Abstract
Refractile bodies are one of the main morphological abnormalities that can be observed in the cytoplasm of human oocytes. In the present studies the characteristics of refractile bodies and the relationship between the size of these structures and developmental competence of the affected oocytes and resulting embryos were examined. The refractile bodies were found to have yellow autofluorescence which was consistent with the typical autofluorescence of lipofuscin. Viewed by transmitted electron microscopy, the refractile bodies showed the conventional morphology of lipofuscin inclusions and consisted of a mixture of lipids and dense granule materials. Large refractile bodies (>5 μm) were positively stained by the Schmorl reaction and were considered to contain lipofuscin. These larger lipofuscin inclusions (>5 μm) were associated with significantly reduced fertilization and unfavorable blastocyst development.
Keywords: Oocyte refractile bodies, Lipofuscin bodies, Autofluorescence, Oocyte fertilization, Blastocyst development
Introduction
Oocyte morphology is an important predictor of pregnancy for intracytoplasmic sperm injection (ICSI) [1–3]. On the other hand, it has been reported that oocyte morphology does not correlate with fertilization rate and embryo quality after ICSI [4, 5]. These inconsistent results may be related to the different criteria used for assessing cytoplasmic dysmorphic phenotypes in oocytes. Since various cytoplasmic abnormalities may derive from different causes, we need to examine independently the influence on development, as well as the etiology for each abnormal phenotype. In this study, we focused on the presence of cytoplasmic refractile bodies previously described by Veeck [6].
According to Veeck, the diameter of a refractile body may be approximately 10 μm under bright-field microscopy, appearing highly refractile because of its composition of lipid material and dense granules. In oocytes, cytoplasmic refractile bodies can include lipid bodies, small autophagic vacuoles, and lipofuscin bodies. Necrotic regions, described by Van Blerkom [7], may also be included, but there are complicated by vacuolation, and are probably quite rare. All of these inclusions may vary in size, ranging from 1 to over 5 μm, and some are autofluorescent. The larger refractile bodies are more likely to contain lipofuscin, although special staining (described in this paper) is required to demonstrate this property. Both mature and immature oocytes have been demonstrated to contain refractile bodies and there was a tendency for their recurrence in the same patient with repeated treatment cycles [6]. Such refractile bodies were usually associated with poor fertilization (2%) when IVF procedures were used [6]. In contrast, oocytes containing refractile bodies were usually fertilized normally using ICSI [4, 8]. However, the relationship between the sizes of the refractile bodies and developmental competence of oocytes has not been clearly defined. Also, the development of these cytoplasmic structures and their relationship to oocyte maturity and viability are not fully understood. Moreover, information on the composition and characteristics of the refractile bodies have not been published previously.
Lipofuscin is an intracellular material discovered more than a century and a half ago. In 1842 it was first described as a brown-yellow pigment in neurons [9]. In 1912 Hueck introduced the term lipofuscin, based on lipo (Greek for fat) and fuscus (Latin for dark), consisting of aggregated polymers derived from the oxidation products of lipids and proteins. Lipofuscin is autofluorescent [10, 11] and related to aging [12] with continuous accumulation over time within postmitotic cells such as neurons [13, 14] and cardiac myocytes [15, 16]. Some experimental data has shown that the cytoplasmic accumulation of lipofuscin in cells is accelerated by an increase in the concentration of oxygen [17, 18] and by inhibitors of lysosomal proteases and lipases [19, 20], suggesting that oxidative stress and the incomplete degradation of autophagocytosed material may cause lipofuscinogenesis by the accumulation of peroxidized lipids and proteins in secondary lysosomes [20].
In this study, we found that the refractile bodies corresponded to the presence of lipofuscin in such inclusions in oocytes. We analyzed the morphological, cytochemical and autofluorescent characteristics of these refractile (lipofuscin) bodies and examined the relationship between their size and the developmental competence of affected oocytes.
Materials and methods
IVF and ICSI procedure
Oocytes were collected from follicular fluid, washed in freshly equilibrated HTF medium (In Vitro Care, San Diego, USA), and incubated at 37°C in 6% CO2/5% O2/89% N2 before being denuded of follicular cells. The pH of the culture medium was checked every week and adjusted to 7.2∼7.4. The denuding procedure for ICSI was performed 2∼5 h after oocyte retrieval. The size of the refractile bodies was recorded prospectively from January 2004 to December 2004 after the denuding procedure and on day 1 after insemination. We have removed the granulosa cells as much as possible to observe for the presence of refractile bodies. It is possible to observe the presence of such bodies after partial removal of the granulosa cells. Measurements of refractile bodies were made from a monitor connected to an Olympus inverted microscope, at ×300 magnification (Olympus, Japan). A microscope objective micrometer (Olympus) which can be used to measure cytoplasmic inclusions as small as 1 μm, was employed in these studies. In the case of ICSI, the size of the refractile bodies was checked again on day 1 after sperm injection. We confirmed that the size of refractile bodies did not change during 1 day in vitro culture. Therefore, the oocytes and zygotes coming from IVF and ICSI were comparable. The detection of refractile bodies was based on DIC bright field microscopy. ICSI was performed on at least 20% of the oocytes in the majority of patients according to our clinic’s sperm evaluation criteria. In brief, when the number of motile sperm count was less than 5 × 106/ml, ICSI was performed for all mature oocytes. When the number of motile sperm count was between 5 × 106/ml and 10 × 106/ml, ICSI was performed for about half of the oocytes and IVF was performed for the remaining oocytes. Oocytes were selected randomly for IVF or ICSI. Insemination for IVF was performed 2∼3 h after the oocyte retrieval. HTF, IVC-two and IVC-three media (In Vitro Care) were used in all cycles for ICSI and IVF. All patients signed an informed consent form, and Institutional Review Board approvals were obtained from Ochanomizu University and Nagai Clinic.
Fluorescence microscopy
Fifteen oocytes that could not be fertilized in the IVF and ICSI procedure, and which contained refractile bodies, were directly mounted in HEPES-buffered HTF medium (In Vitro Care) and examined with an Olympus fluorescence microscope excited at 450–490 nm with an emission filter of 510 nm and at 510∼560 nm with an emission filter of 590 nm.
Spectral imaging
Spectral image analysis was performed using SpectraImage (Applied Spectral Imaging, Migdal HaEmek Israel) combined with an Olympus IX70 microscope (Olympus), equipped with a high-pressure mercury lamp for excitation, and a set of filters for blue-violet excitation (band pass: 420∼480 nm), a dichroic mirror (500 nm), and a cut-on red emission barrier filter (580 nm). The spectral resolution (FWHM; full-width-half-maximum) was 5 at 400 nm (12 at 600 nm). The cells were inspected for less than 1 min at a time to avoid sample bleaching. The maximum intensity was considered to be 100% and then the data were normalized. Four oocytes that contained refractile bodies that failed to fertilize in the IVF or ICSI procedures and three embryos derived from abnormal fertilization such as single or multiple pronuclear formations were used to obtain the spectral images. Oocytes or embryos were analyzed in HEPES-buffered HTF medium kept at 37°C. The spectral images were obtained within 30 min in the HEPES-buffered HTF medium. The background fluorescence of the cytoplasm in the vicinity of the lipofuscin bodies was deducted from the level of detected fluorescence.
Transmitted electron microscopic (TEM) observations of the refractile bodies
We examined ten oocytes containing refractile bodies and three oocytes that were negative for refractile bodies that failed to fertilize using ICSI or IVF procedures. Oocytes were fixed in 2.5% glutaraldehyde in PBS, pH 7.4, for 2 h, rinsed in PBS, and then post-fixed for 2 h with 1% OsO4 in PBS. The oocytes were rinsed thoroughly in distilled water, then dehydrated in ethanol, and finally embedded in Epon resin containing 14% Questo1653 (Okenshoji, Osaka, Japan), 23% ERL4206, 63% nonenyl sussinic anhydride, and 0.5% S-1 (TAAB Laboratories Equipment, Berkshire England). Ultra thin sections were stained with uranyl acetate and lead citrate and then examined using a JEOL-1230 transmission electron microscope (JEOL, Tokyo, Japan).
Schmorl staining
Three oocytes that were positive for refractile bodies (>5 μm) were fixed for 30 min in 3.6% w/v solution of formaldehyde EM (TAAB). Fixed samples were washed with Dulbecco’s PBS (Sigma) three times and dehydrated in graded ethanol and butylalcohol and embedded in paraffin wax. Sections were cut at 3 μm using a Leica Ultracut UCT, and dried into Superfrost microscope slides (Matsunami Glass Ind., Japan). Schmorl staining was performed at BML (Bio Medical Laboratories, Saitama, Japan). Observations were made using an inverted microscope.
Comparative studies
The size of the refractile bodies in all MII oocytes from all patients (n = 1,113) were classified into the three groups (<3, 3–5, >5 μm). Fertilization and cleavage rates were compared in relation to the three groups of cytoplasmic refractile bodies. During the study period, the following three ovarian stimulation protocols were used: uFSH/HMG+GnRH antagonist protocol (n = 97), Clomiphene citrate (CC)+uFSH/HMG+GnRH antagosist protocol (n = 65), and the long protocol (n = 72). The exact ovarian stimulation protocol was chosen based on gynecologist and/or patient preference. Statistical analysis was performed for each stimulation protocol and total number of stimulation protocols. Treatment cycles were classified into the above three groups based on the size of the largest refractile bodies that existed in individual oocytes in each of the treatment cycles. The age of the patients was classified into four groups (<31, 31∼35, 36∼40, >40), and results were compared based on the size of the largest refractile bodies in oocytes recovered from different treatment cycles.
Furthermore, the embryos that were not transferred to patients were cultured for 5∼7 days to determine whether they would develop to blastocysts. The rate of blastocyst development was compared in relation to the size of refractile bodies (<3, 3∼5, >5 μm). All developed blastocysts were cryopreserved according to the patients’ requests. We could not analyze pregnancy rates because frequently embryos containing refractile bodies of different sizes were transferred together. Embryos were transferred on day 2∼5 of development.
Statistical analysis
The statistical package used for data analysis was SPSS Ver12.0, SPSS. Clinical characteristics were analyzed using ANOVA. Observed differences between the results were regarded as statistically significant if the P value was <0.05.
Results
Autofluorescence of refractile bodies
When we observed unstained human oocytes using fluorescent microscopy at blue (450∼490 nm) or at green (510∼560 nm) excitation wavelengths, we found that refractile bodies emitted a yellow or red autofluorescence (Fig. 1A,B). These bodies were detected in oocytes throughout their meiotic maturation (GV, MI, MII) and embryonic development (four-cells, eight-cells, morula, blastocyst). The yellow autofluorescence in the refractile bodies was also confirmed by laser confocal microscopy, excited at either blue (450∼490 nm) or green (510∼560 nm) wavelengths.
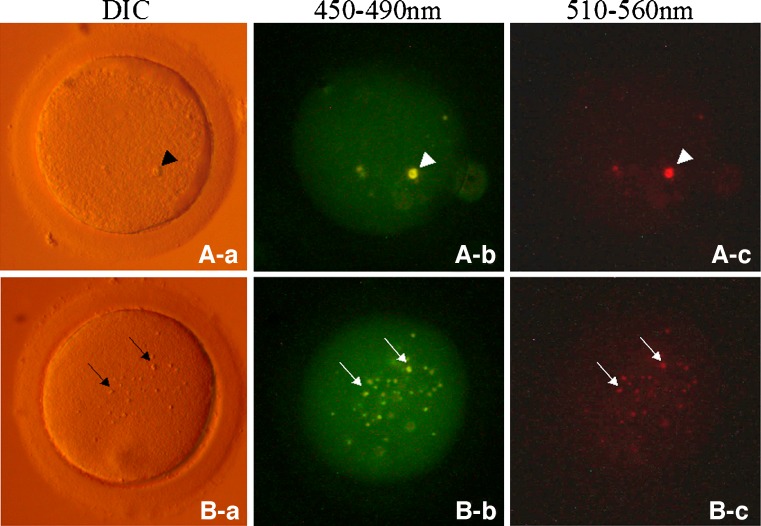
Fig. 1.
Autofluorescent refractile bodies in human oocytes. An arrowhead shows a large refractile body (∼6 μm) in a MII stage human oocyte (A-a). Arrows show small refractile bodies (1∼2 μm) in a MII stage human oocyte (B-a). Both the large and the small refractile bodies in human oocytes exhibited autofluorescence when excited at 450–490 nm (A-b, B-b) or at 510∼560 nm (A-c, B-c)
Ultrastructure of refractile bodies
To observe the ultrastructure of the refractile bodies, oocytes that had a large refractile body (8∼10 μm) were selected using light or fluorescent microscopy. The oocytes were then fixed and embedded in resin (see materials and methods). When an entire series of continuous ultra thin sections of whole oocytes (n = 3) were examined using TEM, a structure composed of fine amorphous electron-dense substances and lipids, encircled partly by a membrane, were observed in each oocyte (Fig. 2A). The size and position of these structures were similar to that of the refractile bodies identified by light microscopy and the ultrastructural features were typical in morphology to lipofuscin. In small foci within refractile bodies, an accumulation of lipid droplets, about 1 μm in diameter (Fig. 2B), was observed (Fig. 2B-b). These lipid foci were not encircled by a membrane.
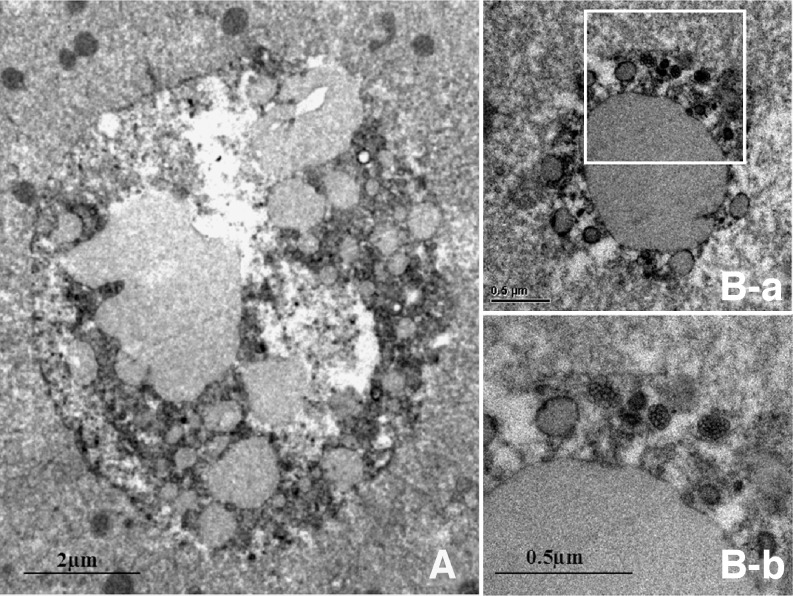
Fig. 2.
Transmission electron micrographs of refractile bodies are shown. A A large refractile body (∼8 μm in diameter) contained a fine amorphous electron-dense substance and lipids. The inclusions were partly encircled by a membrane (not completely surrounding the inclusion; ×6,000). B A corona-shaped small refractile body ∼1.5 μm in diameter (B-a, ×20,000). Further magnification (B-b, ×40,000) shows an accumulation of lipid droplets at the periphery of the large lipid body. Small (1∼2 μm) refractile bodies were not surrounded by a membrane
Schmorl staining
It is well known that lipofuscin is stained by the Schmorl reaction which is a ferricyanide reduction method for reducing substances of lipofuscin [21–24]. To show that the refractile bodies contain lipofuscin, oocytes that contained large refractile bodies (>5 μm), obtained from different patients, were examined using the Schmorl reaction. As shown in Fig. 3, the large bodies were positively stained, indicating that the refractile bodies contained lipofusicn.
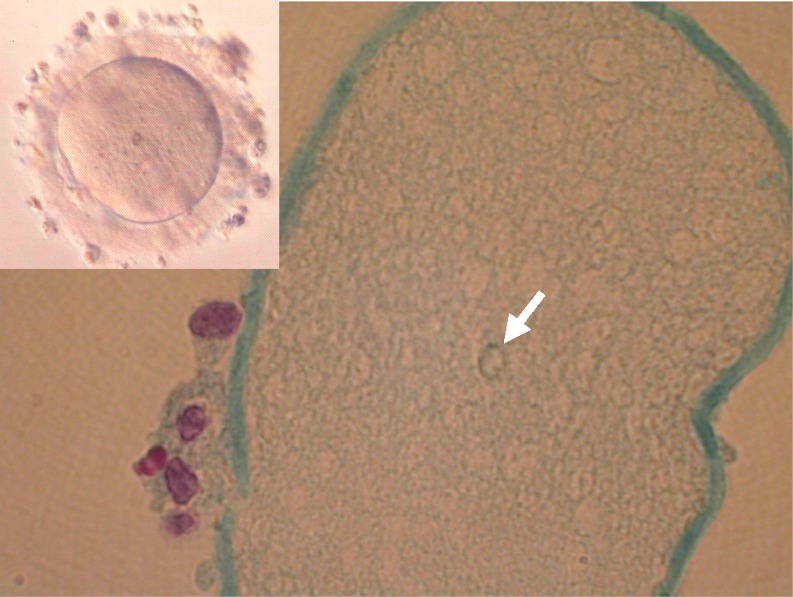
Fig. 3.
Schmorl staining for lipofuscin in human oocytes. Three oocytes containing a large lipofuscin body (>5 μm) obtained from different patients were fixed and embedded in paraffin wax. An arrow shows a lipofuscin body (∼7 μm) positively stained by the Schmorl reaction. The inset shows the oocyte before fixation
Fluorescence spectra of lipofuscin in developing oocytes and embryos
In the spectral image analysis using seven lipofuscin-positive oocytes/embryos, the emission maximum of the refractile bodies (∼5 μm) obtained at each stage of development (GV, MI, MII, cleaved embryo and morula) was 570∼610 nm when excited at 420∼480 nm (Fig. 4A). Differences in fluorescent characteristics of the refractile bodies were not observed during meiotic maturation and early embryonic development (Fig. 4B).
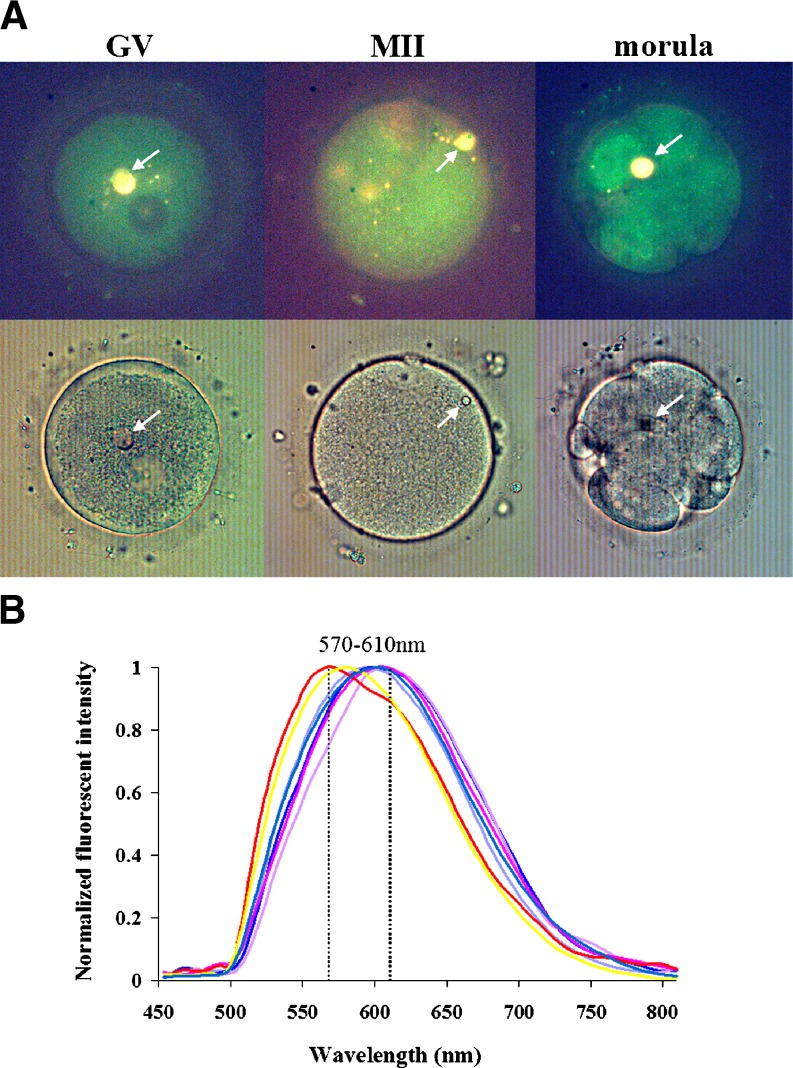
Fig. 4.
Spectral imaging analysis of oocytes and embryos containing refractile bodies-performed with SpectraImage (Applied Spectral Imaging). AArrows in the upper panel show yellow autofluorescence at each stage of development of the oocyte and embryo. The lower panel shows light microscopic images of the same oocytes (or embryo). B In total, seven large refractile bodies (5 μm) at each of the stages of development were analyzed. The maximum emission of the refractile bodies was 570∼610 nm when excited at 420∼480 nm
Comparative studies
To examine the relationship between the size of refractile bodies and fertilization rate or cleavage rate after IVF or ICSI procedures, oocytes were classified into three groups based on the size of their largest refractile bodies (<3, 3∼5, >5 μm). In the case of ICSI, the size of the refractile bodies was checked again on day 1 after sperm injection. We confirmed that the size of refractile bodies did not change during one day of in vitro culture. The results were evaluated on the basis of these three size categories of refractile bodies and the ovarian stimulation protocol used as shown in Table 1.
Table 1.
Comparison between fertilization rate, cleavage rate following IVF and ICSI procedures in oocytes containing different sizes of refractile/lipofuscin bodies and recovered from three stimulation protocols
| The size of the largest refractile/lipofuscin bodies (μm) | CC-antagonist | FSH/HMG-antagonist | Long protocol | ||||||
|---|---|---|---|---|---|---|---|---|---|
| <3 | 3–5 | >5 | <3 | 3–5 | >5 | <3 | 3–5 | >5 | |
| IVF fertilization rate | 86.4% (127/147) | 68.8% (8/11) | 66.6% (2/3) | 66.8% (193/289) | 47.4% (9/19) | 53.3% (8/15) | 84.5% (212/251) | 82.8% (24/29) | 68.0% (15/22) |
| Cleavage rate | 86.4% (127/147) | 68.8% (8/11) | 66.6% (2/3) | 66.1% (191/289) | 47.4% (9/19) | 46.7% (7/15) | 84.5% (212/251) | 82.8% (24/29) | 59.1% (13/22) |
| ICSI fertilization rate | 70.6% (48/68) | 60.0% (3/5) | 0% (0/1) | 69.1% (85/123) | 60.0% (9/15) | 87.5% (7/8) | 64.8% (46/71) | 66.7% (12/18) | 80.0% (4/5) |
| Cleavage rate | 69.1% (47/68) | 60.0% (3/5) | 0% (0/1) | 69.1% (85/123) | 60.0% (9/15) | 87.5% (7/8) | 64.8% (46/71) | 66.7% (12/18) | 80.0% (4/5) |
The size of the largest refractile/lipofuscin bodies in each of the oocytes/embryos were used to classify them into the three groups of <3, 3–5 and >5 μm.
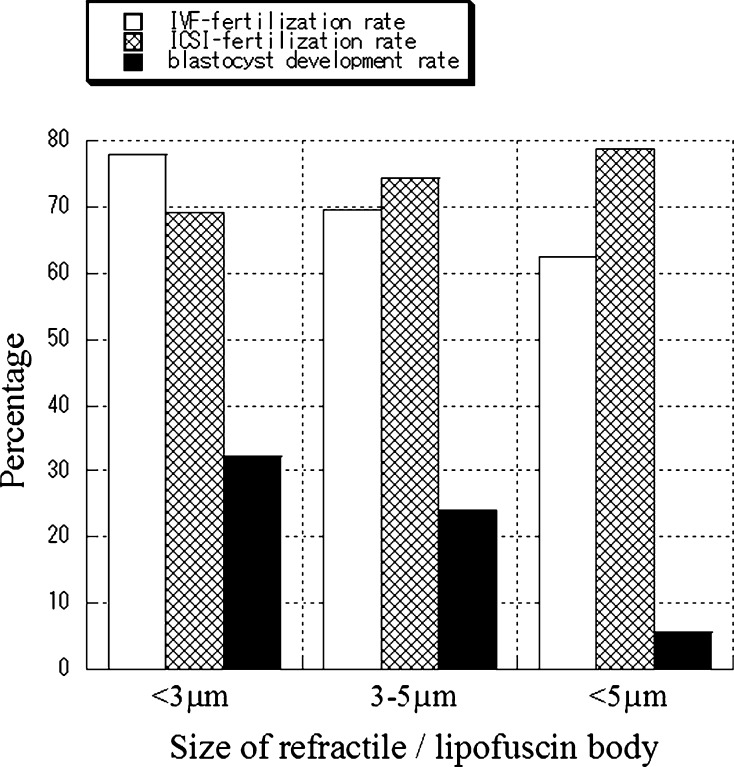
In this study, no significant difference was obtained on fertilization and cleavage rates among three simulation protocols. In total, fertilization and cleavage rates in IVF were significantly lower when the size of the refractile bodies was larger. However, these differences were not seen in the case of ICSI (Fig. 5). To examine the developmental competence of embryos containing refractile bodies of various sizes, embryos that were not transferred to uteri (n = 318) were cultured for 5∼7 days to determine whether or not they would develop to blastocysts. Blastocyst development was found to be 5.6% (1/18) when embryos contained large refractile bodies (>5 μm), 24.2% (8/33) when their refractile bodies were 3∼5 μm and 32.2% (86/267) when they were less than 3 μm. The differences were statistically significant (P < 0.05) between all size categories (Fig. 5).
Fig. 5.
Fertilization success rate and blastocyst development rate from oocytes/embryos containing lipofuscin. In total, fertilization and cleavage rates in IVF were significantly lower when the size of the refractile bodies was larger. These differences were not seen in the case of ICSI. Blastocyst development was found to be significantly lower when the size of the refractile bodies was larger. See detail in the text
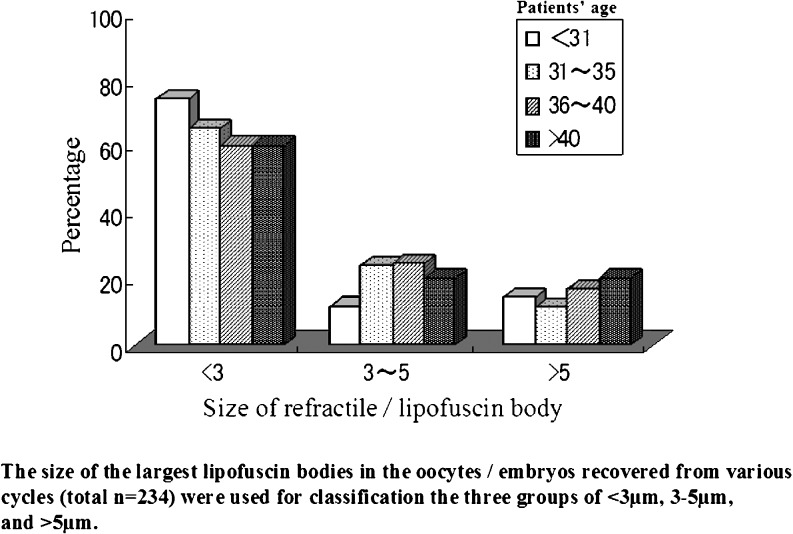
Since lipofuscin is usually observed in aging cells, we examined the occurrence of refractile bodies in relation to the age of patients. The age of patients was classified into four groups (<31, 31∼35, 36∼40, >40). Oocyte categories were classified into three groups based on the size of the largest refractile bodies (<3, 3∼5, >5 μm) that were present in individual oocytes. The occurrence of refractile bodies in each size category (<3, 3∼5, >5 μm) was 74.3, 11.4, 14.3% (patients’ age <31; n = 35), 65.3, 23.8, 10.9% (31∼35; n = 101), 60.0, 24.4, 16.7 (36∼40; n = 78) and 60.0, 20.0, 20.0% (>40; n = 20) and no significant difference was detected among the four age groups (Fig. 6).
Fig. 6.
Relationship between patients’ age and the occurrence of lipofuscin bodies. The age of patients was classified into four groups (<31, 31∼35, 36∼40, >40). Oocyte categories were classified into three groups based on the size of the largest refractile bodies (<3, 3∼5, >5 μm) that were present in individual oocytes. The occurrence of refractile bodies in each size category (<3, 3∼5, >5 μm) was not significantly different among the four age groups. See detail in the text
Discussion
The refractile bodies in oocytes were found to have yellow autofluorescence which was consistent with the typical autofluorescence previously described for lipofuscin (Ex: approximately 440; Em: approximately 600 nm [25]). Our TEM studies on the refractile bodies also showed that they had the conventional morphology of lipofuscin inclusions, composed of a mixture of lipids and dense granular materials. The cytoplasmic refractile bodies were also shown to be positively stained using the Schmorl reaction which is a traditional method for detecting lipofuscin [21]. We have therefore concluded that the refractile bodies, described in the present paper and originally depicted in human oocytes by Veeck [6], correspond to lipofuscin bodies.
Using ICSI, the oocytes that contain refractile/lipofuscin bodies could be fertilized as shown by De Sutter et al. [4] and Serhal et al. [8]. In contrast, when IVF was employed, it was reported that the presence of refractile bodies was associated with poor fertilization (2%) [6]. Our result also showed that a significantly reduced fertilization rate occurred in oocytes with large (>5 μm) refractile/lipofuscin bodies (62.5% compared with 77.8% in oocytes with smaller refractile bodies). The much higher fertilization rates obtained in the present studies, compared to that reported by Veeck [6], may be related to improvements in culture media and sperm preparation over the last 10 years. More importantly, the present studies showed that the presence of large lipofuscin bodies (>5 μm) was associated with significantly lower blastocyst development rates. However, reduced blastocyst developmental was not detected when the size of cytoplasmic lipofuscin bodies was less than 3 μm. We propose that an evaluation for the presence of lipofuscin bodies (>5 μm) in oocytes/embryos could be used for determining oocyte or embryo quality.
Normal IVF is influenced by a multiple-stage process that involves sperm binding to zona pellucida, penetration of the zona, sperm bonding to the plasma membrane of the oocyte, incorporation of sperm into the ooplasm and subsequently decondensation of the sperm and maternal chromatin and pronuclear formation followed by syngamy and the formation of the first mitotic spindle. Some of the early stages of IVF could be disrupted by oxidative stress or cytoplasmic deterioration (for example, induction of a premature cortical granule reaction and damage of plasma membrane which may disturb sperm–egg fusion). ICSI bypasses some of the critical early stages of fertilization. This could account for some of the differences between IVF and ICSI in relation to cytoplasmic disturbances related to lipofuscin formation and associated abnormalities.
Detection of lipofuscin bodies during IVF may be difficult due to the presence of granulosa cells or sperm attached to the zona perucida. In the present study, we found that lipofuscin bodies exhibited autofluorescence with a maximum emission of 570∼610 nm. Thus, it would be possible to detect lipofuscin-containing cytoplasmic inclusions during IVF procedures by using very sensitive cameras [26] with non-toxic levels of blue or green excitation. Alternatively, it may be possible to screen oocytes for refractile bodies using differential interference contrast optics with a 100× oil immersion lens with a high numerical aperture, together with glass culture dishes.
It is well established that the lipofuscin content of many post-mitotic cell types increases progressively during normal senescence. This age-related accumulation of lipofuscin occurs most notably in the liver, neurons, cardiac muscle, and retinal pigment epithelium. However, in this study, we found that no relationship existed between the range of ages studied and lipofuscin formation in human oocytes obtained following ovarian stimulation.
Lipofuscin bodies in human oocytes were detected throughout meiotic maturation (GV, MI, MII stages). This is different from other cytoplasmic abnormalities in human oocytes, such as smooth endoplasmic reticulum clusters which appear only in mature MII stage oocytes [27].
Since the age of patients did not correlate with the occurrence of lipofuscin bodies, the aging of oocytes during inactive phases of oogenesis may not be involved with lipofuscinogenesis. Instead, the accumulation of lipofuscin may occur during the growth phase of the oocytes when dominant follicles are being recruited into the preovulatory pathway. Also, the occurrence of the large lipofuscin bodies may be related to conditions of the developing ovarian follicles, such as perifollicular blood circulation and follicular fluid composition. Further studies will be needed to evaluate this proposal. Since the occurrence of refractile bodies tends to recur in particular patients [6], their formation in relation to special female traits also remains to be studied.
One possible explanation for the occurrence of lipofuscin in normal aging is related to oxidative stress as suggested by some studies [28, 29]. It has also been suggested that proteolytic degradation may be a factor that influences the genesis of lipofuscin during normal aging [30–32]. Another possibility for the occurrence of lipofuscin may be related to lipid metabolism as a source of energy supply. Potential interactions between adipokines and the hypothalamus, pituitary, ovary, oocyte and embryo, and the female reproductive tract such as insulin resistance have been suggested [33]. However, the relationship between lipid metabolism and lipofuscinogenesis has not been evaluated. Consequently, further research will need to determine whether oxidative stress, abnormal lipid metabolism or proteolytic degradation are involved in lipofuscinogenesis in human oocytes.
Acknowledgements
The authors would like to thank Dr. Alexander Lopata for reading and commenting on this manuscript and Mr. Yoshihito Kondo at Tokyo Instruments for technical support on spectral image analysis.
Footnotes
Refractile bodies in human oocytes were found to contain lipofuscin. The largest lipofuscin inclusions (>5 μm) were associated with significantly reduced fertilization and unfavorable blastocyst development.
Contributor Information
Junko Otsuki, Email: midori@nagai-cl.com.
Yasushi Nagai, Email: nagai@nagai-cl.com.
Kazuyoshi Chiba, Email: kchiba@cc.ocha.ac.jp.
References
- 1.Daya S, Gunby J, Casper R. Oocyte morphology as a predictor of pregnancy for intracytoplasmic sperm injection. The 51st annual Clinical Meeting, The socirty of obstetricians and Gynecologists of Canada 1995; Abstr 013-REI, p. 47.
- 2.Alikani M, Palermo G, Adler A, Bertoli M, Blake M, Cohen J. Intracytoplasmic sperm injection in dysmorphic human oocytes. Zygote. 1995;3:283–288. doi: 10.1017/s0967199400002707. [DOI] [PubMed] [Google Scholar]
- 3.Xia Intracytoplasmic sperm injection: correlation of oocyte grade based on polar body, perivitelline space and cytoplasmic inclusions with fertilization rate and embryo quality. Hum Reprod. 1997;12:1750–1755. doi: 10.1093/humrep/12.8.1750. [DOI] [PubMed] [Google Scholar]
- 4.Sutter P, Dozortsev D, Qian C, Dhont M. Oocyte morphology does not correlate with fertilization rate and embryo quality after intracytoplasmic sperm injection. Hum Reprod. 1996;11:595–597. doi: 10.1093/humrep/11.3.595. [DOI] [PubMed] [Google Scholar]
- 5.Balaban B, Urman B, Sertac A, Alatas C, Aksoy S, Mercan R. Oocyte morphology does not affect fertilization rate, embryo quality and implantation rate after intracytoplasmic sperm injection. Hum Reprod. 1998;13:3431–3433. doi: 10.1093/humrep/13.12.3431. [DOI] [PubMed] [Google Scholar]
- 6.Veeck LL. Atlas of the human oocyte and early conceptus. Baltimore: Williams & Wilkins; 1991. pp. 121–166. [Google Scholar]
- 7.Blerkom J. Occurrence and developmental consequences of aberrant cellular organization in meiotically mature human oocytes after exogenous ovarian hyperstimulation. J Electron Microsc Tech. 1990;16:324–346. doi: 10.1002/jemt.1060160405. [DOI] [PubMed] [Google Scholar]
- 8.Serhal PF, Ranieri DM, Kinis A, Marchant S, Davies M, Khadum IM. Oocyte morphology predicts outcome of intracytoplasmic sperm injection. Hum Reprod. 1997;12:1267–1270. doi: 10.1093/humrep/12.6.1267. [DOI] [PubMed] [Google Scholar]
- 9.Hannover A. Mikroskopiske undersögelser af nervesystemet. Kgl. Danske Vidensk. Kabernes Selskobs Naturv. Math. Afh. (Copenhagen) 1842;10:1–112. [Google Scholar]
- 10.Browne RM, Rippin JW. Autofluorescent granular cells in oral mucosal hyperplasias. Histopathology. 1977;1:375–384. doi: 10.1111/j.1365-2559.1977.tb01675.x. [DOI] [PubMed] [Google Scholar]
- 11.Collins VP, Brunk UT. Characterization of residual bodies formed in phase II cultivated human glia cells. Mech Ageing Dev. 1976;5:193–207. doi: 10.1016/0047-6374(76)90018-X. [DOI] [PubMed] [Google Scholar]
- 12.Koneff H. Beiträge zur Kenntniss der Nervenzeilen den peripheren Ganglien. Mitt Naturforsch Gesellsch (Bern) 1886;44:13–14. [Google Scholar]
- 13.Glees P, Hasan M. Lipofuscin in neuronal aging and diseases. Norm Pathol Anat. 1976;32:1–68. [PubMed] [Google Scholar]
- 14.Sohal RS, Donato H., Jr Effect of experimental prolongation of life span on lipofuscin content and lysosomal enzyme activity in the brain of the housefly, Musca domestica. J Gerontol. 1979;34:489–496. doi: 10.1093/geronj/34.4.489. [DOI] [PubMed] [Google Scholar]
- 15.Munnell JF, Getty R. Rate of accumulation of cardiac lipofuscin in the aging canine. J Gerontol. 1968;23:154–158. doi: 10.1093/geronj/23.2.154. [DOI] [PubMed] [Google Scholar]
- 16.Nakano M, Mizuno T, Gotoh S. Accumulation of cardiac lipofuscin in crab-eating monkeys (Macaca fasicularis): the same rate of lipofuscin accumulation in several species of primates. Mech Ageing Dev. 1993;66:243–248. doi: 10.1016/0047-6374(93)90011-F. [DOI] [PubMed] [Google Scholar]
- 17.Sohal RS, Brunk UT. Lipofuscin as an indicator of oxidative stress and aging. Adv Exp Med Biol. 1989;266:17–29. doi: 10.1007/978-1-4899-5339-1_2. [DOI] [PubMed] [Google Scholar]
- 18.Gao G, Ollinger K, Brunk UT. Influence of intracellular glutathione concentration of lipofuscin accumulation in cultured neonatal rat cardiac myocytes. Free Radic Biol Med. 1994;16:187–194. doi: 10.1016/0891-5849(94)90142-2. [DOI] [PubMed] [Google Scholar]
- 19.Marzabadi MR, Sohal RS, Brunk UT. Mechanisms of lipofuscinogenesis: effect of the inhibition of lysosomal proteinases and lipases under varying concentrations of ambient oxygen in cultured rat neonatal myocardial cells. APMIS. 1991;99:416–426. doi: 10.1111/j.1699-0463.1991.tb05170.x. [DOI] [PubMed] [Google Scholar]
- 20.Terman A. The effect of age on formation and elimination of autophagic vacuoles in mouse hepatocytes. Gerontology. 1995;41:319–326. doi: 10.1159/000213753. [DOI] [PubMed] [Google Scholar]
- 21.Pearse AG. Hystochemistry: theoretical and applied. London: Churchill; 1968. [Google Scholar]
- 22.Cornillie FJ, Lauweryns JM. Phagocytotic and iron-storing capacities of stromal cells in the rat endometrium. A histochemical and ultrastructural study. Cell Tissue Res. 1985;239:467–476. doi: 10.1007/BF00219224. [DOI] [PubMed] [Google Scholar]
- 23.Kuwamura M, Hattori R, Yamate J, Kotani T, Sasai K. Neuronal ceroid-lipofuscinosis and hydrocephalus in a chihuahua. J Small Anim Pract. 2003;44:227–230. doi: 10.1111/j.1748-5827.2003.tb00148.x. [DOI] [PubMed] [Google Scholar]
- 24.Huang SZ, Luo YJ, Wang L, Cai KY. Effect of ginkgo biloba extract on livers in aged rats. World J Gastroenterol. 2005;11:132–135. doi: 10.3748/wjg.v11.i1.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Porta EA. Pigments in aging: an overview. Ann N Y Acad Sci. 2002;959:57–65. doi: 10.1111/j.1749-6632.2002.tb02083.x. [DOI] [PubMed] [Google Scholar]
- 26.Matsuura-Tokita K, Takeuchi M, Ichihara A, Mikuriya K, Nakano A. Live imaging of yeast Golgi cisternal maturation. Nature. 2006;441:939–940. doi: 10.1038/nature04737. [DOI] [PubMed] [Google Scholar]
- 27.Otsuki J, Okada A, Morimoto K, Nagai Y, Kubo H. The relationship between pregnancy outcome and smooth endoplasmic reticulum clusters in MII human oocytes. Hum Reprod. 2004;19:1591–1597. doi: 10.1093/humrep/deh258. [DOI] [PubMed] [Google Scholar]
- 28.Gao G, Ollinger K, Brunk UT. Influence of intracellular glutathione concentration of lipofuscin accumulation in cultured neonatal rat cardiac myocytes. Free Radic Biol Med. 1994;16:187–194. doi: 10.1016/0891-5849(94)90142-2. [DOI] [PubMed] [Google Scholar]
- 29.Marzabadi MR, Sohal RS, Brunk UT. Mechanisms of lipofuscinogenesis: effect of the inhibition of lysosomal proteinases and lipases under varying concentrations of ambient oxygen in cultured rat neonatal myocardial cells. APMIS. 1991;99:416–426. doi: 10.1111/j.1699-0463.1991.tb05170.x. [DOI] [PubMed] [Google Scholar]
- 30.Ivy GO, Schottler F, Wenzel J, Baudry M, Lynch G. Inhibitors of lysosomal enzymes: accumulation of lipofuscin-like dense bodies in the brain. Science. 1984;226:985–987. doi: 10.1126/science.6505679. [DOI] [PubMed] [Google Scholar]
- 31.Ivy GO, Kanai S, Ohta M, Smith G, Sato Y, Kobayashi M, et al. Lipofuscin-like substances accumulate rapidly in brain, retina and internal organs with cysteine protease inhibition. Adv Exp Med Biol. 1989;266:31–45. doi: 10.1007/978-1-4899-5339-1_3. [DOI] [PubMed] [Google Scholar]
- 32.Ivy GO, Roopsingh R, Kanai S, Ohta M, Sato Y, Kitani K. Leupeptin causes an accumulation of lipofuscin-like substances and other signs of aging in kidneys of young rats: further evidence for the protease inhibitor model of aging. Ann N Y Acad Sci. 1996;786:12–23. doi: 10.1111/j.1749-6632.1996.tb39048.x. [DOI] [PubMed] [Google Scholar]
- 33.Mitchell M, Armstrong DT, Robker RL, Norman RJ. Adipokines: implications for female fertility and obesity. Reproduction. 2005;130:583–897. doi: 10.1530/rep.1.00521. [DOI] [PubMed] [Google Scholar]