Abstract
Purpose
To study the effects of GnRH antagonist (ganirelix-Orgalutran®) on the endometrium of regularly menstruating women.
Materials and methods
Prospective, self-controlled study. The thirty-five volunteers were studied for two cycles: one as a control and the other, GnRH antagonist-treated cycles in which ganirelix 0.25 mg/d was given daily for 3 days, starting when the largest follicle reached 15 mm. In both cycles, serum estradiol, LH and endometrial thickness were measured when the largest follicle was ≥18 mm. Endometrial biopsy was performed on day 6 after ovulation for histological dating and morphometric study.
Results
No statistical differences between histological dating and the endometrial thickness in the control and GnRH antagonist-treated cycles. All morphometric parameters were also not different. Serum estradiol and LH levels were significantly lower in GnRH antagonist-treated cycles.
Conclusion
GnRH antagonist has no effect on the endometrium of regularly menstruating women as assessed by either histological dating or morphometric analysis.
Keywords: Endometrium, GnRH antagonist, Histological dating, Morphometric analysis
Introduction
Currently, the combination of GnRH agonist and gonadotropins is the most successful treatment regimen for controlled ovarian hyperstimulation (COH) in IVF. The role of GnRH agonist is to prevent premature LH surge which is associated with cycle cancellation. The most commonly prescribed therapeutic regimen is the long GnRH agonist protocol, proved to be the most efficacious [1], but it increases the duration of treatment period (2–3 weeks are needed to obtain desensitization), causes inconvenience to administration, increases the amount of gonadotropin used and the risk of ovarian hyperstimulation syndrome (OHSS) [2]. Furthermore, some women may suffer common problems of hypoestrogenism such as hot flashes, headaches and vaginal dryness during the desensitization period of the long GnRH agonist protocol [3, 4].
The third generation of GnRH antagonists which are devoid of anaphylactoid reactions, have recently been introduced as an alternative for the prevention of premature LH surge. GnRH antagonists may have some advantages over GnRH agonists due to their mechanisms which competitively block the GnRH receptors, therefore, inducing an immediate and rapid suppression in LH and FSH secretion without a ‘flare-up’ period [5].
Nowadays two different compounds of GnRH antagonists are available: cetrorelix (Cetrotide®; Serono) and ganirelix (Antagon® or Orgalutran®; Organon), and both of them have been approved for clinical use in COH. Clinical studies of both GnRH antagonists in COH-IVF have proved their efficacy and safety. Compared with long GnRH agonist protocol, GnRH antagonists reduced the duration of GnRH analogues treatment (4 vs. 22 days) [6], lowered dosages of gonadotropin used, more flexibility and convenience of administration and more rapid reversibility [7–10]. However, from meta-analysis [11, 12], in comparison with the long GnRH agonist protocol, there was a trend toward lower pregnancy rates in GnRH antagonist cycles in spite of no difference in the number of oocytes retrieved, fertilization rate and embryo quality. Therefore, the GnRH antagonist’s effect on the endometrium which plays an important role for embryo implantation cannot be excluded.
Some earlier studies found that in GnRH antagonist (cetrorelix) protocol, the quality of frozen–thawed pronuclear embryos did not decrease, as well as the implantation and pregnancy rates, as compared to those from the long GnRH agonists protocol [13, 14]. In addition, high dosages of another GnRH antagonist, ganirelix, did not adversely affect the potential of the embryos to establish clinical pregnancy in freeze–thaw cycles [15]. These evidences suggest that GnRH antagonist may have no detrimental effect on the embryos and raise the question about the impact of the GnRH antagonist on the endometrium.
Moreover, there were many studies which suggested the role of GnRH at the cellular level in extrapituitary tissue [16]. Recent studies have demonstrated the presence of GnRH and GnRH receptors in the endometrium. Dong et al. [17] and Raga et al. [18] reported the presence of GnRH mRNA gene expression in the endometrium throughout the menstrual cycle, with a significant increase in the secretory phase. The presences of GnRH receptors in the human endometrial and decidual tissues were also demonstrated by Takeuchi et al. [19]. These studies provided physiological evidences that endometrial GnRH may play a paracrine/autocrine role in the early stages of implantation. Furthermore, there are sufficient data which suggest that GnRH antagonist is an inhibitor of cell cycle by decreasing the syntheses of growth factors [16]. As a result, there is a possibility that GnRH antagonists may compromise the endometrial development.
The objective of our study is to investigate the effect of GnRH antagonist (ganirelix-Orgalutran®) on the endometrium of regularly menstruating female volunteers by comparing with the endometrium of the natural cycle of the same women on day 6 after ovulation (theoretically the window of implantation) [20]. In this study, we use endometrial thickness, histological dating and morphometric analysis, which provided an objective and quantitative method of assessing endometrial appearance [21], as the outcomes of our studies.
Materials and methods
Volunteers
This study was conducted in Department of Obstetrics and Gynecology, Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand. Thirty-five healthy, female volunteers between 20 and 35 years of age, with regular menstrual cycles of 28–35 days and normal pelvic examinations were recruited into this study. All volunteers willingly participated this study due to academic purpose. They did not plan to have pregnancy and were advised to refrain from sexual intercourse or to use barrier contraception during the study periods if they had not been performed tubal resection. The volunteers were excluded if they had been using any hormonal contraception and/or an intrauterine device during the 3 months before the trial and/or if they had been on endocrine treatment and/or were having any pelvic pathology.
The study was approved by the Ethics Committee of the Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand, and all volunteers provided their written informed consent before enrollment.
Study design
This study is prospective, self-controlled study. The study periods consisted of three consecutive menstrual cycles, the first natural cycle as a self-controlled cycle, followed by one resting cycle, and then the GnRH antagonist-treated cycle, respectively.
In the control cycle, the volunteers underwent transvaginal sonographic examination using a 7.5-mHz transducer (Aloka, Model SSD-1700; Aloka Co., Ltd; Tokyo, Japan) from day 12 of cycle onwards, to determine the follicular size. If the diameter of the largest follicle was ≥18 mm, the endometrial thickness was measured ultrasonographically and the venous blood samples were obtained to determine the serum levels of E2 and LH. And on the same day, the participants were given 5,000 units of human chorionic gonadotropin (Pregnyl®, N.V. Organon, OSS, The Netherlands) intramuscularly to induce and for timing ovulation. About 40 h after the injection, ovulation was confirmed by ultrasonographic examination, if not, the ultrasonography was performed daily until evidence of ovulation appeared. Finally, on day 6 after the documented day of ovulation, an endometrial biopsy was performed for histological dating and morphometric analysis.
In the GnRH antagonist-treated cycles, transvaginal sonography was performed, starting on day 10 of cycle, to determine whether the leading follicular diameter was at least 15 mm, then GnRH antagonist, ganirelix (Orgalutran®, N.V. Organon, OSS, The Netherlands) 0.25 mg were given subcutaneously daily for 3 days or until the largest follicle reached 18 mm in diameter. The endometrial thickness was then measured and blood samples were obtained, after that the same dose of hCG was given. The ovulation was confirmed and lastly, the endometrial biopsy was performed on day 6 after ovulation as in the control cycles.
Transvaginal sonographic examination
All the ultrasonographic examinations were carried on by one investigator (P.S.). The endometrial thickness was measured by aligning the uterus along the central longitudinal axis, and the thickness was measured from the echogenic interface between the endometrium and myometrium to the opposite interface at the point of maximal thickness [22]. The measurement was taken three times and a mean value was calculated for analysis.
The follicular diameter was determined by calculating the mean of the two perpendicular diameters measured at the largest plane of the follicle. Ovulation was confirmed by documenting at least two of the following parameters: a decrease in follicular diameter, blurring of the follicular border, the presence of internal echoes, and free fluid in the cul-de-sac [23, 24].
Hormone assay
Venous blood samples were allowed to clot; the separated serum samples by centrifugation were stored at −20 °C until assessment for E2 and LH levels. Serum E2 was analyzed by a commercially available solid phase fluoroimmunoassay (DELFIA; Wallac Oy, Turku, Finland) and LH level by a solid phase, two-site fluoroimmunoassay (DELFIA; Wallac Oy, Turku, Finland). The intraassay and interassay coefficients of variation were 4.04 and 6.72% for E2 level and 6.63 and 9.74% for LH level, respectively. The lower limits for detection were 13.6 pg/mL (0.05 nmol/L) for E2 level and 0.05 IU/L for LH level.
Histological dating and morphometric analysis of the endometrium
Aspiration biopsy of the endometrium was performed as an outpatient procedure on day 6 after ovulation using an endometrial cell sampler (Endocell®, Wallach Surgical Device, Inc., USA). Endometrial specimens were taken from the anterior and posterior walls of the uterine fundus to ensure the assessment of an area of maximal physiological development. The specimen was immediately fixed in 10% formalin, embedded in paraffin, sectioned and stained with hematoxylin and eosin for light microscopic evaluation.
Histological dating was evaluated by two experienced gynecologic pathologists (S.T. and S.N.) who were blinded to the treatment groups. The endometrial dating was assessed by the criteria of Noyes et al. [25] which are still considered the gold standard. If their findings were different, the specimens would be reviewed by the two pathologists to reach a common opinion. We considered a difference of 2-or-more days in the histological dating as ‘out-of-phase’ endometrium.
Morphometric analysis was performed by one investigator (P.S.), who was blinded to the clinical data at the time of examination, using an image analysis system (Image-Pro Express® Version 4.0 for Windows, Media Cybernetics, L.P., MD, USA). There were four parameters which were shown to be specifically sensitive to tissue differentiation chosen for this analysis: number of endometrial glands per square millimeter, diameter of endometrial gland (in μm), height of glandular epithelium (in μm), and number of vacuolated cells per 1,000 glandular cells [26, 27].
The number of glands per square millimeter was determined by counting the glands in three areas of 1 mm2 and the mean value was used. The diameter of endometrial gland was determined by randomly selecting four endometrial glands which were cut parallel to their longitudinal axis, each of the glands measured five values of maximal cross-sectional diameter and the mean value was used for calculation. The height of the glandular epithelium was determined by selecting the glandular epithelial cells which extended in the section from cilia to basement membrane [21] and measuring 50 cells of typical columnar shape and calculating a mean value. Finally, the number of vacuolated cells per 1,000 glandular cells was determined by randomly selecting ten glands and counting the vacuolated cells and non-vacuolated cells of glandular epithelium; then the proportion of vacuolated cells per 1,000 total glandular cells was calculated.
Statistical analysis
Data analysis was carried out using the Statistical Package for the Social Sciences for Windows, Release 11.5.0 (SPSS, Inc., Chicago, IL, USA). Comparisons of E2 and LH levels, endometrial thickness and morphometric analysis between the control and the GnRH antagonist-treated cycles were analyzed for statistical significance by paired t test or Wilcoxon Signed Ranks test (depending on whether the data had a normal distribution as determined by SPSS). Histological dating in the control and the GnRH antagonist-treated cycles were compared using McNemar’s test. A P level of <0.05 was considered statistically significant.
Results
A total of 35 volunteers were recruited in this study between January and December 2004. Their mean (± SD) age was 31.7 ± 2.8 years (ranged 26–35). The mean (± SD) body mass index was 22.7 ± 3.9 kg/m2 (ranged 17.0–32.5). Their mean (± SD) parity was 1.4 ± 0.8 (ranged 0–3). Most of these volunteers were parous women (29 of 35; 82.9%). The mean (± SD) interval of menstrual cycle was 29.4 ± 1.5 days (ranged 27–33).
Thirty-three women received a total of three dosages of ganirelix while the other two received only two dosages. There were 62 out of 70 cycles (82.6%) that ovulation occurred within 40 h after hCG injection by ultrasonography detection. All of the remainders (three in the control and five in GnRH antagonist-treated cycles) ovulated within 24 h later.
Totally, there were 10 out of 70 endometrial specimens that were not included for histological dating and morphometric analysis. Six specimens had insufficient endometrial tissue for analysis (two specimens in control cycles and four in GnRH antagonist-treated cycle); among these, one participant had inadequate endometrial tissue in both cycles. The other four specimens (one in control cycles and three in GnRH antagonist-treated cycle) could not be assessed since they were reported as proliferative endometrium in spite of ultrasonographically documented ovulation. Therefore, only 52 endometrial specimens of 26 women could be included for analysis of histological dating and morphometric study.
The serum E2 and LH levels and the endometrial thickness on the day when the largest follicular diameter reached ≥18 mm in control and GnRH antagonist-treated cycles are shown in Table 1. There were no statistically significant difference between the two cycles with regards to the endometrial thickness (Wilcoxon Signed Ranks Test, P = 0.08). However, the serum E2 and LH levels in control cycles were significantly higher than those in the GnRH antagonist cycles (paired t test, P = 0.01 and Wilcoxon Signed Ranks Test, P = 0.02, respectively).
Table 1.
E2 and LH levels, and the endometrial thickness on the day when the largest follicle reached 18 mm in control and GnRH antagonist-treated cycles
| Variable | Control cycles | GnRH antagonist-treated cycles | P value |
|---|---|---|---|
| E2 level (pg/mL) | 680.99 ± 374.81 | 463.93 ± 300.60 | <0.05a |
| LH level (IU/L) | 18.59 ± 21.09 | 9.88 ± 12.94 | <0.05b |
| Endometrial thickness (mm) | 9.7 ± 2.0 | 8.9 ± 2.4 | NSb |
Values are means ± SD.
NS not significant
aPaired t test
bWilcoxon Signed Ranks Test
The results of the histological dating of the endometrium are demonstrated in Table 2. The percentages of out-of-phase endometrium (both delayed and accelerated growth) were not different between the control (21.9%) and GnRH antagonist-treated cycles (14.3%) (McNemar’s test, P = 0.375). As we used the criteria of Noyes for histological dating, the behavior of the glandular epithelium has been key of the first half of the secretory phase [25], we found that 2 out of 32 in the control and 1 out of 28 specimens in the GnRH antagonist-treated cycles had stromal advancement (glandular–stromal dyssynchrony).
Table 2.
Histological dating of the endometrium obtained on day 6 after ovulation in control and GnRH antagonist-treated cycles
| Histological dating | Control cycles n (%) | GnRH antagonist-treated cycles n (%) |
|---|---|---|
| In-phase | 25 (78.1) | 24 (85.7) |
| Delayed growtha | 5 (15.6) | 3 (10.7) |
| Accelerated growthb | 2 (6.3) | 1 (3.6) |
| Total | 32 | 28 |
aDelayed maturation ≥2 days
bAccelerated maturation ≥2 days
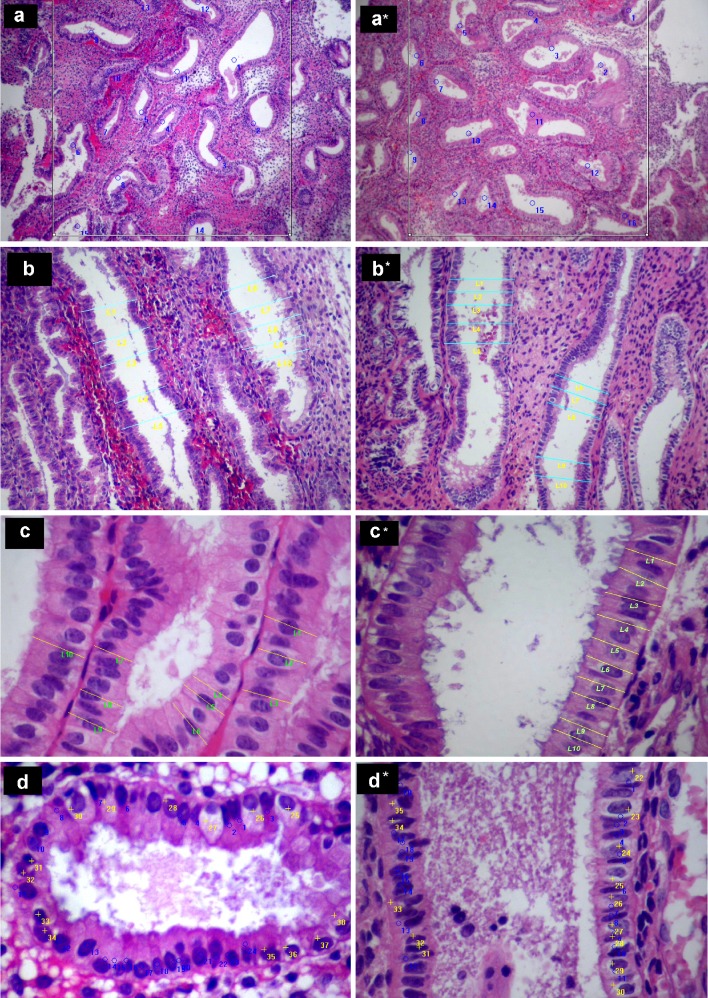
No significant difference in any of the four parameters of morphometric analysis between the two cycles was noted as depicted in Table 3. However, the diameter of endometrial glands trends to be smaller in GnRH antagonist-treated cycles (paired t test, P = 0.052). The illustrations of histological figures of morphometric analysis were demonstrated in Fig. 1.
Table 3.
Morphometric analysis of the endometrium obtained on day 6 after ovulation in control and GnRH antagonist-treated cycles
| Morphometric parameter | Control cycles | GnRH antagonist-treated cycles | P value |
|---|---|---|---|
| No. of glands per mm2 | 15.9 ± 4.4 | 15.6 ± 5.0 | 0.793a |
| Diameter of glands (μm) | 126.5 ± 17.6 | 116.3 ± 17.9 | 0.052a |
| Height of glandular epithelium (μm) | 20.1 ± 3.3 | 21.4 ± 2.6 | 0.087a |
| No. of vacuolated cells per 1,000 glandular cells | 667.0 ± 157.6 | 671.4 ± 111.4 | 0.887a |
Values are means ± SD
aPairedt test
Fig. 1.
Histological appearance, morphometric analysis in endometrial specimens from natural (a, b, c and d) and GnRH-antagonist treated (a*, b*, c* and d*) cycles. a, a* The number of endometrial glands per square millimeter. b, b* The maximal cross-sectional diameter of endometrial glands which were cut parallel to their longitudinal axis. c, c* The height of the glandular epithelial cells. d, d* The number of vacuolated cells (yellow marks) per 1,000 glandular cells
There was no adverse event caused by the procedures or drug injection in all volunteers except one who had ecchymosis at the injection site of the abdominal skin after ganirelix injection.
Discussion
Although the utilization of GnRH antagonists in combination with gonadotropin in COH is increasing, its trend toward lower pregnancy rate has influenced clinicians’ attitudes; therefore many clinicians become reluctant to use this regimen [28, 29]. Many evidences suggest that GnRH antagonist may have detrimental effects on the endometrium which consequently have an impact on embryo implantation. Firstly, the outcome from the ganirelix dose-finding study group [30], demonstrated that high dosages of GnRH antagonist (1 or 2 mg once daily) were associated with low implantation rate (8.8 and 1.5%, respectively) in fresh cycles. Whereas the follow-up report of this study [15] showed that 11 ongoing pregnancies were achieved from 46 cycles after replacement of supernumerary cryopreserved embryos obtained in the stimulation cycles of the same study [30]. Six of these 11 patients had been treated with high dosages of ganirelix (1 or 2 mg; ongoing pregnancy rate = 36.4 and 25%, respectively). These data suggested that high dosages of ganirelix did not adversely affect the quality of oocytes and embryos but the direct effect on the endometrium cannot be ruled out [15]. In addition, the results of Cochrane review which compared the efficacy of GnRH antagonist and long GnRH agonist protocol for COH showed a trend toward lower pregnancy rate in GnRH antagonist cycles despite the similar fertilization rate and number of good quality embryos [11]. Therefore, a decrease in endometrial receptivity presents on plausible mechanism for this trend. Furthermore, the presence of GnRH and its receptors in the endometrium with a significant increase during the early secretory phase which corresponds to the implantation window as in previous reports [17–19] suggesting the role of GnRH during the implantation period. Eventually, the mechanisms of GnRH antagonists are competitively block the GnRH receptors which evidently appear in the endometrium. Therefore, there are enough evidences that support the possibility of the effect of GnRH antagonist on the endometrium which may be responsible for the lower pregnancy rate when compared to the GnRH agonist long protocol.
There were many studies that evaluated the effects of GnRH agonists on the endometrium during COH. Nevertheless, the data on the endometrial morphology in cycles using GnRH antagonist adjunct to gonadotropins are much scarcer. In a study of 55 women undergoing COH with rFSH and GnRH antagonist, endometrial biopsies were performed on the day of oocyte pick-up. All endometria were advanced as compared to the expected chronological date and no pregnancy was established if histological dating was >3 days out-of-phase [31]. The results from another study [32] which evaluated the endometrium of the oocyte donors obtained on the day of oocyte retrieval and again, on day 7 post oocyte retrieval, compared the cycles utilizing GnRH antagonists (ganirelix or cetrorelix) with GnRH agonist, found accelerated endometrial maturation either on the day of oocyte retrieval or 7 days later, but no differences in cycles using GnRH agonist and antagonists. The investigators suppose that the supraphysiological level of E2 and the elevated serum progesterone level may be responsible for the marked advancement in endometrial maturation.
In our study, the endometrium on day 6 after ovulation, which correspond to the window of implantation, of the normal female volunteers were compared between the natural control cycles and the GnRH antagonist-treated cycles. We have found no differences between in-phase and out-of-phase endometrium in the two cycles. Moreover, in contrast to the two aforementioned studies [31, 32], we have found only three accelerated growth (two in natural and one in GnRH antagonist-treated cycles) in the examined endometria. This may be due to our study was not performed in COH cycles and hence no supraphysiological levels of E2 and progesterone that are responsible for endometrial advancement as found in COH cycles.
As we expected, the serum E2 and LH level in the GnRH antagonist-treated cycles were significantly lower than in the control cycle due to the rapid suppression of LH and E2 by GnRH antagonists [33]. However, in our study, the mean LH level in GnRH antagonist cycles was 9.88 IU/L, higher than the mean level from the pharmacodynamic study of ganirelix in female volunteers [34] and also in the COH cycles utilizing GnRH antagonist [6–8]. The possible explanation was the duration of administration of ganirelix in this study (averagely 3 days) which was shorter than the mentioned studies and it usually requires 2–3 days for ganirelix to reach the steady-state level [34].
While the E2 level was significantly lower in the GnRH antagonist cycle, we have found no difference in the endometrial thickness between the two cycles. According to the study of Ueno et al. [35] and Ohno et al. [36], they also found no correlation between E2 level and endometrial thickness, not only in the natural cycle but also in the stimulated cycle.
There were four proliferative endometrium specimens which not included in our analysis despite documented ovulation (one in natural cycle and three in GnRH antagonist cycles). These may be caused by the insufficient progesterone level (not analyzed in our study) or inadequate progesterone receptors to transform proliferative into secretory endometrium. Whether or not GnRH antagonist has any effect on progesterone and progesterone receptors this has not been established. The studies on COH cycles comparing ganirelix and long GnRH agonist protocols [6–8] showed the comparable progesterone level on the day of oocyte retrieval and embryo transfer. However, the data of the effect of GnRH antagonist on progesterone level in natural cycle need further investigation. Nevertheless, proliferative endometrium was reported as high as 19% of the endometrial tissue obtained from the patients with proved ectopic pregnancies [37].
Moreover, our results demonstrate no difference in any parameter of morphometric analysis between the two cycles was found. However, there is no clear evidence to confirm any change in morphometric parameters that would interfere with embryo implantation.
Even though our data suggest that the administration of GnRH antagonist to regularly menstruating volunteers may not affect the endometrium during the implantation window regarding the endometrial thickness, histological dating or morphometric analysis, GnRH antagonist may affect the endometrium at the molecular level which may be responsible for endometrial receptivity such as cell adhesion molecule and cytokines which cannot be detected by macro-cellular histology as in our study. Nevertheless, further studies are needed to determine this particular aspect of the endometrium.
However, the objective of this study is only to find out basic knowledge of the effect of GnRH antagonist on the endometrium, particularly when there is no effect of supraphysiological level of E2 and progesterone from COH. We did not perform the study in COH cycles because to do endometrial sampling in COH cycles during window of implantation may have negative effect on pregnancy rate. And the study in oocyte donors which is an ideal model is restricted by the limit of number of cases in our institute.
In conclusion, this study shows that GnRH antagonist has no effect on the endometrium of regularly menstruating women as assessed by endometrial thickness, histological dating and morphometric analysis while serum E2 and LH levels were lowered by GnRH antagonist as we expected.
Acknowledgements
We would like to thank Mettanando Bhikkhu for editing this manuscript. We are very grateful to Surasith Chaithongwongwatthana, M.D. for his useful advice on statistical analysis and Sarunya Numto, B.Sc. for her assistance in processing the endometrial specimens. This study would not have been possible without the generous cooperation of all volunteers who participated in this study. This study is supported by the Ratchadapiseksompotch Fund, Faculty of Medicine, Chulalongkorn University. Grant number: RA 178/47.
Footnotes
Capsule The endometrium of regularly menstruating volunteers on day 6 post ovulation after receiving GnRH antagonist were not different from the endometrium of natural cycle.
References
- 1.Daya S. Gonadotropin releasing hormone agonist protocols for pituitary desensitization in in vitro fertilization and gamete intrafallopian transfer cycles. Cochrane Database Syst Rev. 2000;2:CD001299. doi: 10.1002/14651858.CD001299. [DOI] [PubMed] [Google Scholar]
- 2.Olivennes F, Cunha-Filho JS, Fanchin R, Bouchard P, Frydman R. The use of GnRH antagonists in ovarian stimulation. Hum Reprod Update. 2002;8:279–90. doi: 10.1093/humupd/8.3.279. [DOI] [PubMed] [Google Scholar]
- 3.Newton C, Slota D, Yuzpe AA, Tummon IS. Memory complaints associated with the use of gonadotropin-releasing hormone agonists: a preliminary study. Fertil Steril. 1996;65:1253–5. doi: 10.1016/s0015-0282(16)58351-4. [DOI] [PubMed] [Google Scholar]
- 4.Warnock JK, Bundren JC, Morris DW. Depressive mood symptoms associated with ovarian suppression. Fertil Steril. 2000;74:984–6. doi: 10.1016/S0015-0282(00)01607-1. [DOI] [PubMed] [Google Scholar]
- 5.Shapiro DB. Introduction. Fertil Steril. 2003;80(suppl 1):S1–7. doi: 10.1016/S0015-0282(03)00766-0. [DOI] [PubMed] [Google Scholar]
- 6.The North American Ganirelix Study Group Efficacy and safety of ganirelix acetate versus leuprolide acetate in women undergoing controlled ovarian hyperstimulation. Fertil Steril. 2001;75:38–45. doi: 10.1016/S0015-0282(00)01638-1. [DOI] [PubMed] [Google Scholar]
- 7.®. Study Group. The European Orgalutran Treatment with the gonadotropin-releasing hormone antagonist ganirelix in women undergoing ovarian stimulation with recombinant follicle stimulating hormone is effective, safe, and convenient: results of a controlled, randomized, multicentre trial. Hum Reprod. 2000;15:1490–8. doi: 10.1093/humrep/15.7.1490. [DOI] [PubMed] [Google Scholar]
- 8.®. Study Group. The European and Middle East Orgalutran Comparable clinical outcome using the GnRH antagonist ganirelix or a long protocol of the GnRH agonist triptorelin for the prevention of premature LH surges in women undergoing ovarian stimulation. Hum Reprod. 2001;16:644–51. doi: 10.1093/humrep/16.4.644. [DOI] [PubMed] [Google Scholar]
- 9.Albano C, Felberbaum RE, Smitz J, Riethmüller-Winzen H, Engel J, Diedrich K, et al. Ovarian stimulation with HMG: results of a prospective randomized phase III European study comparing the luteinizing hormone-releasing hormone (LHRH)-antagonist cetrorelix and the LHRH-agonist buserelin. Hum Reprod. 2000;15:526–31. doi: 10.1093/humrep/15.3.526. [DOI] [PubMed] [Google Scholar]
- 10.Olivennes F, Belaisch-Allart J, Emperaire JC, Dechuad H, Alvarez S, Moreau L, et al. Prospective, randomized, controlled study of in vitro fertilization-embryo transfer with a single dose of a luteinizing hormone-releasing hormone (LH-RH) antagonist (cetrorelix) or a depot formula of an LH-RH agonist (triptorelin) Fertil Steril. 2000;73:314–20. doi: 10.1016/S0015-0282(99)00524-5. [DOI] [PubMed] [Google Scholar]
- 11.Al-Inany H, Aboulghar M. GnRH antagonist in assisted reproduction: a Cochrane review. Hum Reprod. 2002;17:874–85. doi: 10.1093/humrep/17.4.874. [DOI] [PubMed] [Google Scholar]
- 12.Al-Inany H, Abou-Setta AM, Aboulghar M. Gonadotrophin-releasing hormone antagonists for assisted conception. Cochrane Database Syst Rev. 2006;3:CD001750. doi: 10.1002/14651858.CD001750.pub2. [DOI] [PubMed] [Google Scholar]
- 13.Nikolettos N, Al-Hasani S, Felberbaum R, Demirel LC, Riethmüller-Winzen H, Reissmann T, et al. Comparison of cryopreservation outcome with human pronuclear stage oocytes obtained by the GnRH antagonist, Cetrorelix, and GnRH agonists. Eur J Obstet Gynaecol Reprod Biol. 2000;93:91–5. doi: 10.1016/S0301-2115(99)00294-8. [DOI] [PubMed] [Google Scholar]
- 14.Seelig AS, Al-Hasani S, Katalinic A, Schöpper B, Sturm R, Diedrich K, et al. Comparison of cryopreservation outcome with gonadotropin-releasing hormone agonists or antagonists in the collecting cycle. Fertil Steril. 2002;77:472–5. doi: 10.1016/S0015-0282(01)03008-4. [DOI] [PubMed] [Google Scholar]
- 15.Kol S, Lightman A, Hillensjo T, Devroey P, Fauser B, Tarlatzis B, et al. High doses of gonadotrophin-releasing hormone antagonist in in-vitro fertilization cycles do not adversely affect the outcome of subsequent freeze–thaw cycles. Hum Reprod. 1999;14:2242–4. doi: 10.1093/humrep/14.9.2242. [DOI] [PubMed] [Google Scholar]
- 16.Hernandez ER. Embryo implantation and GnRH antagonists: Embryo implantation: the Rubicon for GnRH antagonists. Hum Reprod. 2000;15:1211–6. doi: 10.1093/humrep/15.6.1211. [DOI] [PubMed] [Google Scholar]
- 17.Dong KW, Marcelin K, Hsu MI, Chiang CM, Hoffman G, Roberts JL. Expression of gonadotropin-releasing hormone (GnRH) gene in human uterine endometrial tissue. Mol Hum Reprod. 1998;4:893–8. doi: 10.1093/molehr/4.9.893. [DOI] [PubMed] [Google Scholar]
- 18.Raga F, Casan EM, Kruessel JS, Wen Y, Huang HY, Nezhat C, et al. Quantitative gonadotropin-releasing hormone gene expression and immunohistochemical localization in human endometrium throughout the menstrual cycle. Biol Reprod. 1998;59:661–9. doi: 10.1095/biolreprod59.3.661. [DOI] [PubMed] [Google Scholar]
- 19.Takeuchi S, Futamura N, Minoura H, Toyoda N. Possible direct effect of gonadotropin releasing hormone on human endometrium and decidua. Life Sci. 1998;62:1187–94. doi: 10.1016/S0024-3205(98)00045-9. [DOI] [PubMed] [Google Scholar]
- 20.Acosta AA, Elberger L, Borghi M, Calamera JC, Chemes H, Doncel GF, et al. Endometrial dating and determination of the window of implantation in healthy fertile women. Fertil Steril. 2000;73:788–98. doi: 10.1016/S0015-0282(99)00605-6. [DOI] [PubMed] [Google Scholar]
- 21.Li TC, Lenton EA, Rogers AW, Cooke ID, Dockery P. A new method of histologic dating of human endometrium in the luteal phase. Fertil Steril. 1988;50:52–60. doi: 10.1016/s0015-0282(16)60008-0. [DOI] [PubMed] [Google Scholar]
- 22.Bakos O, Lundkvist Ö, Wide L, Bergh T. Ultrasonographical and hormonal description of the normal ovulatory menstrual cycle. Acta Obstet Gynecol Scand. 1994;73:790–6. doi: 10.3109/00016349409072507. [DOI] [PubMed] [Google Scholar]
- 23.Ritchie WGM. Ultrasound in the evaluation of normal and induced ovulation. Fertil Steril. 1985;43:167–81. doi: 10.1016/s0015-0282(16)48369-x. [DOI] [PubMed] [Google Scholar]
- 24.Ecochard R, Boehringer H, Rabilloud M, Marret H. Chronological aspects of ultrasonic, hormonal, and other indirect indices of ovulation. Br J Obstet Gynaecol. 2001;108:822–9. doi: 10.1016/S0306-5456(00)00194-7. [DOI] [PubMed] [Google Scholar]
- 25.Noyes RW, Hertig AT, Rock J. Dating the endometrial biopsy. Fertil Steril. 1950;1:3–25. doi: 10.1016/j.fertnstert.2019.08.079. [DOI] [PubMed] [Google Scholar]
- 26.Johannisson E, Parker RA, Landgren BM, Diczfalusy E. Morphometric analysis of the human endometrium in relation to peripheral hormone levels. Fertil Steril. 1982;38:564–71. doi: 10.1016/s0015-0282(16)46636-7. [DOI] [PubMed] [Google Scholar]
- 27.Sereepapong W, Suwajanakorn S, Triratanachat S, Sampatanukul P, Pruksananonda K, Boonkasemsanti W, et al. Effects of clomiphene citrate on the endometrium of regularly cycling women. Fertil Steril. 2000;73:287–91. doi: 10.1016/S0015-0282(99)00509-9. [DOI] [PubMed] [Google Scholar]
- 28.Fauser B, Devroey P. Why is the clinical acceptance of gonadotropin-releasing hormone antagonist cotreatment during ovarian hyperstimulation for in vitro fertilization so slow? Fertil Steril. 2005;83:1607–11. doi: 10.1016/j.fertnstert.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 29.Griesinger G, Felderbaum R, Diedrich K. GnRH antagonists in ovarian stimulation: a treatment regimen of clinicians’ second choice? Data from the German national IVF registry. Hum Reprod. 2005;20:2373–5. doi: 10.1093/humrep/dei086. [DOI] [PubMed] [Google Scholar]
- 30.The ganirelix dose-finding study group A double-blind, randomized, dose-finding study to assess the efficacy of the gonadotropin-releasing hormone antagonist ganirelix (Org 37462) to prevent premature luteinizing hormone surges in women undergoing ovarian stimulation with recombinant follicle stimulating hormone (Puregon®) Hum Reprod. 1998;13:3023–31. doi: 10.1093/humrep/13.11.3023. [DOI] [PubMed] [Google Scholar]
- 31.Kolibianakis E, Bourgain C, Albano C, Osmanagaoglu K, Smitz J, Steirteghem A, et al. Effect of ovarian stimulation with recombinant follicle-stimulating hormone, gonadotropin releasing hormone antagonists, and human chorionic gonadotropin on endometrial maturation on the day of oocyte pick-up. Fertil Steril. 2002;78:1025–9. doi: 10.1016/S0015-0282(02)03323-X. [DOI] [PubMed] [Google Scholar]
- 32.Saadat P, Boostanfar R, Slater CC, Tourgeman DE, Stanczyk FZ, Paulson RJ. Accelerated endometrial maturation in the luteal phase of cycles utilizing controlled ovarian hyperstimulation: impact of gonadotropin-releasing hormone agonists versus antagonists. Fertil Steril. 2004;82:167–71. doi: 10.1016/j.fertnstert.2003.11.050. [DOI] [PubMed] [Google Scholar]
- 33.Griesinger G, Felberbaum RE, Schultze-Mosgau A, Diedrich K. Gonadotropin-releasing hormone antagonists for assisted reproductive techniques: are there clinical differences between agents? Drugs. 2004;64:563–75. doi: 10.2165/00003495-200464060-00001. [DOI] [PubMed] [Google Scholar]
- 34.Oberyé JJL, Mannaerts BMJL, Huisman JAM, Timmer CJ. Pharmacokinetic and pharmacodynamic characteristics of ganirelix (Antagon®/Orgalutran®). Part II. Dose-proportionality and gonadotropin suppression after multiples doses of ganirelix in healthy female volunteers. Fertil Steril. 1999;72:1006–12. doi: 10.1016/S0015-0282(99)00414-8. [DOI] [PubMed] [Google Scholar]
- 35.Ueno J, Oehninger S, Brzyski RG, Acosta AA, Philput CB, Muasher SJ. Ultrasonographic appearance of the endometrium in natural and stimulated in-vitro fertilization cycles and its correlation with outcome. Hum Reprod. 1991;6:901–4. doi: 10.1093/oxfordjournals.humrep.a137455. [DOI] [PubMed] [Google Scholar]
- 36.Ohno Y, Hosokawa K, Tamura T, Fujimoto Y, Kawashima M, Koishi K, et al. Endometrial oestrogen and progesterone receptors and their relationship to sonographic appearance. Hum Reprod. 1995;10:708–11. doi: 10.1093/oxfordjournals.humrep.a136020. [DOI] [PubMed] [Google Scholar]
- 37.Ollendorff DA, Fejgin MD, Barzilai M, Ben-Noon I, Gerbie AB. The value of curettage in the diagnosis of ectopic pregnancy. Am J Obstet Gynecol. 1987;157:71–2. doi: 10.1016/s0002-9378(87)80348-4. [DOI] [PubMed] [Google Scholar]