Abstract
Background
Alpha and beta defensins have been isolated from various human tissues and form an important part of the innate immune system. Their role in implantation of the embryo has not yet been studied. This study was designed to detect both alpha and beta defensins in the mid luteal phase endometrium and investigate the correlation between the defensin expression and implantation of the embryo.
Method and results
An experimental study was designed to detect α defensin (HNP1–3) and β defensin (HBD1) in midluteal phase endometrial samples obtained from women attending the IVF unit at the Liverpool Women’s Hospital, UK. Samples were obtained at least two menstrual cycles before IVF treatment was commenced. Immunohistochemical staining was conducted to estimate defensin expression. Some endometrial stromal cells stained positive for HNP1–3 during the midluteal phase. HNP1–3 expression is significantly higher in cases presenting with female factor infertility as compared with purely male factor infertility. A significant increase was not observed in tubal factor or endometriosis when considered separately. Endometrial stromal neutrophils were shown to be the main source of endomatrial HNP1–3. HBD1 was the only beta defensin detected by immunochemical staining in the midluteal phase endometrium. The intensity of staining was significantly different in the endometrial stroma, luminal and glandular epithelia. HBD1 expression is not significantly higher in female factor infertility.
Conclusion
The study confirmed secretion of HNP1–3 by endometrial stromal neutrophils. Glandular epithelium is the main source of HBD1 expression in the human endometrium. HNP1–3 shows increased expression in female factor infertility. HBD1 expression is not higher in female factor infertility. These defensins do not appear to influence implantation.
Keywords: HNP1–3, HBD1, Defensin, Implantation, IVF outcome
Introduction
Defensins are a group of cationic antimicrobial peptides that have a broad spectrum of activity and are considered to be ancient elements of the immune response in all species of life [1, 2]. Two main classes of defensins have been described in humans, α and β. α-defensins have been isolated from the primary granules of rabbit and human neutrophils [3, 4] and are named Human Neutrophil Peptide (HNP) 1, 2, 3 and 4. α-defensin 5, also known as Human Defensin (HD 5), is produced at epithelial sites and is widespread in the female reproductive tract [5, 6]. Within the endometrium, maximal expression of HD 5 has been observed during the early-luteal phase [6].
Four β-defensins, Human Beta Defensins (HBD) 1, 2, 3 and 4 have so far been described [7, 8, 9, 10]. In addition to being secreted by a wide variety of epithelial tissues, expression of HBD1, 2, 3, 4 and 5 have been described in the endometrium [11, 12].
Using real-time quantitative polymerase chain reaction (PCR) studies, it has been shown that peak expression of endometrial β-defensin varies with different phases of the menstrual cycle [12, 13]. mRNA expression of HBD1 in the endometrium is maximum during the mid-luteal phase of the menstrual cycle while that of HBD2 occurs during the menstrual phase. Endometrial HBD3 expression shows two peaks, one during the early-luteal and the other in the late-luteal phases. There is very little expression of HBD3 in the endometrium during the mid-luteal phase. Maximal expression of endometrial HBD4 occurs during the proliferative phase of the menstrual cycle. HBD1 is the only epithelial defensin that shows peak expression during the mid-luteal phase of the menstrual cycle, the time of the cycle when implantation of the embryo occurs.
Implantation of the embryo involves an interaction between the blastocyst and maternal endometrium. The time in which this interaction is optimal and allows adhesion and invasion of the blastocyst is generally termed the “implantation window” occurring between day 19 and day 23 of a normal 28-day menstrual cycle [14]. This corresponds to the mid-luteal phase of the menstrual cycle when progesterone production peaks inducing the appearance of pinopodes in the luminal epithelial cells of the endometrium which are a morphological marker for endometrial receptivity [15]. These progesterone mediated changes may have a beneficial effect on embryo development and implantation.
Luteal phase progesterone has also been shown to influence HBD1 expression in the endometrium and, unlike other defensins, HBD1 production is resistant to exposure to inflammatory molecules [16–18]. The addition of anti-progesterone (RU486) to secretory phase explants has been shown to negate the effects of progesterone on the expression of HBD1 [13]. These observations suggest that HBD1 may have a role in implantation of the embryo.
Leucocyte populations within the endometrium constitute about 8% of the stroma during the proliferative phase of the menstrual cycle increasing to 25% in the late secretory phase [19]. These leucocytes (uterine natural killer cells [uNK cells], macrophages, neutrophils and mast cells) have an established role in endometrial anti-microbial activity [20]. It is not known whether endometrial neutrophils, like blood neutrophils, also produce HNP1–3 and whether these neutrophils, like other endometrial leucocytes, play any role in implantation [1, 21]. Agerbeth et al. have reported the expression of HNP1–3 by the NK cells in the peripheral circulation [22]. It is possible therefore, that the endometrial uNK cells also produce HNP1–3.
This study was designed to investigate the expression of HNP1–3 and the secretion of HBD1, 2, 3, 4 and HD5 in endometrium during the implantation window. It also aimed to study the correlation of HNP1–3 with endometrial leucocytes—uNK cells and neutrophils in women undergoing In-vitro fertilisation (IVF) and Intra Cytoplasmic Sperm Injection (ICSI) treatment and to investigate any difference in defensin expression in women with tubal factor or endometriosis as compared with other causes of infertility. Correlation of defensin expression in women who did or did not become pregnancy from subsequent IVF/ICSI treatment was also investigated.
Materials and methods
The Liverpool Research Ethics Committee granted permission for the project. Women attending the Reproductive Medicine Unit at Liverpool Women’s Hospital for IVF and/or ICSI treatment were asked to participate in the study. An information sheet was given to all women, and informed consent was obtained from those who agreed to participate.
Tissue collection
Twenty-three women were recruited for the study. Women were supplied with a ‘Clear Blue’ ovulation detection kit (Unipath, Bedford, UK) to detect the LH surge. An endometrial biopsy was performed 7–9 days following the surge of Lutenising Hormone (LH) using an endometrial sampler (Wallace Ltd., Hythe, Kent, UK) which corresponded to day 21–23 (implantation window) of the menstrual cycle [14]. All women reported a regular menstrual cycle (25–35 days) and had not received any form of hormonal treatment in the 3 months preceding the biopsy. Following the biopsy, women did not commence IVF treatment for at least two menstrual cycles. Tissue samples were frozen using liquid nitrogen within 20 min of collection and stored at −70°C until processed for immunostaining.
For their subsequent IVF treatment, all women followed the standard long protocol used in the unit; downregulation was achieved with the long downregulation protocol using GnRH-analogue nasal spray (Buserelin; Aventis Pharma; 200 μg twice daily). Stimulation was then commenced using FSH (Menopur, Ferring), the initial dose being dependent on the baseline FSH. When at least three follicles had reached a diameter of >16 mm, human chorionic gonadotropin (hCG; Profasi; Serono; 5000 IU) was given, and oocyte retrieval was performed 36 h later by ultrasound-guided follicle aspiration.
A maximum of two embryos were transferred on the 2nd day of culture and surplus transferable embryos were cryopreserved. The luteal phase was supported by daily rectal or vaginal administration of progestogen pessary (Cyclogest; Shire; Andover, Hants, UK; 200 mg twice daily) beginning the day before embryo transfer and continuing until the day of the pregnancy test. In cases of a positive pregnancy test, an ultrasound scan was arranged to confirm a clinical pregnancy.
Immunohistochemistry
Five micrometer frozen sections were cut using a cryostat and the sections stained using the alkaline-phosphatase anti-(alkaline phosphatase) (APAAP) technique [20]. Sections were fixed for 10 min in acetone and, after washing with Tris-buffered saline (TBS 0.05 mol/l, pH 7.6) were incubated with the appropriate diluted monoclonal antibody for 30 min. The antibodies used are shown in Table 1. Mouse IgG was used in place of the first antibody as a negative control. After two 5 min washes in TBS, bound antibody was detected by the APAAP method using rabbit anti-mouse IgG (diluted 1:25; DAKO Ltd; High Wycombe, Bucks, UK) for a further 30 min, washed in TBS, and then incubated with preformed complex of calf intestinal alkaline phosphate and mouse anti-alkaline phosphatase (APAAP, diluted 1:50; DAKO) for 30 min. Staining was developed with Naphthol AS-MX phosphate and Fast Red (Sigma, Poole, Dorset, UK) with the inclusion of 1 mmol/l levamisole to block any endogenous alkaline phosphatase. Slides were counterstained with haemulum and mounted in Aquamount (BDH, Poole, Dorset, UK).
Table 1.
Antibodies used for immunohistochemical staining
| Antibodies used | Dilution | Manufacturer |
|---|---|---|
| HNP1–3 | 1:3,000 | Serotec, Oxford, UK |
| HBD-1 | 1:100 | Abcam, Cambridge, UK |
| HBD-2 | 1:50 | Abcam |
| HBD-3 | 1:50 | Abcam |
| HBD-4 | 1:50 | Abcam |
| HD-5 | 1:50 | Abcam |
| CD16 | 1:100 | Pharmingen, Oxford, UK |
| CD56 | 1:100 | Serotec |
| IgG | 1:100 | Serotec |
Preliminary experiments were performed to obtain the correct dilution for optimal staining. The manufacturer’s specification for HNP1–3 antibody (this is the only commercial combined antibody to detect HNP1, 2 and 3), suggested a dilution of 1:100. However, this dilution produced intense staining and further dilutions were used. A dilution of 1:3,000 produced optimal positive staining without any background staining.
The staining of the endometrium produced positive stained individual cells (Fig. 1). The number of stromal cells staining positive for HNP1–3 were counted and expressed as a percentage of the total number of stromal cells per field in ten random fields at ×400 magnification. The average percentage of positive cells in all the fields was taken as the final value. Neutrophils (CD16) and uNK cells (CD56) cells were also counted using the same method.
Fig. 1.
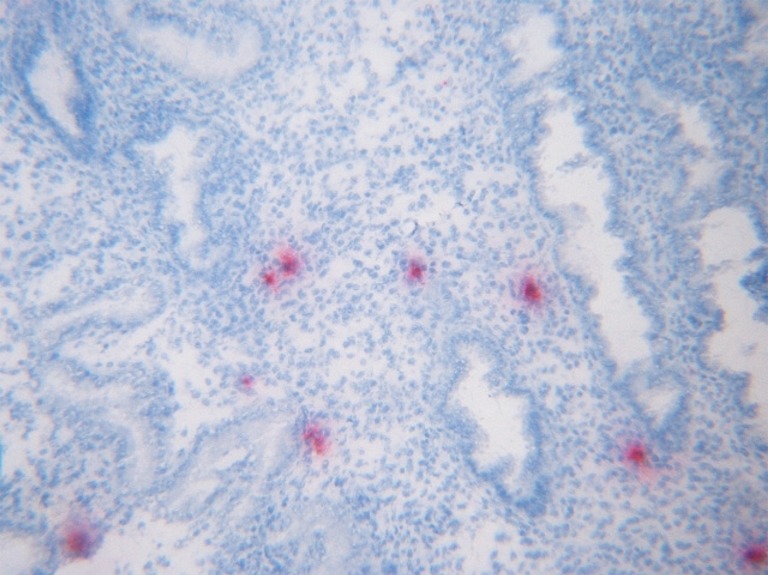
Immunostaining of midluteal endometrium phase with antibodies against HNP1–3. Stromal cells staining positive for HNP1–3 ×400
Unlike HNP1–3, the staining for HBD1 antibody produced diffuse staining of the endometrial glands, luminal epithelium and to some extent, the stroma (Fig. 3). The analysis of this staining was scored using the following system: 0, negative staining; +, minimal intensity of staining; ++, moderate intensity; and +++, very intense staining of the tissue. This method was adapted from the H-score system which could not be used as HBD1 is a secretory product and the staining was not restricted to specific granules within the cells. The intensity was assessed separately in the stroma, luminal and glandular epithelium in ten fields at ×200 magnification.
Fig. 3.
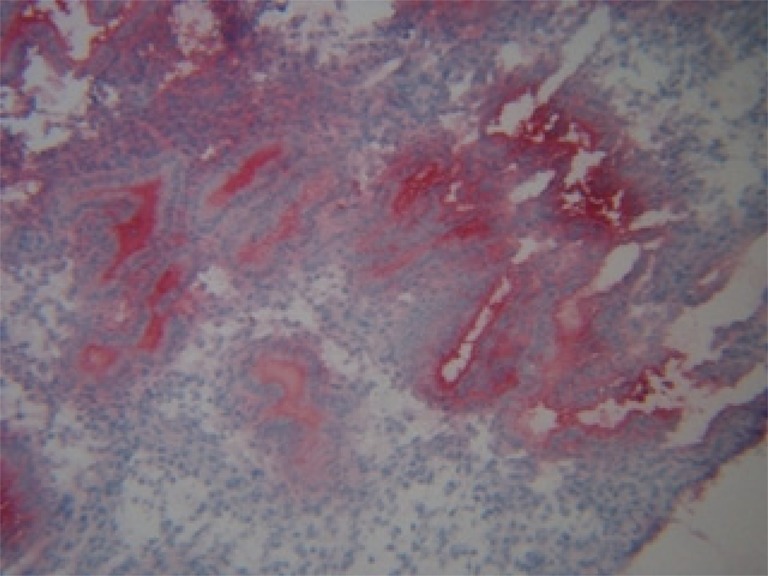
Immunostaining of midluteal endometrium phase with antibodies against HBD1. Positive staining of HBD1. Intense staining of glandular epithelium and secretion (+++), intermediate staining of luminal epithelium (++) and minimal staining of stroma (+, 0) ×200
Statistical analysis
Statistical analysis was performed using the Stats Direct software package, Stats Direct Limited, version 2.3.3. The Shapiro–Wilkes test was used to confirm parametric or non-parametric distribution of data. Parametric data was described as mean (standard deviation [SD]) and nonparametric data as median (interquartile range [IQR]). The unpaired Student’s t-test was used to compare the means and Mann–Whitney U test for medians. Spearman’s correlation test was used to assess relationship between two variables for non-parametric data.
Results
Twenty-three patients were recruited over a 6-month period between January 2004 and July 2004. Fifty-two percent of couples underwent IVF and 48% underwent ICSI treatment. The mean duration of infertility was 4 years. Male factor contributed 43% of cases, tubal factor 39%, endometriosis 17%, and unexplained infertility 9%. Some couples had more than one factor contributing to infertility. There was no difference in the age of the groups of women who did or did not become pregnant following subsequent treatment (Table 2).
Table 2.
Demographic characteristics of the study groups
| Became pregnant (n = 7) | Did not become pregnant (n = 16) | |
|---|---|---|
| Age (years) | 33 (3.7)* | 35 (3.7)* |
| Duration of infertility (years) | 3 (2.0)* | 5 (2.5)* |
| IVF | 4 (57) | 8 (50) |
| ICSI | 3 (43) | 8 (50) |
| Type of infertility | ||
| Primary | 6 (83) | 14 (88) |
| Secondary | 1 (17) | 2 (12) |
Values are mean (SD)* for age and duration of infertility, and n (%) for IVF, ICSI and type of infertility.
The cells staining positive for HNP1–3 were restricted entirely to the endometrial stroma (Fig. 1). The cells staining positive for CD16 were also present in the endometrial stroma (Fig. 2). Staining for HBD1 was most intense in the glandular epithelium and the lumen as expected, HBD1 being a secretory product. The luminal epithelium showed an intermediate intensity of staining and the stroma staining for HBD1 was minimal (Fig. 3). Immunochemical staining for HBD1 at dilution of 1:100 produced optimal staining of the mid-luteal phase endometrial samples. Analysing all tissue samples, HBD1 expression showed significant differences in the intensity of staining in the glandular epithelium, luminal epithelium and stroma (Table 3). Comparison of HBD1 expression in couples with purely male factor infertility with those presenting with female factor infertility showed no significant difference in expression in either group.
Fig. 2.
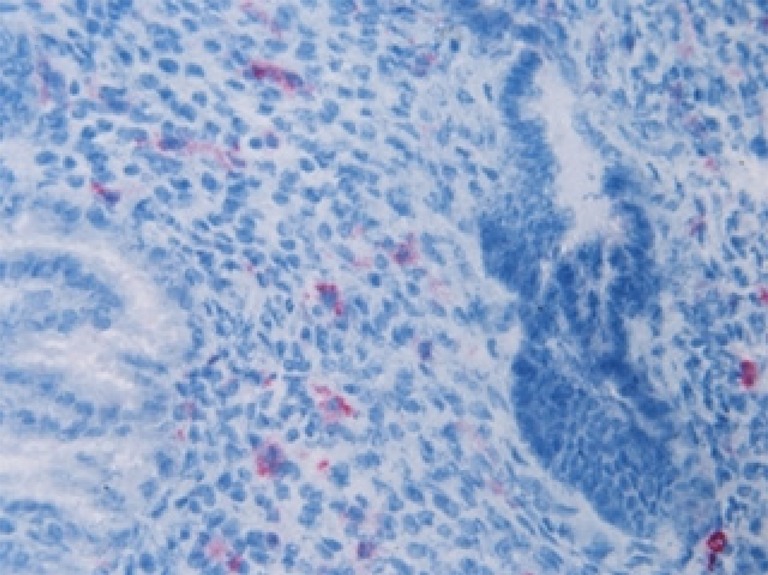
Immunostaining of midluteal endometrium phase with antibodies against CD16. Cells staining positive with CD16 antibody ×400
Table 3.
Intensity of staining for HBD1 in the glandular, luminal epithelium and stroma of the endometrium
| Intensity of staining (n) | Median (range) | P-value | |
|---|---|---|---|
| HBD1(glandular secretion and epithelium) | +++ (13) | +++ (+ to +++) | 0.005 |
| ++ (9) | |||
| + (1) | |||
| 0 (0) | |||
| HBD1(luminal epithelium) | +++ (2) | ++ (+ to +++) | 0.0004 |
| ++ (12) | |||
| + (9) | |||
| 0 (0) | |||
| HBD1 (stroma) | +++ (0) | + (0 to ++) | <0.0001 |
| ++ (3) | |||
| + (11) | |||
| 0 (9) |
P-values compare the difference in the intensity of staining between glandular and luminal epithelium; luminal epithelium and stroma; and between glandular epithelium and stroma.
Positive staining was not demonstrated for HBD2, 3, 4 and HD5 in the midluteal phase endometrium. The commercial antibodies for HBD3 and HD5 tested positive on early secretory phase (day 14) endometrium. HBD4 antibody tested positive on peripheral blood spot. HBD4 antibody could not be tested as the required positive control-pancreatic tissue was not available for research purposes. Figure 4 shows the IgG staining of the luteal phase endometrium as control.
Fig. 4.
Immunostaining of midluteal endometrium phase with antibodies against IgG as control ×400
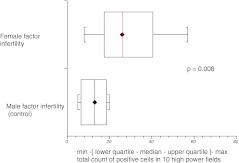
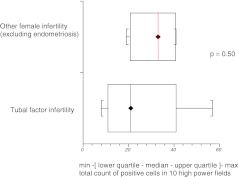
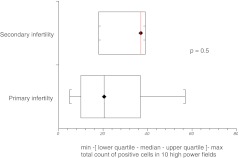
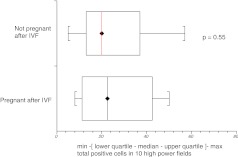
Subgroup analysis based on cause and type of infertility showed that median expression of HNP1–3 was significantly higher in couples with female factor infertility as compared with those presenting with purely male factor infertility (p = 0.008) (Fig. 5). Although tubal factor infertility and endometriosis are generally associated with prominence of inflammatory markers in the pelvis, our results did not show significantly higher HNP1–3 expression in these groups when considered separately. There was no difference in expression of HNP1–3 in women who did or did not become pregnant following subsequent IVF treatment (Figs. 6, 7 and 8).
Fig. 5.
HNP1–3 expression in male and female factor infertility
Fig. 6.
HNP1–3 expression in women with tubal factor infertility as compared with other causes of female infertility (excluding endometriosis)
Fig. 7.
HNP1–3 expression in women with primary and secondary infertility
Fig. 8.
HNP1–3 expression in women who did not become pregnant after IVF treatment
Since both CD16 and CD56 cells are known to produce HNP1–3, counting was performed to establish which was the main cell type secreting HNP1–3. The number of CD16 neutrophils was assessed in all samples. Spearman’s correlation test showed a significant correlation of HNP1–3 positive cells and CD16 cells (r = 0.83). After an initial count of ten slides, Spearman’s correlation test showed no correlation between HNP1–3 and CD56 counts with (r = −0.4).
Discussion
This is the first study to demonstrate the expression of HNP1–3 in the endometrium during the implantation window. This is also the first study to use immunohistochemical techniques to detect β-defensin protein in the endometrium.
Our study showed a significantly higher expression of HNP1–3 in women with female factor infertility as compared with those who presented with male factor infertility. However, women with tubal factor infertility and those with endometriosis did not show significantly higher expression of HNP1–3 as compared women with anovulation. There was no difference in the expression of HNP1–3 irrespective of whether or not the woman achieved a pregnancy following assisted reproduction. This suggests that although there is a trend for presence of higher numbers of inflammatory cells in the endometrium in of female factor infertility, it does not appear to affect future potential for successful implantation of the embryo. Our numbers are small and this difference would need to be substantiated with larger control studies. The endometrial samples were collected at least two cycles before the treatment cycle and therefore may not directly reflect the immunological status of the treatment cycle accurately. This was unavoidable as it would be unethical to sample the luteal phase endometrium during the treatment cycle and at least two menstrual cycles would be necessary to allow any possible reactive changes in the defensin concentration to return to baseline levels prior to treatment.
Correlation tests following the counts of CD16, CD56 and HNP1–3 suggest the stromal neutrophils to be the chief source of HNP1–3 as opposed to the uNK cells. However, this positive statistical correlation may have occurred due to low counts of both CD16 cells and HNP1–3 positive cells. Agerbeth et al, have reported the expression of HNP1–3 by the NK cells in the peripheral circulation [23]. It is possible that uNK cells also produce HNP1–3. Confocal microscopy would confirm the lack of secretion of HNP1–3 by these cells.
HBD1 was the only epithelial defensin detected by immunochemical staining during the mid-luteal phase of the menstrual cycle. Previous studies conducted on endometrial tissue to establish the presence of epithelial defensins HBD1, 2, 3 and 4 have used polymerase chain reaction (PCR) and related techniques to confirm the gene expression and detection of mRNA of HBD1, 2, 3 and 4 [12, 13]. HBD1 is an epithelial defensin and in accordance, its expression was prominent in the glandular and luminal epithelium with only a minimal presence in the stroma. Staining in the glandular epithelium was significantly higher than that in the luminal epithelium and the stroma suggesting the glandular secretions that bathe the endometrial surface to be the main source of HBD1 during the mid-luteal phase of the menstrual cycle. Unlike HNP1–3, expression of HBD1 was not significantly higher in female factor infertility group which is explained by its constitutive expression in the endometrium.
PCR studies have demonstrated peak expression of mRNA of the endometrial HBD1, 2, 3, 4 and HD5 at different phases of the menstrual cycle with lower levels detected during other phases of the cycle [6, 12]. HBD1 mRNA expression is maximum during the mid-luteal phase while expression of HBD2, 3 and 4 mRNA is detected at low levels. Demonstration of only HBD1 in our study may mean that defensin secretion in the endometrium only occurs when the gene expression and mRNA for that particular defensin is at its peak, in this case HBD1. Regulation of defensin secretion in the endometrium may take place at the mRNA level. This could explain the complete absence of staining for HBD2, 3, 4 and HD5 in the mid-luteal endometrium.
Studies on animal and more recently human tissues have established that the defensins play a key role in maintaining the immunity of the body [4, 21, 24]. HNP1–3 has been shown to be active against a wide spectrum of Gram-positive and Gram-negative organisms in the peripheral circulation while the β-defensins are the first line of defence at the epithelial sites and increase several-fold on exposure to infectious agents. Women in preterm labour secondary to infection have been shown to have high concentrations of α-defensins HNP1–3 in the amniotic fluid [25]. The main role of defensins in the endometrium appears to be mainly antimicrobial, limiting the spread of infection and maintaining local defences rather than playing a direct role in implantation.
References
- 1.Ganz T, Selsted ME, Szklarek D, Harwig SS, Daher K, Bainton DF, et al. Defensins. Natural peptide antibiotics of human neutrophils. J Clin Invest. 1985;76:1427–35. doi: 10.1172/JCI112120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoffman JA, Kafatos FC, Janeway CA, Ezekowitz RA. Phylogenetic perspectives in innate immunity. Science. 1999;284:1313–8. doi: 10.1126/science.284.5418.1313. [DOI] [PubMed] [Google Scholar]
- 3.Schroder JM, Harder J. Human beta-defensin 2. Int J Biochem Cell Biol. 1999;31:645–51. doi: 10.1016/S1357-2725(99)00013-8. [DOI] [PubMed] [Google Scholar]
- 4.Lehrer A, Lichtenstein A, Ganz T. Defensins: antimicrobial and cytotoxic peptides of mammalian cells. Anuual Rev Immunol. 1993;11:105–28. doi: 10.1146/annurev.iy.11.040193.000541. [DOI] [PubMed] [Google Scholar]
- 5.Svinarich DM, Wolf NA, Gomez R, Gonik B, Romero R. Detection of human defensin 5 in reproductive tissues. Am J Obstet Gynaecol. 1997;176:470–5. doi: 10.1016/S0002-9378(97)70517-9. [DOI] [PubMed] [Google Scholar]
- 6.Quayle AJ, Porter EM, Nussbaum AA. Gene expression, immunolocalisation and secretion of human defensin-5 in human female reproductive tract. Am J Path. 1998;152(5):1247–58. [PMC free article] [PubMed] [Google Scholar]
- 7.Bensch KW, Raida M, Magert HJ, Schultz-Knappe P, Forssmann WG. HBD 1: a novel beta-defensin from human plasma. FEBS Lett. 1995;368:331–5. doi: 10.1016/0014-5793(95)00687-5. [DOI] [PubMed] [Google Scholar]
- 8.Bals R, Wang X, Wu Z, Freeman T, Bafna V, Zasloff M, et al. HBD 2 is a salt sensitive peptide antibiotic expressed in human lung. J Clin Invest. 1998;102:874–80. doi: 10.1172/JCI2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harder J, Bartels J, Christophers E, Schroder JM. Isolation and characterisation of human beta-defensin 3, a novel human inducible peptide abtibiotic. J Biol Chem. 2001;275:5707–13. doi: 10.1074/jbc.M008557200. [DOI] [PubMed] [Google Scholar]
- 10.Garcia JR, Krause A, Schulz S, Rodriguez-Jimenez J, Kluver E, Adermann K, et al. Human beta-defensin 4: a novel human inducible peptide with a specific salt-sensitive spectrum of antimicrobial activity. Faseb J. 2001;15:1819–21. [PubMed] [Google Scholar]
- 11.King AE, Fleming DC, Critchley HOD, Kelly RW. Regulation of natural antibiotic expression by inflammatory mediators and mimics of infection in human endometrial epithelial cells. Mol Hum Reprod. 2002;8:341–9. doi: 10.1093/molehr/8.4.341. [DOI] [PubMed] [Google Scholar]
- 12.King AE, Fleming DC, Critchley HOD, Kelly RW. Differential expression of natural antimicrobials, beta-defensins 3 and 4, in human endometrium. J Reprod Immunol. 2003;59:1–16. doi: 10.1016/S0165-0378(02)00083-9. [DOI] [PubMed] [Google Scholar]
- 13.Fleming DC, King AE, Williams ARW, Critchley HOD, Kelly RW. Hormonal contraception can suppress natural antimicrobial gene transcription in human endometrium. Fertil Steril. 2003;79(4):856–63. doi: 10.1016/S0015-0282(02)04930-0. [DOI] [PubMed] [Google Scholar]
- 14.Bergh PA, Navot D. the impact of embryonic development and endometrial maturity on the timing of implantation. Fertil Steril. 1992;58:537–42. doi: 10.1016/s0015-0282(16)55259-5. [DOI] [PubMed] [Google Scholar]
- 15.Murphy CR. The cytoskeleton of uterine epithelial cells: a new player in uterine receptivity and plasma membrane transformation. Hum Reprod Update. 1995;6:567–80. doi: 10.1093/humupd/1.6.567. [DOI] [PubMed] [Google Scholar]
- 16.Krisanaprakornit S, Weinberg A, Perez CN, Dale BA. Expression of the peptide antibiotic human beta-defensin 1 in cultured gingival epithelial cells and gingival tissue. Infect Immun. 1998;66:4222–8. doi: 10.1128/iai.66.9.4222-4228.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao C, Wang I, Lehrer RI. Widespread expression of beta-defensin HBD-1 in human secretory glands and epithelial cells. FEBS Lett. 1996;396:319–22. doi: 10.1016/0014-5793(96)01123-4. [DOI] [PubMed] [Google Scholar]
- 18.O’Neil DA, Porter E, Elewaut D, Anderson GM, Eckmann L, Ganz T, Kagnoff MF. Expression and regulation of defensins HBD-1 and HBD-2 in intestinal epithelium. J Immunol. 1999;163:6718–24. [PubMed] [Google Scholar]
- 19.Bulmer JN, Longfellow M, Ritson A. Leucocytes and resident blood cells in endometrium. Ann N Y Acad Sci. 1991;622:57–68. doi: 10.1111/j.1749-6632.1991.tb37850.x. [DOI] [PubMed] [Google Scholar]
- 20.King A, Loke YW. On the nature and function of human granular lymphocytes. Immunol Today. 1995;12:432–5. doi: 10.1016/0167-5699(91)90014-K. [DOI] [PubMed] [Google Scholar]
- 21.King AE, Critchley HOD, Kelly RW. Innate immune defences in the human endometrium. Reprod Biol and Endocrinol. 2003;1:116. doi: 10.1186/1477-7827-1-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Agerbeth B, Charo J, Werr J, et al. The human antimicrobial and chemotactic peptides LL-37 and α-defensins are expressed by specific lmphocyte and monocyte populations. Blood. 2000;96:3086–93. [PubMed] [Google Scholar]
- 23.Mason DY. Immunocytochemical labelling of monoclonal antibodies by the APAAP immunoalkaline phopsphatase technique. In: Bullock GR, Petrusz P, editors. New York: Academic Press; 1985. pp. 25–42.
- 24.Ryley HC. Human antimicrobial peptides. Rev Med Microbiol. 2001;12(3):177–88. [Google Scholar]
- 25.Heine RP, Weisenfeld H, Mortimer L, Greig PC. Amniotic fluid defensins: potential markers of subclinical intrauterine infection. Clin Infect Dis. 1998;27:513–8. doi: 10.1086/514691. [DOI] [PubMed] [Google Scholar]