Abstract
Purpose: This study investigated the effects of HB-EGF on expression of integrin 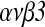 and implantation of embryos.
and implantation of embryos.
Methods: Two-cell embryos were recovered and cultured with or without 10 ng/mL HB-EGF for 96h. Expression of integrin 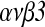 in cultured embryos was examined by real time-RT-PCR and immunofluorescence analysis; embryos were cultured with or without HB-EGF, then transferred into the uteri of pseudo-pregnant female mice in order to analyze their implantation rate.
in cultured embryos was examined by real time-RT-PCR and immunofluorescence analysis; embryos were cultured with or without HB-EGF, then transferred into the uteri of pseudo-pregnant female mice in order to analyze their implantation rate.
Results: HB-EGF improved embryonic hatching and outgrowth during extended culture, and up-regulated expression of integrin 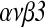 in both the preimplantation embryo and outgrowing blastocyst. Also, integrin
in both the preimplantation embryo and outgrowing blastocyst. Also, integrin 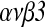 subunits were localized at the pericellular borders and cell–cell contact areas. The number of successful implantation sites of transferred HB-EGF-treated embryos in the uterus was increased when compared to number of implantation sites with non-treated controls.
subunits were localized at the pericellular borders and cell–cell contact areas. The number of successful implantation sites of transferred HB-EGF-treated embryos in the uterus was increased when compared to number of implantation sites with non-treated controls.
Conclusions: HB-EGF may improve implantation by accelerating expression of integrin 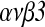 in peri-implantation mouse embryos.
in peri-implantation mouse embryos.
KEY WORDS: Embryonic development, HB-EGF, integrin 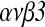 , implantation, mouse.
, implantation, mouse.
INTRODUCTION
Implantation is a critical step in embryogenesis that requires preparation of a receptive uterus and activation of the blastocyst. Both of these processes proceed in a temporally and spatially coordinated manner. The regulated expression of several growth factors and their receptors in the uterus and embryo suggests that these growth factors could serve as local mediators of steroid hormone action during the pre-implantation period (1).
The roles of the EGF family in uterine biology and implantation have been studied more extensively than those of other polypeptide growth factors. The EGF family includes EGF itself, transforming growth factor-α (TGF-α), amphiregulin (AR), HB-EGF, betacellulin (BTC), epiregulin (ER), and heregulins/neu differentiation factors (NDFs). In particular, HB-EGF is a heparin-binding member of the EGF family first identified in conditioned medium from macrophages. HB-EGF is synthesized as a transmembrane precursor that can be cleaved enzymatically to release a soluble 14–20 kDa form. The soluble form of HB-EGF is a potent paracrine and/or autocrine mitogen for fibroblasts, smooth muscle cells (SMC) and keratinocytes (2). HB-EGF is an important paracrine and autocrine regulator of trophoblast activity during implantation and early placentation. Also, HB-EGF improved development of the embryo to the hatching blastocyst stage and promoted trophoblast outgrowth in the mouse. In the mouse, expression of HB-EGF is induced 6–7 h before the attachment reaction solely at the site of blastocyst apposition (3), and continues to be expressed by extravillous cytotrophoblast cells and uterine vascular endothelial cells during the first trimester. Four receptors for the EGF family (erbb/HER) were known. HB-EGF binds to HER1 and HER4, but not to HER2 or HER3 (4). Mouse blastocysts in peri-implantation express erbb1 and erbb4, two receptors that bind to HB-EGF, which suggests that the peri-implantation blastocyst is targeted by HB-EGF during implantation (5). Although, it is not yet clear whether the effects of uterine HB-EGF are mediated in a paracrine and/or juxtocrine manner, the tightly regulated expression of HB-EGF in luminal epithelium and of EGF-receptor in the blastocyst around the time of implantation (6) suggests an important function for this ligand–receptor signaling in embryo–uterine interaction during implantation.
It is clear that mouse primary trophoblast adhesion to the ECM is largely under control of integrins (7). Integrins are heterodimeric glycoproteins that are present on the surface of essentially all cells (8). This class of cell adhesion molecules has been described in both embryo and endometrium (9) at the time of implantation in the mouse and human. The programmatic expression of integrins in placenta and decidua (10) extends the involvement of integrins into the later stages of implantation as well.
Interest in the vitronectin receptor (heterodimeric glycoprotein of integrin (νβ3) has increased as a result of a number of earlier studies that correlate its expression with the window of implantation and its absence in certain types of infertility including luteal phase defect and endometriosis. Recent studies by Simon have demonstrated that embryonic signaling may stimulate expression of this integrin in mouse (11) and human endometrium as well. Initially, the basolateral plasma membrane is replete with integrins that mediate adhesion to the basal lamina, whereas the outward-facing apical domain is largely devoid of integrins, with the exception of integrin (12). Competitive inhibition of the integrin binding site by RGD-containing peptides of neutralization with disintegrins and monoclonal antibodies significantly reduced the number of implantation sites observed in the treated uterine horn compared to the untreated horn (13). Thus, expression of embryonic and endometrial integrin during the window of implantation in the mouse suggests that plays a role in the implantation process.
The expression of critical proteins, such as integrin, is regulated directly or indirectly by steroid hormones (10). Regulation of integrin, which normally appears at the time of implantation, may involve both suppression by the sex steroids, estrogen and progesterone, and stimulation by growth factors (14). Integrin is modulated primarily by the growth factor EGF and HB-EGF in human endometrium (15).
Although there is increasing evidence to support a relationship between these proteins (HB-EGF and integrin ) and implantation, the embryonic correlation of HB-EGF with cell adhesion molecules during implantation remains undefined. The present study was designed to investigate the extent to which HB-EGF participates in developmental competence (embryo development to blastocyst, hatching, adhesion and outgrowth) using an in vitro culture system supplemented with HB-EGF or HB-EGF plus anti-HB-EGF antiserum. We also assessed the effects of HB-EGF on integrin expression during embryonic development using immunofluorescence analysis and real-time RT-PCR.
MATERIALS AND METHODS
Mouse Embryo Preparation
ICR mice (Department of Life Science, Hanyang University Seoul, Korea) were bred in a condition-controlled environment with a 14 h light and a 10 h dark cycle. The 6–8-week-old female mice were superovulated by intraperitoneal injection of 5 IU pregnant mare's serum gonadotrophin (PMSG, Sigma) followed 46 h later by 5 IU human chorionic gonadotrophin (hCG, Sigma) and mated overnight with ICR 10–12-week-old males. Mating was confirmed by the presence of a vaginal plug. Embryos were collected at the morula stage by flushing the uterus with HEPES-buffered M2 medium supplemented with 4 mg/mL bovine serum albumin (BSA, Sigma) with a fine Pasteur pipette at 72 h after the hCG injection (16).
HB-EGF Treatment and Embryo Culture In Vitro
To study cooperative interactions among embryos and the role of HB-EGF on adhesion to the uterus, two-cell stage mouse embryos at 48 h were recovered and pooled in M2 medium containing 0.4% bovine serum albumin (BSA). They were washed three times in the same medium. Embryos were cultured in groups of 5 or 10 in microdrops (25 or 50 μL) of KSOM medium, which is a modified version of medium simplex optimized medium (SOM) with supplemented 3 mg/mL BSA under mineral oil (Sigma) in an humidified atmosphere of 5% CO2 in air at 37°C for 96 h in the presence or absence of various concentrations of HB-EGF (0, 10, and 100 ng/ml) (3) and heparin-binding EGF-like growth factor added at the beginning of cultures. Embryos at the early blastocyst stage, expended blastocyst and hatching/hatched blastocyst stage were collected at 48 and 72 h after initiation of culture, respectively. Embryos were observed to monitor their development at the termination of culture. Each experiment was repeated three times.
Assays for Embryo Attachment and Spreading Area
Embryo attachment and spreading assays were performed using cultured chamber slides. Embryos at the 2-cell stage were obtained by flushing oviducts 48 h after hCG injection. Collected embryos were rinsed in KSOM medium supplemented with 0.4% BSA, and each experimental culture group was supplemented with 1% fetal bovine serum (FBS). Five to eight embryos were placed in each chamber slide and cultured for 96 h. Embryo attachment was identified by gentle washing with phosphate-buffered saline (PBS). Non-attached embryos were washed off with PBS by gentle aspiration; embryos that showed no movement upon washing were considered attached. The extent of spreading was determined by photographing embryos at a magnification of ×200 and printing each at the same size. The area of outgrowth was measured using an image-analyz system (Image-Pro PLUS 4.0 Image & Graphics U.S.A). The result for each treatment represents the mean area values for at least 10 embryos.
Isolation of Total RNA
Total RNA extraction was conducted for each 30 embryos for real-time reverse transcription-polymerase chain reaction (RT-PCR). Embryos were washed three times with Ca2+, Mg2+-free PBS and distilled water treated with 0.1% diethyl-pyrocarbonate (DEPC, Sigma). Extraction was performed using TRIzol (Gibco/BRL) modified form of guanidium thiocyanate-phenol-chloroform RNA extraction protocol.
Real-Time Reverse Transcription-Polymerase Chain Reaction (Real Time RT-PCR)
Reverse transcription was carried out in 10 μL extracted RNA (total 30 embryos), 2 μL of 10× PCR buffer, 2 μL of 10 mM dNTP mixtures, 1.25 pmol oligo (dT) adaptor primer, 4 μL of 25 mM MgCl2, 20 U of RNase inhibitor, and 5 U of AMV (Avian myeloblastosis virus) reverse transcriptase XL (Takara). RT reaction was carried out in the 4800 PCR thermal cycler (Takara) using a program with the following parameters: 42°C, 60 min; 99°C, 5 min. After the reaction was completed, samples were either directly used for real-time PCR or stored at −20°C. Expression of integrins αv mRNA, (forward primer, 5′-GTCTTATACAGAGCCAGACCCG-3′; reverse primer, 5′-CTTCACAGTCAGTGTCAGAGGG-3′) and β3 mRNA, (forward primer, 5′-TGCCGGAAGAGCTGTCACTG-3′; reverse primer, 5′-ACTCCCCCTTTGTAGCGGA-3′) levels using real-time RT-PCR were quantified relative to steady-state ribosomal protein (18S) mRNA, (forward primer, 5′-AGATGATCGAGCCGCGC- 3′; reverse primer, 5′-GCTACCAGGGCCTTT GAGATGGA-3′) levels by using real-time PCR following reverse transcription of total RNA. In preliminary studies, 18s mRNA levels in embryos did not differ following culture. Complementary DNA quantification was performed on a DNA Engine 2 fluorescence detection system (MJ research) using the DyNAmo SYBR green qPCR kit (Finnzymes). Reactions were performed in 20 μL with the final reaction mix containing 4 μL DEPC-treated water, 2 μL forward primer (5 pmole), 2 μL reverse primer (2 pmole), 10 μL premix with SYBR Green and 2 μL of cDNA template. The PCR protocol used an initial denaturing step at 95°C for 10 min followed by 45 cycles of denaturation for 30 s at 95°C, annealing of primers at 60°C (18 s), 60°C (integrins, 62°C (integrins β3) for 30 s and extension at 72°C for 30 s. Fluorescence was measured at the end of each cycle during the 72°C step. The final step was the generation of a melting curve in which the temperature was raised from 65 to 95°C at 0.1°C/s, with the fluorescence being constantly measured, followed by a cooling stage of 40°C for 30 s. The relative quantification of gene expression was analyzed the 2−ΔΔCT method (17).
Immunofluorescence Analysis
Surface distribution of integrins on extended/outgrowth blastocysts was examined using immunofluorescence analysis. Embryos were fixed for 15 min at room temperature in fresh 2% paraformaldehyde in PBS, rinsed twice in PBS, once in 0.15 M glycin in PBS, incubated 1 h in PBS containing 0.1% BSA (PBS-BSA), and then in primary antibody diluted 1:800 in PBS-BSA for 1 h. Embryos were then rinsed three times in PBS-BSA, incubated for 2 h in FITC-conjugated secondary antibody diluted 1:800 in PBS-BSA, given final rinses in PBS-BSA, mounted on slides and viewed under microscope. Parallel samples of early blastocysts were fixed in fresh 2% paraformaldehyde in PBS and permeabilized with 0.2% Triton X-100 in PBS prior to staining to examine integrin distribution in inaccessible parts of the embryo (7). Intensity of fluorescence signal was analyzed by image analysis software (Image-Pro PLUS 4.0 Image & Graphics U.S.A).
Role of HB-EGF in Implantation
To determine the role of HB-EGF in implantation, embryos treated with HB-EGF for 24 h were transferred into uterus. After coitus and documentation of a vaginal plug (Day 1), each female underwent midventral laparotomy on Day 3 of pregnancy between 10:00 and 12:00 h. The uterine horn of each mouse was fixed with forceps, and the needle was very slowly introduced as close as possible to the uterine horn into the cervix. Ten embryos were transferred into each uterine horn. Control animals were prepared with each uterine horn receiving 10 embryos cultured in KSOM. On Day 5 of pregnancy, the uterine horns were injected intravenously with 0.1–0.2 mL of a 0.1% solution of chicago sky bule dye (Sigma) dissolved in 0.85% saline. After 30 min, the mouse was killed. Discrete blue bands around the uterus indicated implantation sites (6). The number of implantation sites in the mouse were counted, then uteri were flushed with PBS to count the number of non-implanted embryos (1).
Statistical Analysisz
Each experiment was carried out with at least three replicates. Embryonic development was compared by the Chi-square test. The surface area of trophoblast outgrowth, relative amount of integrin significant.
RESULTS
Effect of HB-EGF on Development of Preimplantation and Outgrowth Embryos in the Mouse
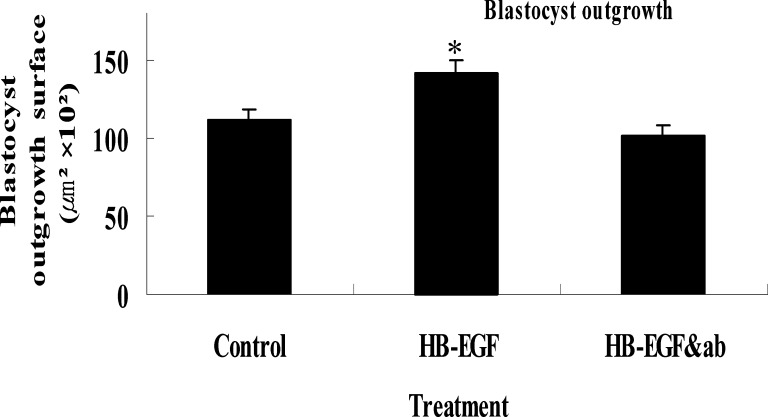
To elucidate the effect of HB-EGF in the development of preimplantation mouse embryos, 2-cell embryos were cultured in the presence or absence of HB-EGF and/or anti-HB-EGF antiserum. HB-EGF from 100 to 1000 ng/mL in KSOM medium showed no significant effects on blastocyst development compared to 10 ng/mL concentration of HB-EGF supplement(data not shown). Co-administration of HB-EGF (10 ng/mL) with anti-HB-EGF antiserum abolished HB-EGF-induced embryo development stimulation. HB-EGF with anti-HB-EGF antiserum showed no significant change in embryo development compared to controls; however, the hatching rates of blastocysts were significantly higher in embryos cultured in media containing HB-EGF at the morula stage than in embryos treated with KSOM only and HB-EGF (10 ng/ml) with anti-HB-EGF antiserum(14.8 and 10.4%, in control). These results indicate that exogenous HB-EGF stimulated development of blastocyst attachment and outgrowth. Effects of HB-EGF on trophoblast outgrowth of mouse blastocyst-derived trophoblast cells has been used by many investigators as an in vitro model for implantation. We used this model to examine effects of HB-EGF on blastocyst attachment and outgrowth in vitro. About 65% of 2-cell stage embryos attached and were outgrown on culture dish surfaces in 1% FBS supplemented media within 96 h of culture, both in the absence and presence of HB-EGF. When 2-cell stage embryos were cultured in KSOM (0.4% BSA) supplemented with 10 ng/mL of HB-EGF and antiserum, the attachment and outgrowth of embryos was similar to that in KSOM (0.4% BSA). However, the addition of HB-EGF to the culture medium significantly accelerated attachment and outgrowth of blastocysts. Also, the mean surface area of trophoblasts displayed similar results (Fig. 1). These results may indicate that exogenous HB-EGF stimulated development of blastocyst attachment and outgrowth (Fig. 2).
Fig. 1.
Blastocyst outgrowth area. Two-cell stage embryos were cultured for 92 h in the presence or absence of HB-EGF or HB-EGF & anti-HB-EGF antiserum. After termination of culture, surface area was measured using image analysis system. Mean±SEM; *p < 0.01.
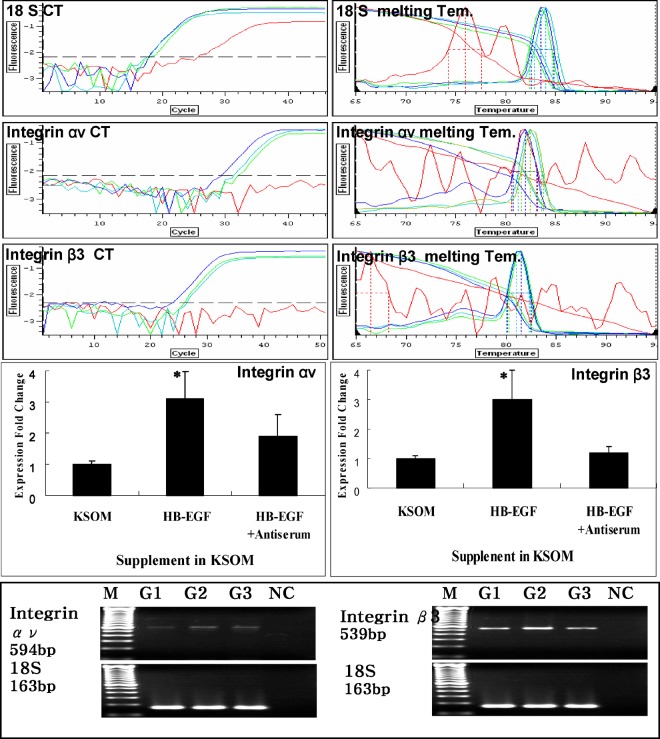
Fig. 2.
Effect of HB-EGF on expression of integrin ανβ3 gene in embryonic development. Green line: post-hCG 48 h 2-cell stage embryos were cultured with KSOM medium; Blue line: post-hCG 48 h 2-cell stage embryos were cultured with KSOM plus HB-EGF (10 ng/mL); Sky blue line: post-hCG 48 h 2-cell stage embryos were cultured with KSOM plus HB-EGF and anti-HB-EGF antiserum (10 ng/mL, 5 μg/μL); Red line: negative control (do not load embryos). Mean±SEM (n=3); *p < 0.05.
Effect of HB-EGF on the Expression of Integrin 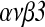 Gene in Early Development
Gene in Early Development
Integrin expression during early development in the mouse was examined using RT-PCR. Integrin and were slightly expressed in the unfertilized egg and late morula stage, but strongly expressed in the blastocyst (7). Expression of these transcripts was greater in the hatching/hatched blastocyst than in the early or late blastocyst, and greatest in the blastocyst outgrowth stage. Real-time RT-PCRs were conducted to investigate the effects of HB-EGF on Integrin and gene mRNA expression in cultured embryos with or without HB-EGF/anti-HB-EGF antiserum(10 ng/mL, 5 μg/μL) in KSOM. Following 96 h of culture, 30 embryos were harvested for real time RT-PCR. In hatching/hatched and outgrowth stage embryos, HB-EGF caused up-regulation of integrin mRNA. Expression fold change analysis of hatching/hatched and outgrowth stage embryos showed up-regulation of integrin αν mRNA expression with HB-EGF treatment, which was significant (p < 0.05), and the relative amount of integrin β3 mRNA expression produced by addition of HB-EGF showed up-regulation in outgrowth stage embryos (p < 0.05).
Immunofluorescence Analysis
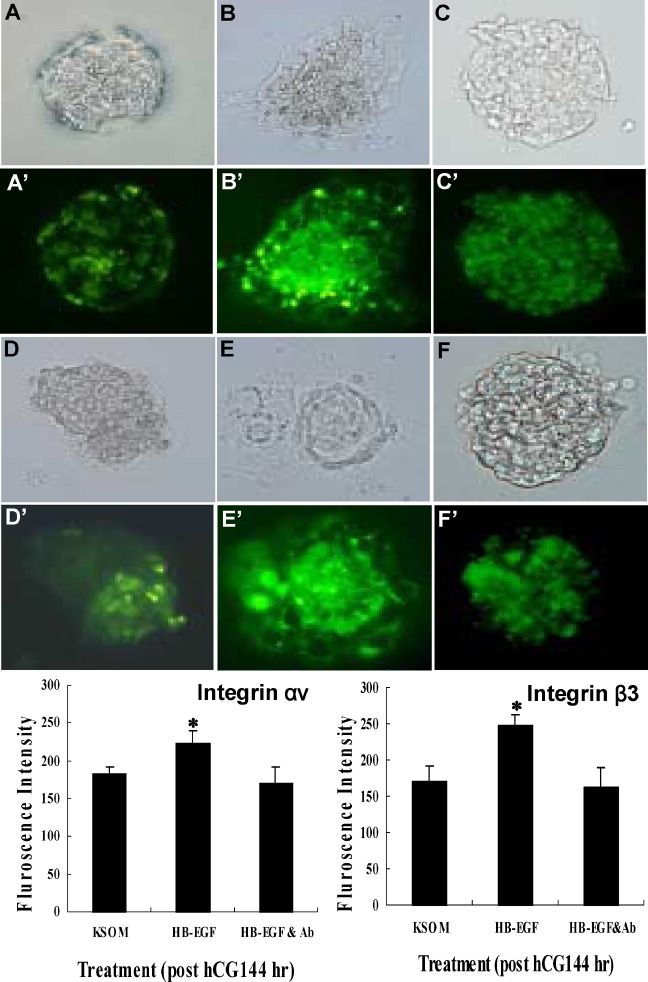
Immunofluorescence analyses were conducted to investigate effects of HB-EGF on Integrin HB-EGF showed higher fluorescence intensity than control (KSOM) and HB-EGF plus anti-HB-EGF antiserum (10 ng/mL, 5 μg/μL) in KSOM (Fig. 3), (Table I).
Fig. 3.
Immunofluorescence intensity and localization of integrin αν and β3 in mouse embryo development. 2-cell embryos (post-hCG 48 h) were cultured in KSOM (A, D), or were cultured in KSOM containing 10 ng/ml HB-EGF (B, E), and 10 ng/ml HB-EGF plus 1 μg/μL of anti-HB-EGF antiserum (C, F). Bright field: A, B, C, D, E, F. Integrin αν antibody stained: A′, B′, C′. Integrin β3 antibody stained: D′, E′, F′. Mean±SEM (n=10); *p < 0.05.
Table I.
Effect of HB-EGF on Attachment and Outgrowth of Mouse Embryos Treated at 2-cell Stage (post hCG 48 h)
| No. (%) of developed embryos | |||||
|---|---|---|---|---|---|
| Treatment | No. of total embryos | Attached embryos | Outgrowth embryos | No. (%) of unattached embryos | |
| Attached/outgrowth (144 h) | Controla | 70 | 27 (36.8±1.8)d | 20 (28.9±1.9) | 23 (37.2±0.3) |
| HB-EGFb | 73 | 21 (28.6±0.9) | 31 (42.5±1.7)* | 21 (28.9±1.6) | |
| HB-EGF& and Abc | 67 | 22 (32.8±0.7) | 19 (28.4±0.9) | 26 (43.5±0.9) | |
aThe control was KSOM only.
bHB-EGF, heparin-binding epidermal growth factor.
cAb, anti-HB-EGF antiserum.
dValues are obtained from three different experiments. Data are expressed as the mean ± SEM.
*Significantly different from control, p < 0.01.
Effect of HB-EGF on Embryonic Implantation
To investigate the role of HB-EGF in embryonic implantation, we transferred embryos treated with/without HB-EGF into uterine horns of mice. The number of implantation sites per mouse was higher in the HB-EGF treated group than in the control (KSOM); in contrast, the number of mice with recoverable embryos was lower in the HB-EGF treated group than in controls (Table II, Fig. 4).
Table II.
Effect of HB-EGF Treatment on Implantation of Embryos
| Treatment | No. of mice | No. (%) of mice with implantation sites | No. of implantation sites/mouse | No. of embryos recovered/mouse |
|---|---|---|---|---|
| Controla | 13 | 10 (77) | 3.4±0.6c | 3.3±0.5 |
| HB-EGFb | 13 | 9 (70) | 4.5±0.7* | 2.3±0.9 |
aThe control was KSOM only.
bHB-EGF, heparin-binding epidermal growth factor.
cValues are obtained from three different experiments. Data are expressed as the mean±SEM.
*Significantly different from control, p < 0.05.
Fig. 4.
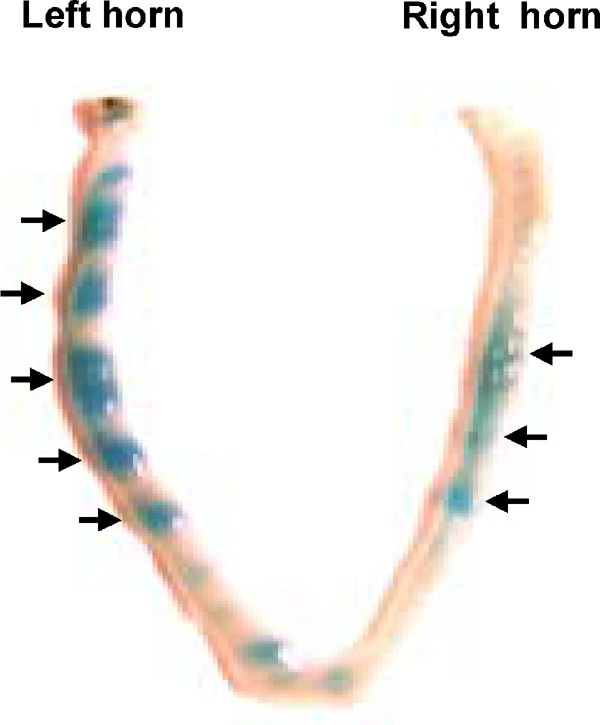
Comparison of implantation sites on the morning of gestation day 3 in mouse uterus for transferred HB-EGF treated or not treated embryos. The number of implantation sites in the treatment group was compared to the number in the control group (KSOM only culture). Uterus transfer with HB-EGF treated embryos is shown in left horn; a control is shown in right horn.
DISCUSSION
Several previous reports supported that HB-EGF might play an important role in blastocyst growth and implantation; this adhesive molecule is also involved in contact/adhesion between the blastula and the endometrial epithelium. Reciprocal expression of integrin in the blastula suggests the involvement of integrin in primary adhesion mediated by ligands such as osteopontin, vitronectins, fibrinogen, fibronectins, and von Willebrand factor (10).
In our study, HB-EGF influenced blastocyst development in-vitro and improved implantation efficiency by up-regulating integrin development and hatching rate. In addition, HB-EGF treatment of embryos increased the outgrowth rate significantly, although, under our culture conditions, any dose-dependent change of development rate was not shown. Specific-dose of HB-EGF operate embryo development at the onset of implantation (16).
HB-EGF modulates epithelial integrins in esophageal and breast cancer cells (20) and was found to specifically increase integrin in variety of other cell types (14), but integrin expression produced by HB-EGF in mouse embryos remains elevated. In the present study, we found that HB-EGF up-regulated expression of integrin, which is believed to be important for implantation in many species for hatching/hatched and outgrowth stage embryos. We speculate that soluble HB-EGF expressed by endometrial cells induces expression of integrin in mouse embryos as a paracrine factor.
By using uterine transfer of HB-EGF treated embryos and in vitro culture, we also found that HB-EGF was involved in embryo attachment and adhesion during implantation. Schmidt et al. reported that blocking HB-EGF or other cell adhesion molecules influenced cell attachment and adhesion. We demonstrated that HB-EGF is involved in the spreading of mouse embryos on decidual cells (21) in in vitro outgrowth assay. The previous finding, as well as our present results suggest that HB-EGF may be important in mediating implantation and embryo–uterine attachment in mouse embryos. However, HB-EGF neutralization by anti-serum in mice does not obviously inhibit the implantation process. Besides HB-EGF, the mouse uterus also expresses Er, BCT and NDF during the implantation period (22), and these molecules can interact with HB-EGF receptors (erbb1, erbb4). If HB-EGF is blocked by anti-serum neutralization, it is supposed that substitution by other growth factors is able to maintain implantation. So, the expression of other growth factors of the EGF family might offer a protective mechanism to ensure a high probability of embryo development and implantation.
CONCLUSIONS
These results indicate that HB-EGF affects embryo development during the hatching/hatched embryo stage to the outgrowth embryo stage. This growth factor stimulates expression of integrin ανβ3 in vitro. Therefore, establishment of implantation may involve a HB-EGF-mediated paracrine mechanism. Further studies will be necessary to demonstrate whether a coordinated reduction in HB-EGF correlates with a decline in other integrin family members and whether such changes are truly associated with implantation problems or recurrent pregnancy loss.
Footnotes
J.J. Lim and D.R. Lee contributed equally to this work.
REFERENCES
- 1.Tamada H, Higashiyama C, Takano H, Kwate N, Inaba T, Sawada T. The effect of heparin-binding epidermal growth factor-like growth factor on preimplantation-embryo development and implantation in the rat. Life Sci. 1999;64:1967–1973. doi: 10.1016/S0024-3205(99)00128-9. [DOI] [PubMed] [Google Scholar]
- 2.Higashiyama S, Abraham JA, Miller J, Fiddes JC, Klagsbrun M. A heparin-binding growth factor secreted by macrophage-like cells that is related to EGF. Science. 1991;251:936–939. doi: 10.1126/science.1840698. [DOI] [PubMed] [Google Scholar]
- 3.Das SK, Wang XN, Paria BC, Damm D, Abraham JA, Klagsbrun M, Andrews GK, Dey SK. Heparin-binding EGF-like growth factor gene is induced in the mouse uterus temporally by the blastocyst solely at the site of its apposition: a possible ligand for interaction with blastocyst EGF-receptor in implantation. Development. 1994;120:1071–1083. doi: 10.1242/dev.120.5.1071. [DOI] [PubMed] [Google Scholar]
- 4.Aviezer D, Yayon A. Heparin-dependent binding and auto-phosphorylation of epidermal growth factor (EGF) receptor by heparin-binding epidermal growth factor-like growth factor but not EGF. Proc Natl Acad Sci. 1994;91:12173–12177. doi: 10.1073/pnas.91.25.12173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paria BC, Elenius K, Klagsbrun M, Dey SK. HB-EGF interacts with mouse blastocysts independently of ErbB1: A possible role for heparin sulfate proteoglycans and ErbB4 in blastocyst implantation. Development. 1999;126:1997–2005. doi: 10.1242/dev.126.9.1997. [DOI] [PubMed] [Google Scholar]
- 6.Paria BC, Huet-Hudson YM, Das SK. Blastocyst's state of activity determines the “window” of implantation in the receptive mouse uterus. Dev Biol. 1993;90:10159–10162. doi: 10.1073/pnas.90.21.10159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sutherland AE, Calarco PG, Damsky CH. Developmental regulation of integrin expression at the time of implantation in the mouse embryo. Development. 1993;119:1175–1186. doi: 10.1242/dev.119.4.1175. [DOI] [PubMed] [Google Scholar]
- 8.Albelda SM, Buck CA. Integrins and other cell adhesion molecules. FASEB J. 1990;11:2868–2880. [PubMed] [Google Scholar]
- 9.Tabibzadeh S. Patterns of expression of integrin molecules in human endometrium throughout the menstrual cycle. Hum Reprod. 1992;6:876–882. doi: 10.1093/oxfordjournals.humrep.a137753. [DOI] [PubMed] [Google Scholar]
- 10.Lessey BA. Regulated expression of HB-EGF in the human endometrium: a potential paracrine role during implantation. Mol Reprod Dev. 2002;62:446–455. doi: 10.1002/mrd.10129. [DOI] [PubMed] [Google Scholar]
- 11.Simon C, Martin JC, Pellicer A. Paracrine regulators of implantation. Baillieres Best Pract Res Clin Obstet Gynaecol. 2000;14(5):815–826. doi: 10.1053/beog.2000.0121. [DOI] [PubMed] [Google Scholar]
- 12.Hierck BP, Thorsteinsdottir S, Niessen CM, Freund E, Iperen LV, Feyen A, Hogervorst F, Poelmann RE, Mummery CL, Sonnenberg A. Variants of the alpha 6 beta 1 laminin receptor in early murine development: distribution, molecular cloning and chromosomal localization of the mouse integrin alpha 6 subunit. Cell Adhes Commun. 1993;1:33–53. doi: 10.3109/15419069309095680. [DOI] [PubMed] [Google Scholar]
- 13.Cullinan E, Illera MJ, Gui Y, Yuan L, Beyler SA, Lessey BA. Blockade of the alpha(v)beta(3) integrin adversely affects implantation in the mouse. Biol Reprod. 2000;62:1285–1290. doi: 10.1095/biolreprod62.5.1285. [DOI] [PubMed] [Google Scholar]
- 14.Somkuti SG, Yuan L, Fritz MA, Lessey BA. Epidermal growth factor and sex steroids dynamically regulate a marker of endome-trial receptivity in ishikawa cells. J Clin Endocrinol Metab. 1997;82:2192–2197. doi: 10.1210/jc.82.7.2192. [DOI] [PubMed] [Google Scholar]
- 15.Apparao KBC, Murray MJ, Fritz MA, Meyer WR, Chambers AF, Truong PL, Lessey BA. Osteopontin and its receptor (v(3 integrin are co expressed in the human endometrium during the menstrual cycle but regulated differentially. J Clin Endocrinol Metab. 2001;86:4991–5000. doi: 10.1210/jc.86.10.4991. [DOI] [PubMed] [Google Scholar]
- 16.Hong SH, Nah HY, Lee JY, Kim JH, Kim CH, Chea HD, Kang BM, Kim MK. Effect of heparin-binding growth factor on the expression of MMP-9 and ATPase γ-subunit mRNA in the mouse embryo. Kor J Fertil Steril. 2001;28:87–93. [Google Scholar]
- 17.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 18.Seshagiri PB, Mishra A, Ramesh G, Rao RP. Regulation of peri-implantation embryo development in golden hamster: role of growth factors. J Reprod Immunol. 2002;53:203–213. doi: 10.1016/S0165-0378(01)00086-9. [DOI] [PubMed] [Google Scholar]
- 19.Martin KL, Barlow DH, Sargent IL. HB-EGF significantly improves human blastocyst development and hatching in serum-free medium. Hum Reprod. 1998;13:1645–1652. doi: 10.1093/humrep/13.6.1645. [DOI] [PubMed] [Google Scholar]
- 20.Narita T, Kawakami-Kimura N, Sato M, Matsuura N, Higashiyama S, Taniguchi N, Kannagi R. Alteration of integrins by heparin-binding EGF-like growth factor in human breast cancer cells. Oncology. 1996;53:374–381. doi: 10.1159/000227591. [DOI] [PubMed] [Google Scholar]
- 21.Yoshimura M, Nishikawa A, Nishiura T, Ihara Y, Kanayama Y, Matsuzawa Y, Taniguchi N. Cell spreading in Colo 201 by staurosporin is alpha 3 beta 1 integrin-mediated with tyrosine phosphorylation of Src and tensin. J Biol Chem. 1995;270(5):2298–2304. doi: 10.1074/jbc.270.5.2298. [DOI] [PubMed] [Google Scholar]
- 22.Lim H, Das SK, Dey SK. ErbB genes in the mouse uterus: cell-specific signaling by epidermal growth factor (EGF) family of growth factors during implantation. Dev Biol. 1998;204:97–110. doi: 10.1006/dbio.1998.9072. [DOI] [PubMed] [Google Scholar]