Abstract
Purpose: Intraovarian arterial blood flows before and after follicular rupture in ovulation induced cycles were examined by transvaginal color flow Doppler imaging. The changes observed in the intraovarian arterial resistance before and after ovulation in relation to the regularity of menstruation and several other parameters were analyzed.
Methods: In a prospective study, 22 patients undergoing infertility treatment in our center were recruited. Patients were divided into two subgroups, according to their menstrual regularity (regular menstrual group and oligomenorrheal group) and 42 cycles were studied. All patients received the same FSH low-dose stimulation treatment. The relationship between the post and preovulatory arterial pulsatility indexes (PI) was analyzed. Cycles that showed a decrease in their PI after ovulation of 10% or more were considered “profoundly decreased cycles.” Cycles from the same patients without ovulatory stimulation (natural cycles) were used as control.
Results: In the regular menstrual group the rate of profoundly decreased cycles during FSH stimulation was 63.2%, which was similar to the rate observed in natural cycles. In the oligomenorrheal group the rate of profoundly decreased cycles during natural cycles was 14.3%, but in FSH ovulation induction cycles this rate was significantly increased (47.6%, p < 0.05). In addition, the pregnancy rate per cycle in the oligomenorrheal group was significantly higher than that in the regular menstrual group (p = 0.03).
Conclusions: Oligomenorrheal patients presented a higher rate in the decrease of their PI values after FSH stimulation, which is essential to achieve pregnancy. This elevated rate of reduction implies that oligomenorrheal patients have increased incidence of disturbance in their ovulatory process when compared to normal cycling patients. Thus, ovulation induction with FSH, in oligomenorrheal patients resulted in a higher pregnancy rate in this group.
KEY WORDS: Low-dose FSH step-up protocol, oligomenorrhea, ovulation induction, pregnancy.
INTRODUCTION
A variety of approaches are now available for the restoration of ovulation in infertile women with irregular menses, oligomenorrhea, or amenorrhea. For infertile women with ovulatory dysfunction, ovulation induction plays an important role in achieving pregnancy, and it is now a conventional treatment for these patients, worldwide. In contrast to its benefits, the induction of ovulation in women with ovulatory dysfunction is currently associated with a high incidence of ovarian hyperstimulation syndrome (OHSS) and multiple pregnancies (1). Therefore, the administration of low doses of FSH in a stepwise fashion (step-up, step-down protocol or a combination of both step-up and step-down protocols) has proven to be effective for significantly reducing these risks.
Recently, we reported the results of a study using transvaginal color flow Doppler imaging to measure the intraovarian arterial blood flow resistance, before and after follicular rupture in infertile women, during natural cycles (2). Doppler imaging is one of the best available methods for evaluating the follicular environment during ovulation and has the advantage of being a noninvasive procedure. We observed that a reduction in intraovarian blood vessel resistance was necessary to achieve pregnancy. Some investigators have reported that the intraovarian arterial blood flow resistance decreases after follicular rupture (3,4). However, in our study half of the infertile women with regular menstrual cycles showed increased, not decreased pulsatility index (PI) values after ovulation. Furthermore, only a small number of pregnancies were established in these women, who showed an increase in PI values after ovulation (2). This suggests that only half of infertile patients with regular menstrual cycles acquired a suitable follicular environment for ovulation and pregnancy.
Welner et al. examined the effects of human menopausal gonadotropin treatment on the pregnancy rate of patients with long-standing infertility of unknown etiology. They found that the rates of conception and birth resulting from human menopausal gonadotropin therapy were significantly higher than those without it (5). Thus, ovulation induction seems to improve the unfavorable follicular environment in the natural cycles of patients whose infertility stems from unknown causes.
In the present study, we examined the intraovarian arterial blood flow before and after follicular rupture using transvaginal color flow Doppler imaging in ovulation inducted cycles of patients having regular or irregular menstrual cycles. We observed the changes in the rates of intraovarian arterial resistance before and after ovulation in relation to the infertility treatment outcomes.
MATERIALS AND METHODS
Patients
A total of 42 cycles from 22 infertile patients who were undergoing infertility treatment at the Division of Reproductive Medicine, Department of Perinatal Medicine and Maternal Care, National Center for Child Health and Development, were examined in this study, from July 2002 to September 2004. All patients had normal pelvis and patent fallopian tubes, as determined by transvaginal sonography and hysterosalpingography, respectively. Only patients whose husbands’ semen presented normal values according to the WHO criteria were included. Endometriosis was diagnosed by laparoscopic examination. Male factor infertility and severe endometriosis were excluded from this study. Patients with polycystic ovary syndrome, diagnosed by basal serum gonadotropin and testosterone levels and vaginal ultrasound examination were also excluded from the study. Informed consent was obtained from all patients and the study was approved by the Institutional Review Board of the National Center for Child Health and Development.
In 21 cycles of nine patients with oligomenorrhea, ovulation induction was used due to ovulatory dysfunction. In addition, in 21 cycles of 13 patients with regular menstrual cycles, ovulation induction was used as a treatment of unexplained infertility. Cycles from the same patients without any treatment (natural cycles) were used as control. Oligomenorrhea was defined as menstrual cycles with intervals of 35 or more days (6).
Ovulation Induction
All patients received an FSH low-dose step-up protocol (7). The treatment began on day 3 of one spontaneous cycle with a standard ultrasound examination. The low-dose FSH step-up regimen started with the administration of 75 IU/day of purified FSH (Fertinorm P: Serono Japan, Tokyo) as the starting dose. Fourteen days after the start of stimulation, if follicles had not reached 10 mm in diameter, the dose was increased by 50% of the initial dose, i.e., 37.5 IU. Further dose adjustments were carried out if necessary, at 7-day intervals.
Ultrasound and Doppler Examination
All patients were examined by transvaginal color flow Doppler imaging within 3 days before and after the follicular rupture confirmed by ultrasound scan, the positive LH surge day, or the human chorionic gonadotropin (hCG; Mochida, Tokyo, Japan) administration day. At least one scan was performed within 48 h after the leading follicle had ruptured. The presence of either a corpus luteum or clouded endometrium was used as ultrasonographic criteria for presuming ovulation, in addition to the basal body temperature. Daily testing of urinary LH with one ovulation predictor kit (Dotest LH, Rohto Pharmaceutical Co., Ltd., Osaka) was started on cycle day 11. When an LH surge was detected by urinary testing, natural intercourse was instructed on the same day or artificial insemination with the husband's semen (AIH) was scheduled for the following day.
The morphology of the uterus and adnexa was studied by B-mode sonography. Color Doppler was used to visualize the intraovarian blood flow, and pulsed Doppler signals were obtained using a 2 mm volume cursor. The examination was performed by one examiner (K.N.), with the patient in a lithotomy position, using a Mochida Sonovista-Color II (Mochida, Tokyo, Japan), with a 6.5-MHz transvaginal probe for imaging and a 6.5-MHz pulsed Doppler system for blood flow analysis. The same follicle was easily detected by its size, location, and growth rate before ovulation through daily transvaginal ultrasound monitoring. Therefore, the examiner was able to observe the changes in one specific perifollicular artery of a leading follicle on every measurement. After ovulation, this vessel, near the corpus luteum derived from the chosen follicle, was also easily detected by its size and location. Moreover, single follicular growth was confirmed in most cases in this study.
Flow velocity waveforms from a perifollicular artery of the leading follicle were recorded. Blood flow impedance was expressed as the pulsatility index (PI), calculated electronically from smooth curves fitted to the waveforms over three cardiac cycles according to the formula: 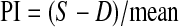 , where S is the peak Doppler shifted frequency; D the minimum Doppler shifted frequency; and mean, the mean maximum Doppler shifted frequency over the cardiac cycles. When the angle between the ultrasound beam and the longitudinal axis of the vessel is optimal for Doppler measurement, the pulsatility index is a convenient parameter despite the scanning angle as the angle parameter is not included in the PI formula. Perifollicular arterial blood flow reflects the cardiac cycle flow. One perifollicular arterial flow corresponds to one cardiac cycle flow and the peripheral arterial blood waveforms look smoother when compared to central arteries. Wall filters (100 Hz) were used to eliminate low frequency signals arising from noise. The intra-assay coefficient variability was 4.3%.
, where S is the peak Doppler shifted frequency; D the minimum Doppler shifted frequency; and mean, the mean maximum Doppler shifted frequency over the cardiac cycles. When the angle between the ultrasound beam and the longitudinal axis of the vessel is optimal for Doppler measurement, the pulsatility index is a convenient parameter despite the scanning angle as the angle parameter is not included in the PI formula. Perifollicular arterial blood flow reflects the cardiac cycle flow. One perifollicular arterial flow corresponds to one cardiac cycle flow and the peripheral arterial blood waveforms look smoother when compared to central arteries. Wall filters (100 Hz) were used to eliminate low frequency signals arising from noise. The intra-assay coefficient variability was 4.3%.
We defined PI decreasing rate as the intraovarian arterial PI value after follicular rupture compared with the one before, and this rate was calculated as follows: [1−(intraovarian arterial PI value after follicular rupture/intraovarian arterial PI value before follicular rupture)]×100. Based on these criteria, we divided the cycles of infertility treated patients into two groups; “profoundly decreased group” and “not profoundly decreased group” (the latter included cycles with increased PI) according to the intraovarian arterial PI values before and after follicular rupture. If the PI rate of decrease was 10% or more of the total decrease it was included in the profoundly decreased group. All the other values were qualified as not profoundly decreased rates.
Hormone Assay
FSH, LH, and PRL were determined using commercially available ELISA kits (IMMULIZE 2000; Diagnostic Products Corporation, LA, CA). Blood samples for FSH, LH, and PRL measurements were obtained between the second and fourth days of the menstrual cycle during the intraovarian arterial PI values evaluation. Serum estradiol (E2) and progesterone (P) were assayed in the midluteal phase using commercially available RIA kits (E2: IMMULIZE 2000; Diagnostic Products Corporation, LA, CA, and P: DPC Progesterone; Diagnostic Products Corporation, LA, CA).
A pregnant cycle was defined as one that presented a urinary positive hCG between the 14th and 20th days after the confirmed follicle rupture.
Statistics
All data are presented as real numbers, percentages, or the 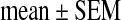 (standard error of the mean). The significance of the differences was determined by Mann–Whitney U test and χ2 test.
(standard error of the mean). The significance of the differences was determined by Mann–Whitney U test and χ2 test.
RESULTS
During the study period, 42 cycles in 22 patients treated with ovulation induction by the low-dose FSH step-up regimen resulted in the establishment of seven pregnancies. The clinical characteristics concerning the low-dose FSH step-up regimen for all patients are summarized in Table I. The mean numbers of developed follicles that reached ≥14 mm in diameter and of ovulated follicles were 1.7 ± 0.2 and 1.2 ± 0.1, respectively. The pregnancy rate per patient was 31.8% and no multiple-pregnancy occurred.
Table I.
Clinical Characteristics of the Low-Dose FSH Step-Up Regimen
| Number of patients | 22 |
| Number of treatment cycles | 42 |
| Age (years)* | 32.3 ± 0.7 |
| Duration of stimulation (days)* | 12.9 ± 0.6 |
| Number of ampoules required* | 15.5 ± 1.0 |
| Mean number of follicles (≥ 14 mm on hCG)* | 1.7 ± 0.2 |
| Number of ovulated follicles* | 1.2 ± 0.1 |
| Ovulation rate (%) | 100 |
| Pregnancy rate (per cycle; %) | 16.7 |
| Pregnancy rate (per patient; %) | 31.8 |
| Miscarriage rate (%) | 0 |
| Multiple pregnancy rate (%) | 0 |
| Incidence of severe OHSS (%) | 4.8 |
*Mean ± S.E.M.
All patients could be divided into two subgroups according to their menstrual regularity. Thirteen patients with regular menstrual cycles were included in the regular menstrual group, while the other nine patients with oligomenorrhea were included in the oligomenorrheal group. The clinical outcomes of both groups were compared and the results are summarized in Table II. The mean age of the oligomenorrheal group was lower than the regular menstrual group, but not significantly different. The mean duration of ovulation induction in the oligomenorrheal group was significantly longer than that in the regular group. As a result, oligomenorrheal patients needed more FSH ampules to induce ovulation than the regular menstrual ones. Although the ovulation rate and the number of ovulated follicles were similar in both groups, the pregnancy rate per cycle in the oligomenorrheal group was significantly higher than that in the regular group (p = 0.03).
Table II.
Outcome of Ovulation Induction with Low-Dose FSH Step-Up Regimen of Patients with Regular Menstrual Cycles or Oligomenorrhea
| Regular menstrual group | Oligomenorrheal group | P value | |
|---|---|---|---|
| Number of treatment cycles | 21 | 21 | — |
| Age (years)* | 33.2 ±0.5 | 31.3 ±1.0 | 0.09 |
| Duration of stimulation (days)* | 11.3 ±0.4 | 14.8 ±1.1 | 0.004 |
| Number of ampoules required* | 12.3 ±0.7 | 18.8 ±1.9 | 0.002 |
| Number of follicles (≥ 14 mm on hCG)* | 1.5 ±0.1 | 2.0 ±0.3 | 0.20 |
| Number of ovulated follicles* | 1.2 ±0.1 | 1.3 ±0.2 | 0.59 |
| Ovulation rate (%) | 100 | 100 | — |
| Pregnancy rate (per cycle; %) | 4.8 | 28.6 | 0.03 |
| Multiple pregnancy rate (%) | 0 | 0 | — |
| Miscarriage rate (%) | 0 | 0 | — |
| Incidence of severe OHSS (%) | 0 | 9.5 | 0.91 |
*Mean ± S.E.M.
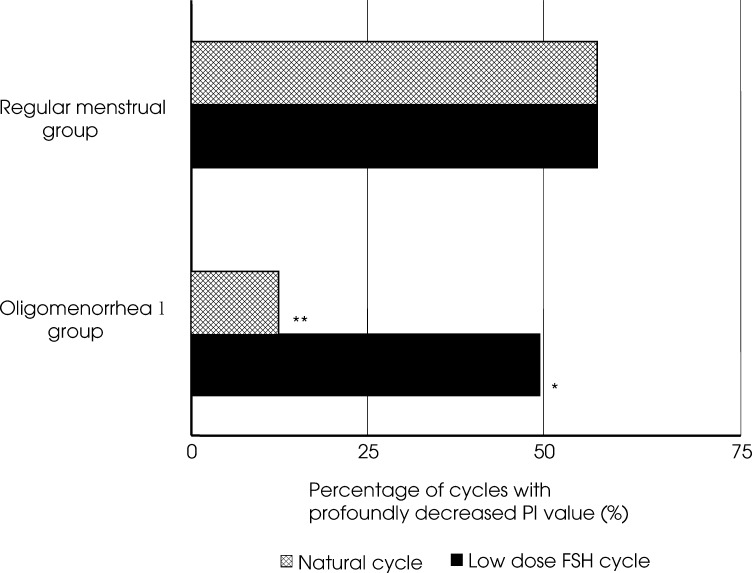
In the regular menstrual group, the percentage of cycles with postovulatory PI values showing a decrease of more than 10% during stimulation was 63.2% and this percentage was similar to natural cycles. On the other hand, in the oligomenorrheal group the percentage of cycles with postovulatory PI values showing decrease of more than 10% during natural cycles was 14.3%, but during ovulation induction cycles with the low-dose FSH step-up regimen this percentage was significantly higher (47.6%; p < 0.05). In natural cycles, the percentage of cycles with postovulatory PI values showing a decrease of more than 10% was significantly lower in the oligomenorrheal group than in the regular menstrual group (p < 0.05; Fig. 1).
Fig. 1.
In the regular menstrual group, the percentage of cycles with postovulatory PI values showing more than 10% decrease during the FSH low-dose step-up cycle was 63.2%, similar to natural cycles. On the other hand, in the oligomenorrheal group the percentage of cycles with postovulatory PI values showing more than 10% decrease during natural cycles was 14.3%, but during ovulation induction cycles with the FSH low-dose step-up regimen this percentage was significantly increased (47.6%; *vs. natural cycle in oligomenorrheal group; p < 0.05). In natural cycles, the percentage of cycles with postovulatory PI values of more than 10% decrease in the oligomenorrheal group was significantly lower than that in the regular menstrual group (** vs. natural cycle in regular menstrual group; p < 0.05).
DISCUSSION
In a standard population, follicular recruitment depends on the endogenous FSH threshold and the width of the selection window, and parameters are dependent on the initial dose of exogenous gonadotropin administered (8). Homburg et al. reported that the administration of low doses of exogenous FSH could reduce excessive ovarian response and improve the quality of the retrieved oocytes from IVF patients (9). In regard to oocyte quality, low-dose stimulation leads to a significant enhancement in the fertilization rate and compensates for the lower number of oocytes retrieved. In addition, a low dose stimulation protocol significantly improves embryo quality, as both fresh and cryopreserved embryos have higher implantation rates (10).
It has been reported that intraovarian blood flow changes dramatically during ovulation (3, 4,11). Kurjak et al. indicated that the resistance index in the postovulatory phase showed a significant decrease in relation to the preovulatory phase (3). Campbell et al. also demonstrated that intrafollicular PI values decreased after follicular rupture but the differences were not significant (4). Though previous reports have indicated that intraovarian blood flow resistance decreases after ovulation, we have recently reported that approximately half of our infertile patients with regular menstrual cycles presented an increase or slight decrease of intraovarian PI values after follicular rupture during natural cycles (2). To evaluate the influence of the PI decrease in the outcome of our patients, we divided the treatment cycles into two groups: the profoundly decreased and the not profoundly decreased groups.
The pregnancy rate in the profoundly decreased group was significantly higher than that in the not profoundly decreased group. Thus, a decrease of 10% or more in postovulatory PI values appears to be a very important phenomenon in establishing a suitable environment for achieving pregnancy.
Therefore, in addition to our previous report, we conclude that a decrease in intraovarian arterial PI values after ovulation indicates the establishment of a suitable follicular environment during ovulation. Consequently, ovulation induction for patients who belong to the not profoundly decreased group was used in order to establish a suitable follicular environment. The percentage of cycles with profoundly decreased PI values in the oligomenorrheal group was only 14.3%. This figure is much lower than that of the regular menstrual group and implies that oligomenorrheal patients have not only longer menstrual intervals but also disturbed ovulation mechanisms. This percentage, however, increased to 47.6% when ovulation induction was used. As a result, a higher pregnancy rate could be achieved in oligomenorrheal patients.
On the other hand, in the regular menstrual group, the percentage of cycles with profoundly decreased PI values after ovulation was almost the same between ovulation inducted and natural cycles. This result prompted us to suspect that ovulation induction, especially our low-dose FSH step-up regimen was not effective for patients with unexplained infertility and regular menstrual cycles. Moreover, adverse follicular environment during ovulation probably is not a cause of infertility in these patients. It is also possible that a larger dose of gonadotropin might be necessary to improve the process of follicle development in idiopathic infertile patients. Welner et al. had already reported that a higher pregnancy rate could be achieved with higher doses of gonadotropins in cases of long-standing infertility of unknown etiology (5).
The decrease in arterial PI values after ovulation appears to reflect perifollicular angiogenesis, increased permeability of perifollicular blood vessels, and the regulation of intrafollicular oxygen level. Previous reports have shown that Doppler indices (resistance and pulsatility indices and systole/diastole ratio), which are markers of a downstream impedance to blood flow, serve as significant and negative correlation measurement of metabolic activity, such as intrafollicular 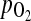 and
and 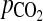 (12). Therefore, we can say that a slight decrease in perifollicular arterial PI value indicates that intrafollicular hypoxia progressed and that the developed follicles, including oocytes and cumulus-oocytes complexes were exposed to a low oxygen stress's environment. Consequently, oocyte quality was affected and, as a result, this slight decrease in PI value resulted in a poor pregnancy outcome.
(12). Therefore, we can say that a slight decrease in perifollicular arterial PI value indicates that intrafollicular hypoxia progressed and that the developed follicles, including oocytes and cumulus-oocytes complexes were exposed to a low oxygen stress's environment. Consequently, oocyte quality was affected and, as a result, this slight decrease in PI value resulted in a poor pregnancy outcome.
Still, how ovulation induction can improve the intrafollicular environment is not clearly understood. One recent report demonstrated that follicular 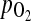 has a positive and significant correlation with the peak of E2 serum level and a negative and significant correlation with the perifollicular PI value (12). Therefore, we can assume that elevated serum estradiol concentrations due to ovulation induction caused an increased intrafollicular
has a positive and significant correlation with the peak of E2 serum level and a negative and significant correlation with the perifollicular PI value (12). Therefore, we can assume that elevated serum estradiol concentrations due to ovulation induction caused an increased intrafollicular 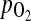 , decreased perifollicular PI value, and consequently improved the intrafollicular hypoxia and increased fecundity.
, decreased perifollicular PI value, and consequently improved the intrafollicular hypoxia and increased fecundity.
In conclusion, although most of oligomenorrheal patients have some disturbance in the ovulatory process, the use of a low-dose FSH step-up regimen reduced the perifollicular arterial blood flow resistance in these patients and consequently increased the pregnancy rate.
ACKNOWLEDGMENTS
This study was partially supported by a grant to the National Center for Child Health and Development (14 KOU-4) from the Ministry of Health, Labor and Welfare, Japan.
REFERENCES
- 1.Homburg R, Levy T, Ben-Rafael Z. A comparative prospective study of conventional regimen with chronic low-dose administration of follicle-stimulating hormone for anovulation associated with polycystic ovary syndrome. Fertil Steril. 1995;63:729–733. doi: 10.1016/s0015-0282(16)57473-1. [DOI] [PubMed] [Google Scholar]
- 2.Nakagawa K, Ozawa N, Takamatsu K, Takahashi Y, Irahara M, Yoshimura Y, Saito H. A reduction in intraovarian arterial blood flow resistance after ovulation is necessary to achieve pregnancy in natural cycle. J Assist Reprod Genet. 2005;22:9–14. doi: 10.1007/s10815-005-0814-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kurjakck A, Kupesic-Urek S, Schulman H, Zalud I. Transvaginal color flow Doppler in the assessment of ovarian and uterine blood flow in infertile women. Fertil Steril. 1991;56:870–873. doi: 10.1016/s0015-0282(16)54657-3. [DOI] [PubMed] [Google Scholar]
- 4.Campbell S, Bourne TH, Waterstone J, Reynolds KM, Crayford TJB, Jurkovic D, Okokon EV, Collins WP. Transvaginal color blood flow imaging of the periovulatory follicle. Fertil Steril. 1993;60:433–438. [PubMed] [Google Scholar]
- 5.Welner S, DeCherney AH, Polan ML. Human menopausal gonadotropins: A justifiable therapy in ovulatory women with long-standing idiopathic infertility. Am J Obstet Gynecol. 1988;158:111–117. doi: 10.1016/0002-9378(88)90789-2. [DOI] [PubMed] [Google Scholar]
- 6.Bayer SR, Apler MM, Penzias AS: The Boston IVF Handbook of Infertility. New York, The Parthenon, 2002, p 12
- 7.Andoh K, Mizunma H, Liu X, Kamijo T, Yamada K, Ibuki Y. A comparative study of fixed-dose, step-down and low-dose step-up regimens of human menopausal gonadotropin for patients with polycystic ovary syndrome. Fertil Steril. 1998;70:840–846. doi: 10.1016/S0015-0282(98)00308-2. [DOI] [PubMed] [Google Scholar]
- 8.Schipper I, Hop WC, Fauser BC. The follicle-stimulating hormone (FSH) threshold/window concept examined by different interventions with exogenous FSH during the follicular phase of normal menstrual cycle: Duration, rather than magnitude, of FSH increase affects follicle development. J Clin Endocrinol Metab. 1998;83:1292–1298. doi: 10.1210/jc.83.4.1292. [DOI] [PubMed] [Google Scholar]
- 9.Homburg R, Howels CM. Low-dose FSH therapy for anovulatory infertility associated with polycystic ovary syndrome: Rationale, results, reflections and refinements. Hum Reprod Update. 1999;5:493–499. doi: 10.1093/humupd/5.5.493. [DOI] [PubMed] [Google Scholar]
- 10.Marci R, Senn A, Essole S, Chanson A, Loumaye E, de Grandi P, Germond M. A low-dose stimulation protocol using highly purified follicle-stimulating hormone can lead to high pregnancy rates in in vitro fertilization patients with polycystic ovaries who are at risk of high ovarian response to gonadotropins. Fertil Steril. 2001;72:1131–1135. doi: 10.1016/S0015-0282(01)01788-5. [DOI] [PubMed] [Google Scholar]
- 11.Tan SL, Zaidi J, Campbell S, Doyle P, Collins W. Blood flow changes in the ovarian and uterine arteries during the normal menstrual cycle. Am J Obstet Gynecol. 1996;175:625–631. doi: 10.1053/ob.1996.v175.a73865. [DOI] [PubMed] [Google Scholar]
-
12.Huey S, Abuhamad A, Barosso G, Hsu MI, Kolm P, Mayer J, Oehninger S: Perifollicular blood flow Doppler indices, but not follicular
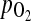 ,
, 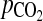 or pH, predict oocyte developmental competence in in vitro fertilization. Fertil Steril 1999;72:707–712 [DOI] [PubMed]
or pH, predict oocyte developmental competence in in vitro fertilization. Fertil Steril 1999;72:707–712 [DOI] [PubMed]