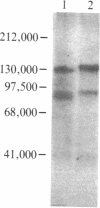
Abstract
The insulin-binding activity and insulin-dependent tyrosine protein kinase activity of the insulin receptor have been purified 2000-fold to homogeneity from human placental membranes. The purified receptor has one high-affinity binding site for insulin per mol of receptor. Its Vmax for phosphorylating angiotensin is 80 nmol of phosphate per min per mg of protein at 23 degrees C. The procedure used to purify the receptor includes chromatography on wheat germ agglutinin-agarose and on insulin-Sepharose. The purified receptor was eluted from insulin-Sepharose with 0.5 M NaCl and 1 mM dithiothreitol at pH 5.5. The addition of dithiothreitol was essential for recovery of the protein kinase. A silver-stained gel of the reduced purified receptor showed two major bands, Mr 95,000 (beta subunit) and Mr 135,000 (alpha subunit). The component of Mr 95,000 comigrated with the autophosphorylated beta subunit of the receptor. The latter was phosphorylated exclusively on tyrosine residues by an intramolecular process. In the presence of insulin, approximately 2 mol of phosphate was incorporated per mol of beta subunit. Two major beta subunit tryptic phosphopeptides were resolved by high-pressure liquid chromatography after autophosphorylation of the purified receptor in the presence or absence of insulin. It is concluded that the insulin binding and the insulin-dependent protein kinase are intrinsic components of the same oligomer since (i) they copurify to homogeneity, (ii) the purified receptor protein kinase is immunoprecipitated by polyclonal and monoclonal antibodies to the human insulin receptor, and (iii) phosphorylation of the beta subunit of the receptor occurs by an intramolecular reaction.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avruch J., Nemenoff R. A., Blackshear P. J., Pierce M. W., Osathanondh R. Insulin-stimulated tyrosine phosphorylation of the insulin receptor in detergent extracts of human placental membranes. Comparison to epidermal growth factor-stimulated phosphorylation. J Biol Chem. 1982 Dec 25;257(24):15162–15166. [PubMed] [Google Scholar]
- Bensadoun A., Weinstein D. Assay of proteins in the presence of interfering materials. Anal Biochem. 1976 Jan;70(1):241–250. doi: 10.1016/s0003-2697(76)80064-4. [DOI] [PubMed] [Google Scholar]
- Casnellie J. E., Harrison M. L., Pike L. J., Hellström K. E., Krebs E. G. Phosphorylation of synthetic peptides by a tyrosine protein kinase from the particulate fraction of a lymphoma cell line. Proc Natl Acad Sci U S A. 1982 Jan;79(2):282–286. doi: 10.1073/pnas.79.2.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuatrecasas P. Affinity chromatography and purification of the insulin receptor of liver cell membranes. Proc Natl Acad Sci U S A. 1972 May;69(5):1277–1281. doi: 10.1073/pnas.69.5.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuatrecasas P. Interaction of concanavalin A and wheat germ agglutinin with the insulin receptor of fat cells and liver. J Biol Chem. 1973 May 25;248(10):3528–3534. [PubMed] [Google Scholar]
- Cuatrecasas P. Isolation of the insulin receptor of liver and fat-cell membranes (detergent-solubilized-( 125 I)insulin-polyethylene glycol precipitation-sephadex). Proc Natl Acad Sci U S A. 1972 Feb;69(2):318–322. doi: 10.1073/pnas.69.2.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita-Yamaguchi Y., Choi S., Sakamoto Y., Itakura K. Purification of insulin receptor with full binding activity. J Biol Chem. 1983 Apr 25;258(8):5045–5049. [PubMed] [Google Scholar]
- Hedo J. A., Harrison L. C., Roth J. Binding of insulin receptors to lectins: evidence for common carbohydrate determinants on several membrane receptors. Biochemistry. 1981 Jun 9;20(12):3385–3393. doi: 10.1021/bi00515a013. [DOI] [PubMed] [Google Scholar]
- Hedo J. A., Kasuga M., Van Obberghen E., Roth J., Kahn C. R. Direct demonstration of glycosylation of insulin receptor subunits by biosynthetic and external labeling: evidence for heterogeneity. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4791–4795. doi: 10.1073/pnas.78.8.4791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T., Sefton B. M. Transforming gene product of Rous sarcoma virus phosphorylates tyrosine. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1311–1315. doi: 10.1073/pnas.77.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs S., Hazum E., Shechter Y., Cuatrecasas P. Insulin receptor: covalent labeling and identification of subunits. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4918–4921. doi: 10.1073/pnas.76.10.4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs S., Shechter Y., Bissell K., Cuatrecasas P. Purification and properties of insulin receptors from rat liver membranes. Biochem Biophys Res Commun. 1977 Aug 8;77(3):981–988. doi: 10.1016/s0006-291x(77)80074-0. [DOI] [PubMed] [Google Scholar]
- Kasuga M., Fujita-Yamaguchi Y., Blithe D. L., Kahn C. R. Tyrosine-specific protein kinase activity is associated with the purified insulin receptor. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2137–2141. doi: 10.1073/pnas.80.8.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasuga M., Fujita-Yamaguchi Y., Blithe D. L., White M. F., Kahn C. R. Characterization of the insulin receptor kinase purified from human placental membranes. J Biol Chem. 1983 Sep 25;258(18):10973–10980. [PubMed] [Google Scholar]
- Kasuga M., Karlsson F. A., Kahn C. R. Insulin stimulates the phosphorylation of the 95,000-dalton subunit of its own receptor. Science. 1982 Jan 8;215(4529):185–187. doi: 10.1126/science.7031900. [DOI] [PubMed] [Google Scholar]
- Kasuga M., Zick Y., Blith D. L., Karlsson F. A., Häring H. U., Kahn C. R. Insulin stimulation of phosphorylation of the beta subunit of the insulin receptor. Formation of both phosphoserine and phosphotyrosine. J Biol Chem. 1982 Sep 10;257(17):9891–9894. [PubMed] [Google Scholar]
- Kasuga M., Zick Y., Blithe D. L., Crettaz M., Kahn C. R. Insulin stimulates tyrosine phosphorylation of the insulin receptor in a cell-free system. Nature. 1982 Aug 12;298(5875):667–669. doi: 10.1038/298667a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Massague J., Pilch P. F., Czech M. P. A unique proteolytic cleavage site on the beta subunit of the insulin receptor. J Biol Chem. 1981 Apr 10;256(7):3182–3190. [PubMed] [Google Scholar]
- Massague J., Pilch P. F., Czech M. P. Electrophoretic resolution of three major insulin receptor structures with unique subunit stoichiometries. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7137–7141. doi: 10.1073/pnas.77.12.7137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massagué J., Czech M. P. Role of disulfides in the subunit structure of the insulin receptor. Reduction of class I disulfides does not impair transmembrane signalling. J Biol Chem. 1982 Jun 25;257(12):6729–6738. [PubMed] [Google Scholar]
- Pang D. T., Shafer J. A. Stoichiometry for the binding of insulin to insulin receptors in adipocyte membranes. J Biol Chem. 1983 Feb 25;258(4):2514–2518. [PubMed] [Google Scholar]
- Parikh I., Sica V., Nola E., Puca G. A., Cuatrecasas P. Affinity chromatography of estrogen receptors. Methods Enzymol. 1974;34:670–688. doi: 10.1016/s0076-6879(74)34089-x. [DOI] [PubMed] [Google Scholar]
- Petruzzelli L. M., Ganguly S., Smith C. J., Cobb M. H., Rubin C. S., Rosen O. M. Insulin activates a tyrosine-specific protein kinase in extracts of 3T3-L1 adipocytes and human placenta. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6792–6796. doi: 10.1073/pnas.79.22.6792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth J. Methods for assessing immunologic and biologic properties of iodinated peptide hormones. Methods Enzymol. 1975;37:223–233. doi: 10.1016/s0076-6879(75)37018-3. [DOI] [PubMed] [Google Scholar]
- Roth R. A., Cassell D. J. Insulin receptor: evidence that it is a protein kinase. Science. 1983 Jan 21;219(4582):299–301. doi: 10.1126/science.6849137. [DOI] [PubMed] [Google Scholar]
- Roth R. A., Mesirow M. L., Cassell D. J. Preferential degradation of the beta subunit of purified insulin receptor. Effect on insulin binding and protein kinase activities of the receptor. J Biol Chem. 1983 Dec 10;258(23):14456–14460. [PubMed] [Google Scholar]
- Schultz R. M., Bleil J. D., Wassarman P. M. Quantitation of nanogram amounts of protein using [3H]dinitrofluorobenzene. Anal Biochem. 1978 Nov;91(1):354–356. doi: 10.1016/0003-2697(78)90850-3. [DOI] [PubMed] [Google Scholar]
- Shia M. A., Pilch P. F. The beta subunit of the insulin receptor is an insulin-activated protein kinase. Biochemistry. 1983 Feb 15;22(4):717–721. doi: 10.1021/bi00273a001. [DOI] [PubMed] [Google Scholar]
- Shia M. A., Rubin J. B., Pilch P. F. The insulin receptor protein kinase. Physicochemical requirements for activity. J Biol Chem. 1983 Dec 10;258(23):14450–14455. [PubMed] [Google Scholar]
- Siegel T. W., Ganguly S., Jacobs S., Rosen O. M., Rubin C. S. Purification and properties of the human placental insulin receptor. J Biol Chem. 1981 Sep 10;256(17):9266–9273. [PubMed] [Google Scholar]
- Smith C. J., Rubin C. S., Rosen O. M. Insulin-treated 3T3-L1 adipocytes and cell-free extracts derived from them incorporate 32P into ribosomal protein S6. Proc Natl Acad Sci U S A. 1980 May;77(5):2641–2645. doi: 10.1073/pnas.77.5.2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadtmauer L. A., Rosen O. M. Phosphorylation of exogenous substrates by the insulin receptor-associated protein kinase. J Biol Chem. 1983 Jun 10;258(11):6682–6685. [PubMed] [Google Scholar]
- Switzer R. C., 3rd, Merril C. R., Shifrin S. A highly sensitive silver stain for detecting proteins and peptides in polyacrylamide gels. Anal Biochem. 1979 Sep 15;98(1):231–237. doi: 10.1016/0003-2697(79)90732-2. [DOI] [PubMed] [Google Scholar]
- Van Obberghen E., Kowalski A. Phosphorylation of the hepatic insulin receptor: stimulating effect of insulin on intact cells and in a cell-free system. FEBS Lett. 1982 Jul 5;143(2):179–182. doi: 10.1016/0014-5793(82)80094-x. [DOI] [PubMed] [Google Scholar]
- Van Obberghen E., Rossi B., Kowalski A., Gazzano H., Ponzio G. Receptor-mediated phosphorylation of the hepatic insulin receptor: evidence that the Mr 95,000 receptor subunit is its own kinase. Proc Natl Acad Sci U S A. 1983 Feb;80(4):945–949. doi: 10.1073/pnas.80.4.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YALOW R. S., BERSON S. A. Immunoassay of endogenous plasma insulin in man. J Clin Invest. 1960 Jul;39:1157–1175. doi: 10.1172/JCI104130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zick Y., Kasuga M., Kahn C. R., Roth J. Characterization of insulin-mediated phosphorylation of the insulin receptor in a cell-free system. J Biol Chem. 1983 Jan 10;258(1):75–80. [PubMed] [Google Scholar]