Abstract
Purpose: To investigate whether sterile filtered light paraffin oil (SPO) overlaying is superior to washed light mineral oil (WMO) in supporting the in vitro developmental competence of bovine follicular oocytes. In addition, the effects of the two types of oil overlaying were compared with oil overlaying plus co-culture (CC) on bovine embryo development in vitro.
Methods: Bovine follicular oocytes retrieved from abattoir-derived ovary were in vitro matured, fertilized and cultured in 50 μL drops overlayed with WMO or SPO and were subsequently evaluated for development rates. In second experiment, day 2 embryos grown under WMO overlaying were further cultured for 6 days in the presence (WMO+CC and SPO+CC) or absence of adult ear skin fibroblast-based co-culture system overlaid with WMO or SPO. Blastocysts from each group were evaluated for total nuclei number or were further cultured for 48 h to evaluate post-hatching development.
Results: SPO overlaying resulted in significant higher (p < 0.05) development rate to morula (44.8% versus 30.6%) and blastocyst (32.8% versus 21.7%) than WMO. Also, treatment of the day 2 embryo cultures with SPO overlaying or oil plus CC (WMO+CC or SPO+CC groups) reached significantly higher development rates from the morula stage compared to embryo cultures treated with the WMO overlaying (p < 0.05). However, the development rates of the SPO treatment group (morula: 72.7%; blastocyst: 53.1%) were slightly high compared to development of the culture treated with WMO+CC (69.6 and 50.4%, respectively). This similar developmental competence pattern was also observed in cell number and embryo hatching rate.
Conclusion: SPO overlaying is superior to WMO and WMO+CC in supporting in vitro development of bovine embryos. The development rates are further enhanced when embryos are cultured in co-culture system overlaid with SPO. Thus, this data suggest that overlaying oil can significantly influence the pre-implantation embryo development in vitro.
Key Words: Bovine IVM/IVF/IVC embryo, developmental competence, sterile filtered light paraffin oil, washed light mineral oil.
INTRODUCTION
Successful in vitro pre-implantation embryo development requires a culture environment similar to the in vivo environment as much as possible; specifically, effective basic culture medium, various protein supplements, and a co-culture system using a somatic cell feeder layer (1–3) are necessary. Researchers have optimized culture systems for various embryo stages and species (4–6), thus advancing in vitro culture technology for blastocyst development. The most commonly used culture environment has been the micro drop culture method using oil rather than large scale medium, with the exception of certain specific culture methods. Micro drop cultures are enable to control of a small number of noble embryos under the microscopic boundary, which reduces accumulation of toxic components in the medium and induces cellular interaction via released cytokines by embryos. Micro drop culture generally facilitates higher developmental competence than large scale medium (7). On the other hand, oil overlaying is necessary for micro drop culture. Oil overlaying protects against dehydration and hydrogen concentration variation in the in vitro medium environment. Several studies investigating the effect of oil quality on embryo development (4,8–14) demonstrated that oil washing or sterilization effectively enhances oil quality, and that high-grade oil can remove toxic material from the embryo. Therefore, use of the most appropriate oil appears to be associated with development of high quality embryos.
This study was designed to investigate whether sterile filtered light paraffin oil (SPO) overlaying is superior to washed light mineral oil (WMO) in supporting the in vitro developmental competence of bovine follicular oocytes fertilized and cultured in the presence or absence of somatic cell co-culture system.
MATERIALS AND METHODS
Production of Bovine IVM/IVF/IVC Blastocysts
The culture procedures employed for production of pre-implantation embryos from bovine follicular oocytes were similar to those of Rosenkrans et al. (15). Briefly, bovine ovaries were obtained from a local abattoir and transported to the laboratory, within 2–3 h, in saline (0.9% NaCl/L) maintained at 32±2°C. Cumulus oocyte complexes (COCs) were collected from visible follicles (2–6 mm) of ovaries with an 18-gauge needle attached to a disposable syringe, washed with TALP-HEPES medium containing 1 μg/mL of bovine serum albumin (BSA; Fraction V, Sigma) and cultured in 50 μL maturation drops composed of TCM-199 (Gibco) supplemented with 10% (v/v) fetal bovine serum (FBS), sodium pyruvate (0.2 mM), FSH (1 μg/mL), estradiol-17β (1 μg/mL), and gentamycin (25 μg/mL) and incubated at 39°C with 5% CO2. After incubation for 22–24 h in IVM medium, COCs were inseminated using 2 μL of highly motile sperm (25×106 spermatozoa/mL) recovered from frozen-thawed semen separated on a discontinuous percoll column and 2 μL of heparin (2 μg/mL) and PHE (18.2 M Penicillamine, 9.1 M Hypotaurine and 1.8 M Epinephrine) were also added in 44 μL fertilization drop. At 44±2 h, the inseminated oocytes were stripped free of any adherent sperm and cleaved embryos (≥2-cell) were cultured in 50 μL drops of the CR1aa medium supplemented with 3 mg/mL of fatty acid-free BSA. On day 4 (96 h after IVF), media was changed into CR1aa supplemented with 10% FBS.
Experimental Design
Exp. 1: In Vitro Development of Bovine Follicular Oocytes Under Different Types of Oil Overlaying.
To examine the effect of different oil overlaying on in vitro development rates, bovine follicular oocytes were cultured in 50 μL drop overlaid with washed light mineral oil (WMO, Sigma M8410, St. Louis, MO) using 0.9% saline or sterile filtered light paraffin oil (SPO, Ovoil, Zander IVF, Vero Beach, FL) at 39°C in a 5% CO2 incubator. Also, in vitro fertilization and in vitro culture were carried out continuously under the same oil for 8 days, respectively.
Exp. 2: In Vitro Development of Bovine Embryos Under Different Oil Overlaying Plus Co-culture Conditions.
The effects of oil overlaying (WMO, SPO) or/and co-culture with adult ear skin fibroblasts from the cleaved embryos (WMO+CC, SPO+CC) were evaluated on development of day 2 embryos (four to eight cell). After 48 h, developed embryos were cultured in new 50 μL medium drop or co-culture drop with CR1aa supplemented with 10% FBS. Some of these in vitro cultured day 8 blastocysts were further cultured for 48 h to determine the effect of the different oil and co-culture environments on embryo hatching development. Other blastocysts were characterized for total cell number and inner cell mass by differential labeling.
Differential Staining of Blastocysts
Cell number of blastomeres, inner cell mass (ICM) and trophectoderm (TE) in blastocysts were counted by differential staining according to the method of Thouas et al. (16). Blastocysts were first incubated in 500 μL of Solution 1 (BSA-free Hepes buffered TCM-199 medium with 1% Triton X-100 and 100 μL/mL propidium iodide) for up to 30 s. Blastocysts were then immediately transferred into 500 μL of solution 2 (fixative solution of 100% ethanol with 25 μg/mL bisbenzimide (Hoechst 33258)) and stored at 4°C overnight. Fixed and stained blastocysts were then transferred directly from solution 2 to glycerol. Blastocysts were mounted and assessed for cell number using epifluorescence microscopy.
Statistical Analysis
Differences in the developmental rates between treatment and control groups were assessed using the Chi-square test (p < 0.05).
RESULTS
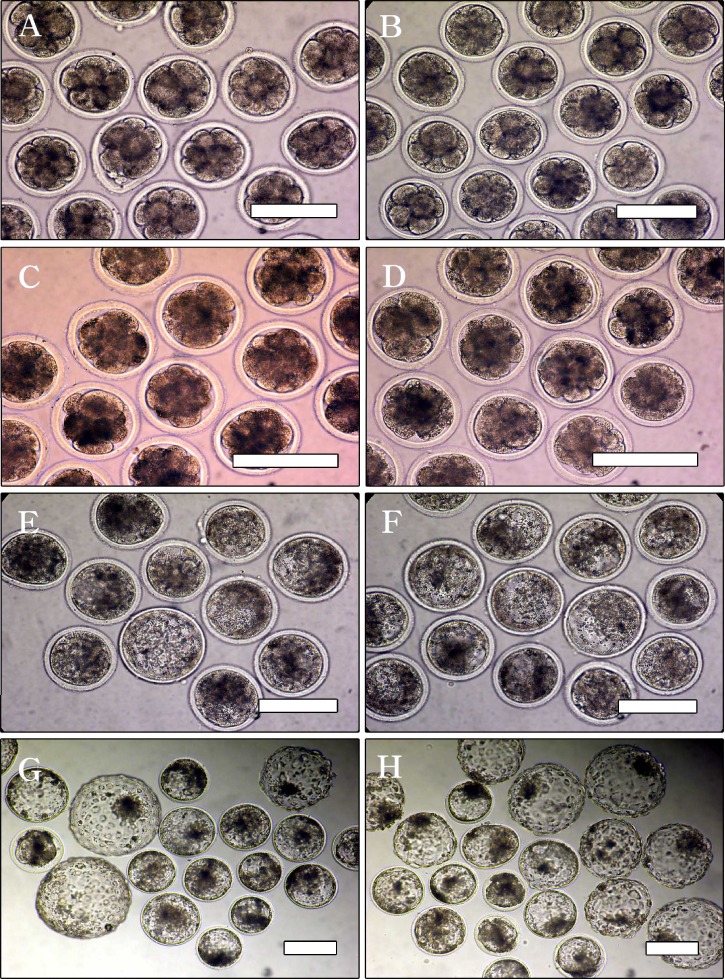
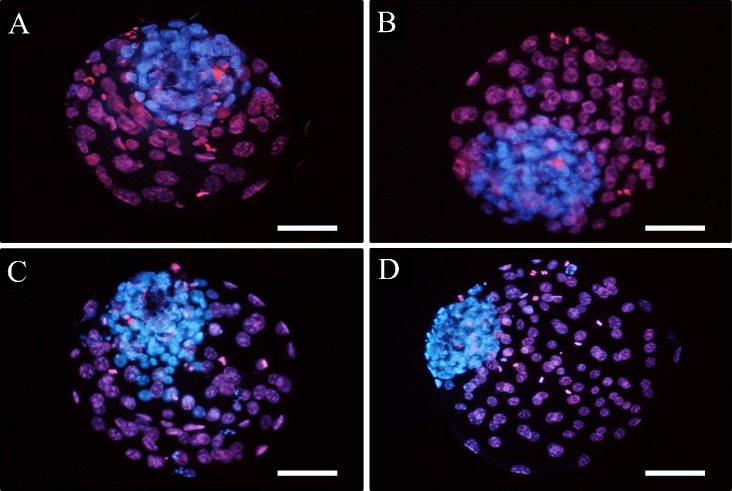
When the developmental competence of follicular oocytes grown under the different types of oil (WMO or SPO) overlaying was examined, as shown in Table I, there were no significant differences in cleavage rate on day 2 (72.0 versus 75.0%) and development rates on day 4 (57.8 versus 58.3%) between groups. However, from day 6, morula and blastocyst development rates in the SPO overlaying group (44.8 and 32.8%) were significantly higher than those in the WMO overlaying group (30.6 and 21.7%) (p < 0.05). The developmental differences were more pronounced at the later development stage in day 2 embryos (four to eight cell) grown under the combination condition of oil overlaying (either oil) and co-culture, as shown in Table II and Fig. 1. Through the seven replications, when the selected four to eight cell embryos were assigned to one of four groups, enhanced development was confirmed in all the groups except for the WMO group (WMO < WMO+CC=SPO < SPO+CC). Although the development rates were similar after 48 h irrespective of treatment group (73.9, 81.5, 81.5 and 85.7%, respectively), there were significant differences in development from the morula stage (58.0, 69.6, 72.7 and 76.1%, respectively) and in development from the blastocyst stage (39.1, 50.4, 53.1 and 54.3%, respectively) among the groups (p < 0.05). Interestingly, development rates between the WMO+CC and SPO groups were not significantly different. Thus, the absence of CC conditions, SPO overlaying helped improve the developmental competence of bovine embryos. This developmental pattern continued to post-hatching development. As shown in Table III, hatching and hatched blastocyst development rates in the WMO+CC group (57.5 and 37.5%) and SPO group (53.8 and 36.3%) were not significantly different. Significant differences were also observed in the total cell number and inner cell mass number determined by differential staining, as indicated in Table II and Fig. 2, among some (n=20) of the in vitro produced blastocyst groups (p <0. 05). Total cell numbers were significantly increased by oil type (WMO: 128.0±16.4; SPO: 147.6±23.6) and by oil combined with co-culture (WMO+CC: 152.9±22.3; SPO+CC: 172.0±38.0) (p < 0.05), but there were no significant differences in ICM in the remaining three groups (WMO: 39.4±7.2; WMO+CC: 48.5±8.3 and SPO: 45.6±6.1), while ICM was higher in the SPO+CC group (62.8±9.3). However, ICM proportion from the total cell numbers was found not to be different among the groups (WMO: 30.8%; WMO+CC: 31.7%; SPO: 30.9% and SPO+CC: 36.5%).
Table I.
In Vitro Development of Bovine Follicular Oocytes Under Different Types of Oil Overlaying
| No. (%) of embryos developed tob | |||||
|---|---|---|---|---|---|
| Oila | Day 2 ≥2 cell | Day 4 ≥8 cell | Day 6 ≥morula | Day 8 ≥blastocyst | |
| No. of oocytes inseminated | |||||
| Washed light mineral oil | 250 | 180 (72.0) | 104 (57.8) | 55 (30.6)a | 39 (21.7)a |
| Sterile filtered light paraffin oil | 256 | 192 (75.0) | 112 (58.3) | 86 (44.8)b | 63 (32.8)b |
aWMO; used after washing with 0.9% saline, Sigma M8410; SPO; ready-to use, Vitrolife Ovoil™-100.
bMeans in the column without common letters are significantly different (p < 0.05).
Table II.
In Vitro Development of Bovine Embryos Under Different Oil Overlaying Plus Co-culture Conditions
| Treatment | No. (%) of embryos developed toa | |||||
|---|---|---|---|---|---|---|
| Oilb | Co-culturec | Day 4 ≥8 cell | Day 6 ≥morula | Day 8 ≥blastocyst | ||
| No.of embryos cultured | No. of total cellsa (ICM) | |||||
| WMO | wo/ | 276 | 204 (73.9) | 160 (58.0)a | 108 (39.1)a | 128.0±16.4a(39.4±7.2)a |
| w/ | 260 | 212 (81.5) | 181 (69.6)b | 131 (50.4)b | 152.9±22.3b(48.5±8.3)a | |
| SPO | wo/ | 275 | 224 (81.5) | 200 (72.7)b | 146 (53.1)b | 147.6±23.6b(45.6±6.1)a |
| w/ | 280 | 240 (85.7) | 213 (76.1)b | 152 (54.3)b | 172.0±38.0c(62.8±9.3)b | |
aMeans in the column without common letters are significantly different (a–b, a–c; p < 0.05).
bWMO; washed light mineral oil, M8410; SPO; sterile filtered light paraffin oil, Ovoil™-100.
cEmbryos were co-cultured with adult ear skin fibroblast cells.
Fig. 1.
In vitro development of bovine day 2 embryos covered with washed light mineral oil (A, C, E and G) or sterile filtered light paraffin oil (B, D, F and H). Four- to eight-cell embryos (A and B) developed similarly until day 4 (8–16 cells; C and D) and then showed gradual differences in developmental capacity from day 6 (morula-blastocyst, E and F) to day 8 (G and H) according to oil variation. Sterile filtered paraffin oil supports increased and improved embryo production in vitro. Scale bars: 200 μm.
Table III.
Effect of Oil Overlaying Plus Co-culture on the Hatching Development of Day 8 Blastocysts
| No. (%) of developed embryos after 48 hb | ||||
|---|---|---|---|---|
| Oila | Co-culture | ≥Hatching | Hatched | |
| No. of day 8 blastocysts | ||||
| WMO | wo/ | 60 | 24 (40.0)a | 14 (23.3)a |
| w/ | 64 | 37 (57.8)ab | 24 (37.5)ab | |
| SPO | wo/ | 80 | 43 (53.8)ab | 29 (36.3)ab |
| w/ | 80 | 54 (67.5)b | 37 (46.3)b | |
aWMO; washed light mineral oil, M8410; SPO; sterile filtered light paraffin oil, Ovoil™-100.
bMeans in the column without common letters are significantly different (a–b; p < 0.05).
Fig. 2.
Differential stained inner cell mass and trophectoderm cells of bovine day 8 blastocysts developed from washed light mineral oil overlay (A), WMO overlay plus co-culture (B), sterile filtered light paraffin oil overlay (C) or SPO overlay plus co-culture (D). All embryos were healthy with numerous cell numbers. Scale bars: 100 μm.
DISCUSSION
This study demonstrated that high-quality sterile filtered light paraffin oil is superior to the conservative oil overlaying and co-culture system has positive effect on the in vitro development (i.e., blastocyst development, hatching development rates and cell number) of pre-implantation embryos. Alteration in oil overlaying represents a change in microenvironment to which relatively less viable embryos might be more sensitive when cultured for a long duration of 9–11 days, as generally they are. Such circumstance become more relevant when only a small and novel number of embryos, such as cloned embryos, micromanipulated embryos, human embryos etc., are available for culture to produce viable offspring. Selecting the best quality overlaying are therefore, of particular importance to improve development rates of embryos produced by in vitro systems.
Oil has been widely used in the formulation of micro drop embryo culture systems. The role of oil in embryo development has been demonstrated in many species including mice (4,11–12), pigs (14) and cows (9,13). Oil overlaying also helps to protect the embryos by neutralizing the toxic artifacts in culture systems (4). On the contrary, oil also has negative effects on embryo culture as it might reduce the full maturation potential of pig oocytes during in vitro meiotic maturation by interacting with steroid hormones, such as oestradiol and progesterone, in the medium (14). In general, oil types used for embryo culture are paraffin, mineral or silicon oil. Toxic components of oil are often washed before their use. Numerous attempts has also been conducted to improve oil quality for embryo culture. Washing removes toxic components from oil (12), with highly variable results dependent on oil type or treatment. Sterile filtered oil has recently become expensive, and sterile filtering of oil is not feasible in laboratory. However, selection of superior oil types may facilitate culture of healthier embryos or offspring.
In this study, we compared the overlaying effect of WMO and sterile filtered light paraffin oil on bovine follicular oocyte or embryo development in vitro. As expected, embryo development rates were continuously influenced by the oil type. Although a difference in development was not observed in the immediate early stage, improved quantitative and qualitative results were obtained from the morula stage in the SPO overlaying group compared to the WMO overlaying group. In addition, we confirmed the similar development pattern in mouse embryo cultures (data not shown). Also, when we examined the difference in developmental competence between oil overlaying and the oil overlaying plus co-culture environment, we found that oil with co-culture system can improve embryo development better than oil overlaying without somatic cell co-culture. In general, somatic cell co-culture is one method for overcoming the in vitro developmental block of cultured mammalian embryos. In a report by Yuh-Ming Hwu et al. (6), co-culture cells may secrete embryotrophic factors such as growth factors, cytokines or oviduct-specific glycoproteins. In our study, the best regime for in vitro bovine embryo development is the use of paraffin oil plus co-culture (SPO+CC). Total cell number and ICM cell numbers were significantly increased by the co-culture effect compared with the oil overlaying group (p < 0.05).
However, it cannot be ignored that co-culture systems are not considered appropriate model systems for investigation of effects of newly added medium compositions. Furthermore, prepared cell conditions can be variable, and the work is really laborious. In this study, we demonstrate that simple SPO overlaying supports developmental competence equivalent to WMO+CC in bovine embryo development in vitro.
CONCLUSIONS
SPO overlaying is superior to WMO and WMO+CC in supporting in vitro development of bovine embryos. The development rates are further enhanced when embryos are cultured in co-culture system overlaid with SPO. Thus, this data suggest that overlaying oil can significantly influence the pre-implantation embryo development in vitro.
ACKNOWLEDGMENT
This study was supported by a grant (01-PJ10-PG8-01EC01-0010) of the Korea Health 21 R&D Project, Ministry of Health & Welfare, Republic of Korea.
Footnotes
Jin Cheol Tae, Eun Young Kim, These two authors contributed equally to this work
REFERENCES
- 1.Bavister BD, Rose-Hellekant TA, Pinyopummintr T. Development of in vitro matured/in vitro fertilized bovine embryos into morulae and blastocysts in defined culture media. Theriogenology. 1992;37:127–146. doi: 10.1016/0093-691X(92)90251-L. [DOI] [Google Scholar]
- 2.Rief S, Sinowatz F, Stojkovic M, Einspanier R, Wolf E, Prelle K. Effects of a novel co-culture system on development, metabolism and gene expression of bovine embryos produced in vitro. Reproduction. 2002;124:543–556. doi: 10.1530/rep.0.1240543. [DOI] [PubMed] [Google Scholar]
- 3.Fukuda Y, Ichikawa M, Naito K, et al. Birth of normal calves resulting from bovine oocytes matured, fertilized, and cultured with cumulus cells in vitro up to the blastocyst stage. Biol Reprod. 1990;42:114–119. doi: 10.1095/biolreprod42.1.114. [DOI] [PubMed] [Google Scholar]
- 4.Lee ST, Cho MY, Kim EJ, Kim TM, Lee CK, Han JY, Lim JM. Renovation of a drop embryo cultures system by using refined mineral oil and the effect of glucose and/or hemoglobin added to a serum-free medium. J Vet Med Sci. 2004;66(1):63–66. doi: 10.1292/jvms.66.63. [DOI] [PubMed] [Google Scholar]
- 5.Fukui Y, McGowan LT, James RW. Factors affecting the in vitro development to blastocysts of bovine oocytes matured and fertilized in vitro. J Reprod Fertil. 1991;92:125–131. doi: 10.1530/jrf.0.0920125. [DOI] [PubMed] [Google Scholar]
- 6.Hwu Y, Lee RK, Chen C, Su J, Chen Y, Lin S. Development of hatching blastocysts from immature human oocytes following in-vitro maturation and fertilization using a co-culture system. Hum Reprod. 1998;13:1916–1921. doi: 10.1093/humrep/13.7.1916. [DOI] [PubMed] [Google Scholar]
- 7.Fukui Y, Lee ES, Araki N. Effect of medium renewal during culture in two different culture systems on development to blastocysts from in vitro produced early bovine embryos. J Anim Sci. 1996;74:2752–2758. doi: 10.2527/1996.74112752x. [DOI] [PubMed] [Google Scholar]
- 8.Miller KF, Goldberg JM, Collins RL. Covering embryo cultures with mineral oil alters embryo growth by acting as a sink for an embryo toxic substance. J Assist Reprod Genet. 1994;11:342–345. doi: 10.1007/BF02214139. [DOI] [PubMed] [Google Scholar]
- 9.Van Soom A, Langendonckt A, Mahmoudzadeh AR, Deluyker H, Dessy F, Kruif A. Effect of oil quality on in vitro embryonic development in the bovine. Theriogenology. 1994;41:325. doi: 10.1016/S0093-691X(05)80235-3. [DOI] [Google Scholar]
- 10.Erbach GT, Bhatnagar P, Baltz JM, Biggers JD. Zinc is a possible toxic contaminant of silicone in microdrop cultures of preimplantation mouse embryos. Hum Reprod. 1995;10:3248–3254. doi: 10.1093/oxfordjournals.humrep.a135897. [DOI] [PubMed] [Google Scholar]
- 11.Borque C, Pintado B, Garcia P, Sanchez R. Effect of washing oil on in vitro development of mouse embryos. Theriogenology. 1996;45:206. doi: 10.1016/0093-691X(96)84679-6. [DOI] [Google Scholar]
- 12.Provo MB, Herr C. Washed paraffin oil becomes toxic to mouse embryos upon exposure to sunlight. Theriogenology. 1998;49:214. doi: 10.1016/S0093-691X(98)90567-2. [DOI] [Google Scholar]
- 13.Van Soom A, Mahmoudzadeh AR, Christophe A, Ysebaert MT, Kruif A. Silicone oil used in microdrop culture can affect bovine embryonic development and freezability. Reprod Domest Anim. 2001;36:169–176. doi: 10.1046/j.1439-0531.2001.00281.x. [DOI] [PubMed] [Google Scholar]
- 14.Shimade M, Kawano N, Terada T. Delay of nuclear maturation and reduction in developmental competence of pig oocytes after mineral oil overlay of in vitro maturation media. Reproduction. 2002;124:557–564. doi: 10.1530/rep.0.1240557. [DOI] [PubMed] [Google Scholar]
- 15.Rosenkrans CF, Jr, Zeng GQ, Mcnamara GT, Schoff PK, First NL. Development of bovine embryos in vitro as affected by energy substrates. Biol Reprod. 1993;49:459–462. doi: 10.1095/biolreprod49.3.459. [DOI] [PubMed] [Google Scholar]
- 16.Thouas GA, Korfiatis NA, French AJ, Tervit HR. Simplified technique for differential staining of inner cell mass and trophectoderm cells of mouse and bovine blastocysts. Reprod Biomed Online. 2000;3:25–29. doi: 10.1016/S1472-6483(10)61960-8. [DOI] [PubMed] [Google Scholar]