female ages, as, indeed, has been observed in the last few years (Table 1 and Fig. 1). A shift towards more “older” patients in IVF programs can also be expected from improving pregnancy rates. As a consequence of quick pregnancy success in younger women, the more difficult cases (such as older patients) remain disproportionally in the system, while younger patients are quickly discharged [4].
Fig. 1.
US IVF cycles at various age groups, 1999–2004: a absolute numbers; b relative percentage increases
Total fresh (non-donor) IVF cycles increased between 1999 and 2004 by 43.3% (Table 1, Fig. 1). Increases were, however, not equally distributed between age groups: the older the patients, the larger was the observed increase in IVF utilization, with women below age 35 showing the lowest increase at 37.6%, and women above age 42 the highest, at 79.2% [1, 5]. In the 5 years, between 1999 and 2004, the percentage of women above age 42 in the whole IVF population thus increased by a full quarter, from 4 to 5%. In the same time period, the youngest age group of patients, below age 35, decreased from 45.1 to 43.3% of the IVF population.
Fertility and IVF outcomes at advanced age
The success of IVF, of course, declines with older female age [1, 5]. This is believed to be a reflection of declining female fecundity (and increasing miscarriage rates) with advancing age. Declining female fertility has statistically been correlated to the decrease in ovarian follicle numbers [6, 7]. Indeed, when female age-dependent fertility and national age-based IVF outcome curves are compared, they are practically identical. Both suggest a steady decline from early age, which accelerates at 37–38 years of age, when approximately 25,000 follicles are left within ovaries. Similar age-based curves have also been reported for intrauterine inseminations, and, inversely, for miscarriages [8].
It has been suggested that advanced reproductive age is not strictly defined but that infertility, overall, becomes more pronounced after age 35 [8]. The age of 37–38 years, when the female fertility decline accelerates, would appear like a more logical point of definition for the beginning of advanced reproductive age. When advanced reproductive age becomes too advanced for further treatment attempts, has, however, not been well defined. Some, mostly anecdotal criteria have intermittently appeared in the literature and will be further discussed below.
After age 40, and especially after age 42, pregnancy rates with all forms of infertility treatment, including, IVF, are poor. The Practice Committee of the ASRM, indeed, notes that “only oocyte donation has been shown to be effective in women older than 40 years of age …” [8], thus implying that all other treatments (including IVF) have limited value in addressing decreasing fertility after age 40.
While female age represents a crucial, and independent, predictor of IVF success, other parameters may be similarly important. For example, baseline (b-)FSH levels [9, 10], and other ovarian function criteria [11, 12] are, to varying degrees, predictive of IVF outcomes. They, however, also reemphasize the potential relativity of such criteria and the strong relevance of age, since, for example, abnormally high b-FSH levels at younger ages are less ominous than at older ages [13, 14].
It is this relativity of baseline FSH levels in younger women, which induced so-far the only discussion in the literature of what constitutes appropriate criteria for IVF treatment in women with diminished ovarian reserve [9, 15–17]. Unanimity amongst authorities to give younger women with diminished ovarian reserve access to IVF, contrary to then established practice patterns, unfortunately did not result in similar reconsiderations in older women. As a consequence, in analogy to what happened to younger women until 2004, older women still are not given full access to IVF by many centers.
When Scott suggested that practically any pregnancy chance warrants that younger women be given access to IVF [9], his argument was widely accepted. The 2004 US national IVF data, however, demonstrate that, even women of very advanced reproductive ages (up to age 44) demonstrate pregnancy rates above what should be considered “minimal.” These data suggest at age 43 a clinical pregnancy rate of 10.8% (live birth rate 5.5%); at age 44, a pregnancy rate of 7.4% (live birth rate of 3.3%); and above age 44, a pregnancy rate of 3.8% (live birth rate 0.8%) [1]. Table 2 summarizes 2004 US IVF pregnancy rates above age 40 [1] and 2004–2006 rates from our own IVF program, which specializes in the treatment of women with diminished ovarian reserve. Most current patients of advanced female who are reported to the US national registry for IVF programs, are, of course, highly selected [18]. We, nevertheless, believe that current national US outcome data in this age group can still be further improved.
Table 2.
Clinical pregnancy rates and delivery rates in women of advanced ages: 2004 US national data and 2004–2006 CHR data
| Age (years) | 40 | 41 | 42 | 43 | 44 | >44a |
|---|---|---|---|---|---|---|
| US data | ||||||
| 2004 | 23.0/16.1 | 19.2/2.5 | 14.8/8.4 | 10.8/5.5 | 7.4/3.3 | 3.8/0.8b |
| CHR data | ||||||
| 2004 | 15.4/15.4 | 23.1/15.4 | 14.3/14.3 | 14.3/14.3 | 0 | 0 |
| 2005 | 12.5/12.5 | 7.1/7.1 | 0 | 9.1/9.1 | 9.5/4.8 | na |
| 2006 | 27.3/na | 25.0/na | 16.7/na | 40.0/na | 16.6/na | 20.0/na |
aThe graphic depiction of pregnancy outcomes for the U.S. in the 2004 report for the first time demonstrates clinical pregnancies and live births after age 45 individual numbers are, however, not cited in the report
bPregnancy/Live Birth Rates per retrieval
na, Data not yet available
IVF outcomes at advanced ovarian ages
Since approximately 10% of females appear to suffer from premature ovarian aging (POA) [19], such women already attain levels of diminished ovarian reserve at unusually young ages. Though, chronologically still young, they behave functionally much older. Since these younger women with “older” ovaries functionally are similar to older women with ovaries that aged at a physiologically normal pace, it would seem clinically and ethically correct to treat them similarly. When this is done, younger patients will, if properly stimulated, still demonstrate outcome advantages [20]. These outcome differences may reflect outright differences in ovarian reserve (i.e., the pool of available oocytes) or be simply reflective of the younger age of oocytes in younger women, as also demonstrated by normal, age-appropriate aneuploidy rates of embryos [21].
POA is frequently overlooked, falsely leading to a diagnosis of so-called unexplained infertility [4]. Amongst infertile populations, like those seen in fertility centers, the prevalence of POA can be surprisingly high [10, 19], and will ultimately depend on the diagnostic criteria for POA. When we based the diagnosis on abnormally elevated age-specific b-FSH levels (in contrast to standard b-FSH levels, which for all age groups usually consider cut offs at 10–12 mIU/ml), almost one half of infertile women in our Center, under age 32 years, qualified for the diagnosis of POA, with the prevalence decreasing considerably with advancing female age [10].
What is current practice?
A detailed literature search for data using advanced female age (and/or ovarian age) as criterion for entry into IVF, involving Medline and PubMed, was surprisingly small. One recent publication marginally attempted to address the topic (2002 Practice Committee Report of the American Society for Reproductive Medicine, entitled “Aging and infertility in women: a committee opinion”), though failed to address this specific issue [8].
In a classical paper Hull et al. reported in 1996 that embryo implantation and live birthrates drop in women above age 40, –6.1 and 3.5%, respectively [22]. In the same year, Marcus and Brindsen reviewed the utilization of IVF in women above age 40 and concluded that practically all IVF parameters plummet above age 40. In a discussion of alternatives, they pointed towards oocyte donation in the presence of reduced ovarian reserve. The same authors also reviewed the evolving practice of selecting those older women, who, based on specific selection criteria, could be given access to IVF, while those who did not qualify, of course, would not [23].
Since this form of patient selection has since become routine in most IVF centers, it is important to recognize that published outcome data in older women, of course, reflect a strong selection bias. This bias can go both ways: By selecting women with the best possible ovarian reserve, one has to assume that published outcome data in women above age 40 are not reflective of all women in that age group. Indeed, this observation would suggest that IVF outcomes for all women above age 40 should be poorer than reported. A recent study from the Cornell group supports this contention: They report excellent pregnancy rates (21.1%) above age 45, though delivery rates were low (3.1% per retrieval) due to a very high miscarriage rate (85.3%). Most importantly, however, this outcome was predicated on patient selection, which a-priori eliminated 127 out of 288 patients (44%) [18].
Yet, an opposing argument can be made, as well: As many IVF programs cancel IVF cycles before retrieval, unless patients develop minimum numbers of preovulatory follicles, it is quite likely that amongst cancelled cycles, at least some would have led to conception, had they not been cancelled. Patients who might have achieved pregnancy may, thus, never be given such a chance because they never will meet a program’s minimum follicle number to reach oocyte retrieval.
That this circumstance exists quite frequently has become very apparent in our IVF program, which under current criteria allows access to IVF up to baseline FSH levels of 40 mIU/ml, and no longer requires minimum follicle numbers to reach retrieval in women with severely diminished ovarian reserve who have received maximal ovarian stimulation. Since our program serves a large number of patients with significantly elevated baseline FSH levels and very small follicle numbers, we were able to analyze recent IVF outcomes in close to 100 (year-2006) patients, previously denied access to retrieval elsewhere. Amongst those, during 2006, in excess of 30% were discharged from our program with an ongoing pregnancy after one, or more, attempts at IVF, and supplemental treatments (Gleicher N and Barad D, Unpublished data). This outcome is mostly the consequence of an improved IVF pregnancy rate of 23.5% in over 50 IVF cycles in women at ages 40–46 (see Table 2) and a small additional, spontaneous pregnancy rate, seen in that same patient group, after supplementation with dehydroepiandrosterone (DHEA), as reported elsewhere in detail [24, 25].
Others also recently reported surprisingly good pregnancy rates in women above age 40, with abnormally elevated FSH levels: Thornton et al. reported clinical pregnancy rates between 5.3 and 16.3% at FSH levels between 10 and 14 mIU/ml [26]. Hernandez et al. recently concluded that the aging of the IVF population mandates a reevaluation and found pregnancy chances to be reasonable till age 44 [27].
In contrast, Watt et al. based on an analysis of only 85 IVF cycles between ages 40 and 42, and 19 patients between 43 and 44 years of age, concluded that the number of IVF cycles in women above age 40 should be limited [28]. Osmanagaoglu et al. from the prestigious group at the Dutch-speaking Brussels Free University in Belgium, based on only 26 patients, and 66 IVF cycles, concluded that “women aged >43 years do not have a realistic chance of achieving a delivery with their own oocytes” [29].
These kinds of statements, of course, influence practice patterns. Based on a careful analysis of published data, such statements, however, currently appear unsupported by evidence. Appropriately conducted prospective studies appear indicated before any clinical approach towards older woman should be considered as established. Any systemic exclusion of women at advanced reproductive ages from IVF would, therefore, as of this point, appear inappropriate. Unfortunately, such practice has, apparently, nevertheless, become engrained.
Patients report two principal reasons (for which we have been unable to locate support in the literature) why IVF centers deny IVF access to older women: A first alleges that in older women (mostly defined as above age 42) IVF is no longer indicated since pregnancy, and delivery rates are too low, often defined as 2 and 1%, respectively. A second premise alleges that in older women intrauterine insemination cycles are more effective than IVF.
Age-specific IVF outcome data (Table 2), of course, refute the first argument. The second rational lacks supportive data, and recent preliminary data actually demonstrate superiority of IVF over inseminations in women above age 40. [30] Why many women of advanced female age still are refused IVF, therefore, remains an enigma.
Who should pay?
A medical procedure has to satisfy risk/benefit and cost effectiveness criteria to be considered clinically indicated. Such evaluations have, however, only rarely been performed in association with IVF, in general [31], and do not exist at all for the utilization of IVF in older populations. Concerns about risks and/or cost effectiveness, therefore, should not be reason why IVF is refused. Older women also should be seen as having an absolute right to choose IVF in their attempts to pursue personal happiness in their lives, even if risk/benefit and cost/benefit assessments were unsupportive [31]. Individuals assume medical risks for minimal, or no, outright medical benefits all the time (for example, in cosmetic surgery).
Medical insurance companies and/or, where applicable, government, of course, can define criteria for financial coverage and have the right to deny insurance coverage, when a procedure does not meet objective risk/benefit and/or cost effectiveness criteria. Criteria have, however, to match those for other medical circumstances, if restrictions, under US law, are not to be considered age discriminatory. US, medical insurance companies are aware of this risk and many, therefore, do not use female age as criterion to deny IVF coverage. Those that do, like companies that deny IVF access to young women with mildly elevated FSH levels, could probably be challenged in the Courts.
There is simply no good reason why a 35 year old woman, with a theoretical clinical pregnancy chance of 10%, should receive insurance coverage, but a 43 year old, with identical chance, should not.
The reporting of program rates
Because IVF outcomes change with female age, it has become tradition to report program results stratified for female age. Federally mandated annual outcome reporting in the US, indeed, has, since its inception, been based on such a format [1]. Interestingly, the pages, summarizing national, as well as individual program outcomes, report only till age 42. Outcomes for women, above age 42, are reported separately and in less detail. This age break in the format of annual reporting, at least subliminally, suggests that IVF outcomes, in women above age 42, are considered less important.
To give such an impression is in our opinion counterproductive, since IVF outcomes at ages 43–46 currently probably represent the most interesting of all federally published outcome data, as they define a potential cutting edge of IVF practice. As Table 2 demonstrates, outcomes in older patients (like in all age groups) have been improving over time. In practical terms this means that, over time, acceptable pregnancy rates have been achieved in progressively older women. This progress can be expected to continue, with the logical conclusion that rigid age criteria simply do not make sense, since progress in outcomes mandates constant adjustments. Withholding of IVF access to women of advanced reproductive ages in the end only results in a self fulfilling prophecy, since, as fewer older women are given access, continuous progress is inhibited.
What should be done
Access to IVF should not be unrestricted, but for fair and universal for women of all ages. Who is given access should not be based on unsupported beliefs, should not be left to insurance carriers to decide and should not be motivated by attempts at protecting an IVF program’s overall pregnancy rate through the selection of favorable patients. Access to IVF should neither be based on age, nor on FSH levels, but on age- (and FSH-) specific outcome expectations. In other words, the decisive factor should not be how old a patient, or how high her FSH level is, but what the expectation is that a patient can achieve a minimally acceptable outcome.
What such a minimal outcome expectation should be, remains, of course, open to discussion. As noted earlier, Scott suggested a very minimal threshold for younger women [9]. Others may disagree with such low minimum demands. Indeed, what such minimum success rates should be may vary, depending on the health system in which IVF is offered. For example, a government supported system, like in most European countries, may very well define a higher threshold than a mostly privately funded system, like in the United States. However, whatever the final consensus, two criteria will have to be met: Minimum outcome expectations should be the same at all ages; and they require constant review and adjustments, as IVF outcomes continue to improve.
Conclusions
What should be considered a milestone in assisted reproduction, has basically gone unnoticed: The 2004 National Summary and Fertility Clinic Reports for the first time offer evidence of pregnancies, and live births above age 45 [1]. Even though reported numbers have remained minimal, the mere fact that the national reporting effort in the US now extends beyond age 45, is reflective of changes in practice patterns with older women. Such changes need to be encouraged and need to be carefully recorded.
Ideally, studies should be conducted, which in non-discriminatory fashion, allow women above age 42, with reasonable b-FSH levels, access to IVF. Within such a study concept, the definition of reasonable baseline FSH levels, of course, remains to be determined. Since women with significantly reduced ovarian reserve can be expected to produce only few follicles, it is also essential to design such studies with minimal exclusion criteria from retrieval, if biases are to be avoided. Outcomes are to be recorded in 1-year age increments, since significant changes can be expected year to year.
Only such carefully designed, prospective studies will allow for a definition of who should, or should not, be given access to IVF. Moreover, such an approach will also allow the profession to better define current limits of the possible. Improvements in care then, of course, can, in controlled fashion, attempt to move those borders towards older age. Allowing older women to conceive, represents one of the principal remaining cutting edge issues in reproductive medicine.
References
- 1.Wright VC, Chang J, Jeng G, Chen M, Macaluso M. Assisted reproductive technology surveillance—United States, 2004. MMWR Surveill Summ. 2007;56(6):1–22. [PubMed] [Google Scholar]
- 2.Hamilton BE, Martin JA, Sutton PD. Births: preliminary data for 2003. Natl Vital Stat Rep. 2004;53(9):1–17. [PubMed] [Google Scholar]
- 3.Hamilton BE, Martin JA, Sutton PD. Births: preliminary data for 2002. Natl Vital Stat Rep. 2003;51(11):1–20. [PubMed] [Google Scholar]
- 4.Gleicher N, Barad D. Unexplained infertility: does it really exist? Hum Reprod. 2006;21(8):1951–5. doi: 10.1093/humrep/del135. [DOI] [PubMed] [Google Scholar]
- 5.Assisted Reproductive Technology Success rates. National Summary and Fertility Clinic Reports. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention December 2006.
- 6.Faddy MJ. Follicle dynamics during ovarian ageing. Mol Cell Endocrinol. 2000;163(1–2):43–8. doi: 10.1016/S0303-7207(99)00238-5. [DOI] [PubMed] [Google Scholar]
- 7.Faddy MJ, Gosden RG. A mathematical model of follicle dynamics in the human ovary. Hum Reprod. 1995;10(4):770–5. doi: 10.1093/oxfordjournals.humrep.a136036. [DOI] [PubMed] [Google Scholar]
- 8.ASRM Aging and infertility in women: a committee opinion. Fertil Steril. 2002;78(1):215–9. doi: 10.1016/S0015-0282(02)03212-0. [DOI] [PubMed] [Google Scholar]
- 9.Scott RT., Jr Diminished ovarian reserve and access to care. Fertil Steril. 2004;81(6):1489–92. doi: 10.1016/j.fertnstert.2003.10.051. [DOI] [PubMed] [Google Scholar]
- 10.Barad DH, Weghofer A, Gleicher N. Age-specific levels for basal follicle-stimulating hormone assessment of ovarian function. Obstet Gynecol. 2007;109(6):1404–10. doi: 10.1097/01.AOG.0000264065.37661.a0. [DOI] [PubMed] [Google Scholar]
- 11.Frattarelli JL, Levi AJ, Miller BT, Segars JH. A prospective assessment of the predictive value of basal antral follicles in in vitro fertilization cycles. Fertil Steril. 2003;80(2):350–5. doi: 10.1016/S0015-0282(03)00664-2. [DOI] [PubMed] [Google Scholar]
- 12.Rooij IA, Broekmans FJ, te Velde ER, et al. Serum anti-Mullerian hormone levels: a novel measure of ovarian reserve. Hum Reprod. 2002;17(12):3065–71. doi: 10.1093/humrep/17.12.3065. [DOI] [PubMed] [Google Scholar]
- 13.Chuang CC, Chen CD, Chao KH, Chen SU, Ho HN, Yang YS. Age is a better predictor of pregnancy potential than basal follicle-stimulating hormone levels in women undergoing in vitro fertilization. Fertil Steril. 2003;79(1):63–8. doi: 10.1016/S0015-0282(02)04562-4. [DOI] [PubMed] [Google Scholar]
- 14.Rooij IA, Bancsi LF, Broekmans FJ, Looman CW, Habbema JD, te Velde ER. Women older than 40 years of age and those with elevated follicle-stimulating hormone levels differ in poor response rate and embryo quality in in vitro fertilization. Fertil Steril. 2003;79(3):482–8. doi: 10.1016/S0015-0282(02)04839-2. [DOI] [PubMed] [Google Scholar]
- 15.Rooij IA, Jong E, Broekmans FJ, Looman CW, Habbema JD, te Velde ER. High follicle-stimulating hormone levels should not necessarily lead to the exclusion of subfertile patients from treatment. Fertil Steril. 2004;81(6):1478–85. doi: 10.1016/j.fertnstert.2003.10.054. [DOI] [PubMed] [Google Scholar]
- 16.Wolff EF, Taylor HS. Value of the day 3 follicle-stimulating hormone measurement. Fertil Steril. 2004;81(6):1486–8. doi: 10.1016/j.fertnstert.2003.10.055. [DOI] [PubMed] [Google Scholar]
- 17.Toner JP. Modest follicle-stimulating hormone elevations in younger women: warn but don't disqualify. Fertil Steril. 2004;81(6):1493–5. doi: 10.1016/j.fertnstert.2003.11.038. [DOI] [PubMed] [Google Scholar]
- 18.Spandorfer SD, Bendikson K, Dragisic K, Schattman G, Davis OK, Rosenwaks Z. Outcome of in vitro fertilization in women 45 years and older who use autologous oocytes. Fertil Steril. 2007;87(1):74–6. doi: 10.1016/j.fertnstert.2006.05.081. [DOI] [PubMed] [Google Scholar]
- 19.Nikolaou D, Templeton A. Early ovarian ageing: a hypothesis. Detection and clinical relevance. Hum Reprod. 2003;18(6):1137–9. doi: 10.1093/humrep/deg245. [DOI] [PubMed] [Google Scholar]
- 20.Gleicher N, Barad D. “Ovarian age-based” stimulation of young women with diminished ovarian reserve results in excellent pregnancy rates with in vitro fertilization. Fertil Steril. 2006;86(6):1621–5. doi: 10.1016/j.fertnstert.2006.04.046. [DOI] [PubMed] [Google Scholar]
- 21.Weghofer A, Barad D, Li J, Gleicher N. Aneuploidy rates in embryos from women with prematurely declining ovarian function: a pilot study. Fertil Steril. 2007;88(1):90–4. doi: 10.1016/j.fertnstert.2006.11.081. [DOI] [PubMed] [Google Scholar]
- 22.Hull MG, Fleming CF, Hughes AO, McDermott A. The age-related decline in female fecundity: a quantitative controlled study of implanting capacity and survival of individual embryos after in vitro fertilization. Fertil Steril. 1996;65(4):783–90. doi: 10.1016/s0015-0282(16)58214-4. [DOI] [PubMed] [Google Scholar]
- 23.Marcus SF, Brinsden PR. In-vitro fertilization and embryo transfer in women aged 40 years and over. Hum Reprod Update. 1996;2(6):459–68. doi: 10.1093/humupd/2.6.459. [DOI] [PubMed] [Google Scholar]
- 24.Barad D, Gleicher N. Effect of dehydroepiandrosterone on oocyte and embryo yields, embryo grade and cell number in IVF. Hum Reprod. 2006;21(11):2845–9. doi: 10.1093/humrep/del254. [DOI] [PubMed] [Google Scholar]
- 25.Brill H, Barad DH, Gleicher N.O-289: Dehydroepiandrosterone (DHEA) supplementation and pregnancy outcome: effect on pregnancy rate and speed of conception Fertil Steril 2006863, Supplement 1S124–S5. 10.1016/j.fertnstert.2006.07.32917055807 [DOI] [Google Scholar]
- 26.Thornton K, Ryley D, Alper M, Ezcurra D. 2):S141. S. IVF outcome in women over age 40 years with elevated cycle day 3 FSH (≥10 mIU/mL) Fertil Steril. 2006;86(Suppl 2):S141. [Google Scholar]
- 27.Hernandez J, Sanabria V, Chinea E, Palumbo A. Reproductive outcome in women 40 and older undergoing in vitro fertilization (IVF) Fertil Steril. 2006;86(Suppl 2):S166–7. doi: 10.1016/j.fertnstert.2006.07.444. [DOI] [Google Scholar]
- 28.Watt AH, Legedza AT, Ginsburg ES, Barbieri RL, Clarke RN, Hornstein MD. The prognostic value of age and follicle-stimulating hormone levels in women over forty years of age undergoing in vitro fertilization. J Assist Reprod Genet. 2000;17(5):264–8. doi: 10.1023/A:1009458332567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Osmanagaoglu K, Tournaye H, Kolibianakis E, Camus M, Steirteghem A, Devroey P. Cumulative delivery rates after ICSI in women aged >37 years. Hum Reprod. 2002;17(4):940–4. doi: 10.1093/humrep/17.4.940. [DOI] [PubMed] [Google Scholar]
- 30.Awonuga A, Merhi Z, Grazi R. Intrauterine inseminations versus in vitro fertilization in infertile women 40 years and older. Fertil Steril. 2006;86(Suppl 2):S143. doi: 10.1016/j.fertnstert.2006.07.384. [DOI] [Google Scholar]
- 31.Gleicher N, Barad D. The relative myth of elective single embryo transfer. Hum Reprod. 2006;21(6):1337–44. doi: 10.1093/humrep/del026. [DOI] [PubMed] [Google Scholar]