Abstract
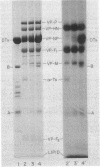
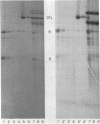
Diphtheria toxin ( DTx ) is an extremely potent inhibitor of protein synthesis. It is secreted as a linear polypeptide, which is cleaved to produce disulfide-linked A and B fragments. Fragment A, the inhibitor of protein synthesis, requires fragment B, the recognition subunit, for entry into intact cells. Fragment B has been proposed to form a transmembrane channel through which A gains access to the cytosol. If it were demonstrated that the B subunit had an exclusive association with membrane lipid acyl chains, this might indicate that A is secluded in a proteinaceous B channel. However, our results from intramembranous photolabeling studies show that both subunits of DTx enter the hydrocarbon domain of the bilayer. Toxin cleavage is not required for penetration. Decreasing pH leads to increased binding and hence indirectly to increased penetration. Parallel permeability studies indicate that cleaved DTx does indeed form pores (24 A in diameter) and they are larger than those previously reported (5 A) with native toxin. The data suggest that these are dimeric structures. Cleaved DTx is much more effective than intact DTx at pore formation. Thus, we conclude that, while pore formation is a feature of toxin-membrane interaction, the pore structure does not protect A from contact with lipid side chains and may in fact consist of both the A and B domains in a dimeric configuration, (AB)2.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alving C. R., Iglewski B. H., Urban K. A., Moss J., Richards R. L., Sadoff J. C. Binding of diphtheria toxin to phospholipids in liposomes. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1986–1990. doi: 10.1073/pnas.77.4.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boquet P., Silverman M. S., Pappenheimer A. M., Jr, Vernon W. B. Binding of triton X-100 to diphtheria toxin, crossreacting material 45, and their fragments. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4449–4453. doi: 10.1073/pnas.73.12.4449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramhall J. S., Shiflett M. A., Wisnieski B. J. Mapping the membrane proteins of Newcastle-disease virus with a photoreactive glycolipid probe. Biochem J. 1979 Feb 1;177(2):765–768. doi: 10.1042/bj1770765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramhall J., Ishida B., Wisnieski B. Photolabile and paramagnetic reagents for the investigation of transmembrane signaling events. J Supramol Struct. 1978;9(3):399–406. doi: 10.1002/jss.400090310. [DOI] [PubMed] [Google Scholar]
- Donovan J. J., Simon M. I., Draper R. K., Montal M. Diphtheria toxin forms transmembrane channels in planar lipid bilayers. Proc Natl Acad Sci U S A. 1981 Jan;78(1):172–176. doi: 10.1073/pnas.78.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draper R. K., Simon M. I. The entry of diphtheria toxin into the mammalian cell cytoplasm: evidence for lysosomal involvement. J Cell Biol. 1980 Dec;87(3 Pt 1):849–854. doi: 10.1083/jcb.87.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer J. D., Bloomfield V. A. Subunit arrangement of cholera toxin in solution and bound to receptor-containing model membranes. Biochemistry. 1982 Jun 22;21(13):3227–3231. doi: 10.1021/bi00256a030. [DOI] [PubMed] [Google Scholar]
- Hu V. W., Esser A. F., Podack E. R., Wisnieski B. J. The membrane attack mechanism of complement: photolabeling reveals insertion of terminal proteins into target membrane. J Immunol. 1981 Jul;127(1):380–386. [PubMed] [Google Scholar]
- Hu V. W., Wisnieski B. J. Photoreactive labeling of M13 coat protein in model membranes by use of a glycolipid probe. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5460–5464. doi: 10.1073/pnas.76.11.5460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida B., Cawley D. B., Reue K., Wisnieski B. J. Lipid-protein interactions during ricin toxin insertion into membranes. Evidence for A and B chain penetration. J Biol Chem. 1983 May 10;258(9):5933–5937. [PubMed] [Google Scholar]
- Kagan B. L., Finkelstein A., Colombini M. Diphtheria toxin fragment forms large pores in phospholipid bilayer membranes. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4950–4954. doi: 10.1073/pnas.78.8.4950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leppla S., Dorland R. B., Middlebrook J. L. Inhibition of diphtheria toxin degradation and cytotoxic action by chloroquine. J Biol Chem. 1980 Mar 25;255(6):2247–2250. [PubMed] [Google Scholar]
- Luckey M., Nikaido H. Specificity of diffusion channels produced by lambda phage receptor protein of Escherichia coli. Proc Natl Acad Sci U S A. 1980 Jan;77(1):167–171. doi: 10.1073/pnas.77.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H., Rosenberg E. Y. Effect on solute size on diffusion rates through the transmembrane pores of the outer membrane of Escherichia coli. J Gen Physiol. 1981 Feb;77(2):121–135. doi: 10.1085/jgp.77.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Proia R. L., Hart D. A., Holmes R. K., Holmes K. V., Eidels L. Immunoprecipitation and partial characterization of diphtheria toxin-binding glycoproteins from surface of guinea pig cells. Proc Natl Acad Sci U S A. 1979 Feb;76(2):685–689. doi: 10.1073/pnas.76.2.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RENKIN E. M. Filtration, diffusion, and molecular sieving through porous cellulose membranes. J Gen Physiol. 1954 Nov 20;38(2):225–243. [PMC free article] [PubMed] [Google Scholar]
- Sandvig K., Olsnes S. Diphtheria toxin entry into cells is facilitated by low pH. J Cell Biol. 1980 Dec;87(3 Pt 1):828–832. doi: 10.1083/jcb.87.3.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandvig K., Olsnes S. Entry of the toxic proteins abrin, modeccin, ricin, and diphtheria toxin into cells. II. Effect of pH, metabolic inhibitors, and ionophores and evidence for toxin penetration from endocytotic vesicles. J Biol Chem. 1982 Jul 10;257(13):7504–7513. [PubMed] [Google Scholar]
- Szoka F., Jr, Papahadjopoulos D. Procedure for preparation of liposomes with large internal aqueous space and high capture by reverse-phase evaporation. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4194–4198. doi: 10.1073/pnas.75.9.4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi M., Montecucco C. Lipid insertion of cholera toxin after binding to GM1-containing liposomes. J Biol Chem. 1981 Nov 10;256(21):11177–11181. [PubMed] [Google Scholar]
- Uchida T., Gill D. M., Pappenheimer A. M., Jr Mutation in the structural gene for diphtheria toxin carried by temperate phage . Nat New Biol. 1971 Sep 1;233(35):8–11. doi: 10.1038/newbio233008a0. [DOI] [PubMed] [Google Scholar]
- Wisnieski B. J., Bramhall J. S. Labeling of the active subunit of cholera toxin from within the membrane bilayer. Biochem Biophys Res Commun. 1979 Mar 15;87(1):308–313. doi: 10.1016/0006-291x(79)91680-2. [DOI] [PubMed] [Google Scholar]
- Wisnieski B. J., Bramhall J. S. Photolabelling of cholera toxin subunits during membrane penetration. Nature. 1981 Jan 22;289(5795):319–321. doi: 10.1038/289319a0. [DOI] [PubMed] [Google Scholar]
- Yoshimura F., Zalman L. S., Nikaido H. Purification and properties of Pseudomonas aeruginosa porin. J Biol Chem. 1983 Feb 25;258(4):2308–2314. [PubMed] [Google Scholar]