Abstract
Purpose: To prospectively evaluate serum and follicular fluid leptin, estradiol, and progesterone levels during in vitro fertilization.
Methods: Prospective observational study measuring serum levels at six points during the IVF cycle and follicular fluid at the time of retrieval.
Results: Serum leptin and estradiol levels both significantly increased for the individual patients during the IVF stimulation process. None of the leptin levels differed based on pregnancy outcome. BMI significantly correlated with all leptin levels. Follicular fluid estradiol correlated with serum estradiol only in pregnant patients (r = 0.97, p<0.01) and was unrelated in non-pregnant patients (r=−0.15, p=0.81).
Conclusion: Serum and follicular leptin levels are highly correlated. Leptin levels increase during the IVF cycle and vary between patients based on maternal BMI, but do not correlate with other serum hormone levels or pregnancy outcome. Pregnancy outcome success was reflected in the relationship between follicular fluid and serum levels of estradiol, independent of leptin levels.
Keywords: Leptin, Follicular fluid, Infertility, In vitro fertilization, Gonadotropins, Pregnancy, Implantation, Estradiol, Progesterone
Introduction
Leptin, a protein secreted from adipocytes and many other tissues of mammals, is known to induce satiety. Leptin is found to a greater extent in obese people than in those with a normal body mass index (BMI). Leptin has been shown to affect the reproductive axis in various ways. Studies have documented leptin’s importance in initiating puberty, and its important relationship with gonadotropins and other hormones [1–3]. Leptin has been found in reproductive tissues to include the placenta, endometrium and ovary [3]. Recently, serum leptin levels were demonstrated to fluctuate in a manner similar to other hormones of the female reproductive axis [4].
Henson et al. demonstrated an increase in leptin receptors around the time of ovulation in humans, leading to the hypothesis that this protein may be necessary for peri-implantational events [2]. Another study has shown a possible relationship with the implantation process by demonstrating an increase in leptin receptor levels in healthy non-pregnant women during the menstrual cycle reaching a peak near ovulation, then declining at menstruation [5]. Likewise, other studies have demonstrated a relationship between women with polycystic ovarian syndrome and serum leptin levels [6, 7].
Recent studies have evaluated relationships of follicular fluid and serum leptin levels with IVF outcomes. Some studies have shown no correlation of follicular fluid or serum leptin with IVF outcome or sex-steroid levels [8, 9]. This is in contrast to other studies which found that low serum and follicular fluid levels of leptin negatively correlated with embryo quality, implantation rates, and pregnancy rates [10–13]. Follicular fluid concentrations of leptin, estradiol, and progesterone have been evaluated by few studies [10–13].
The role and predictive value of serum and follicular fluid leptin levels remains to be fully elucidated. Based on the available literature, we designed our study to evaluate serum and follicular fluid leptin, estradiol and progesterone levels in relationship to patient characteristics, serum hormone levels and IVF success rates. Our goal was to evaluate serum leptin throughout the gonadotropin stimulation process at six different points and follicular fluid leptin, estradiol and progesterone levels at the time of ooctye retrieval in order to determine how leptin changes and if leptin correlates with steroid hormones, IVF stimulation and IVF outcomes.
Materials and methods
Patients between the ages of 19 and 42 years were included independent of their diagnoses or reproductive history. Because of the poor response documented in the literature, patients with elevated FSH levels (≥12 mIU/mL) and women >42 years of age were excluded [16–18].
All patients started oral contraceptive pills (OCs) (30 μg ethinyl estradiol, 0.15 mg norgestrel) the evening of menstrual day 3. The patients continued the OCs for 21 days prior to pituitary suppression with GnRH-a (500 μg) (Luprolide acetate, Lupron; TAP Pharmaceuticals, North Chicago, IL, USA). There was a seven day overlap of the GnRH-a with the OCs. After 14 days of GnRH-a, the dose was decreased to 250 μg and stimulation with exogenous gonadotropins was initiated 5 days later. When the largest cohort of follicles reached the 16 to 18 mm range, a single 10,000 IU intramuscular dose of human chorionic gonadotropin (hCG) were administered. Transvaginal follicular aspiration took place 35 to 36 h later. On post-retrieval day 3 or 5, embryos were transferred vaginally with transabdominal ultrasound guidance using a Wallace transfer catheter (Cooper Surgical, Shelton, CT).
The study protocol was approved by the human use committee at Tripler Army Medical Center, Honolulu, HI. Investigators adhered to policies for protection of human subjects as prescribed in 45 CFR 46.
Fasting serum leptin concentrations were measured along with estradiol concentrations on IVF baseline date (defined as the day the GnRH-a dose was decreased to 250 μg), day 6 of gonadotropin stimulation, day of human chorionic gonadotropin (hCG) administration, day after hCG administration, 7 days after oocyte retrieval, and 14 days after oocyte retrieval. All serum measurements occurred in the fasting state between 7:00 am and 8:00 am. Follicular fluid levels of leptin were measured for each patient on the day of oocyte retrieval. All vaginal oocyte retrievals occurred between 7:00 am and 10:00 am. As there are multiple patients getting vaginal oocyte retrievals per day it was impossible to have all done between 7:00 am and 8:00 am. The largest follicle was aspirated into an empty sterile tube and leptin concentration of the follicular fluid was assessed.
The main outcome measures of serum leptin and follicular fluid leptin, estradiol and progesterone concentrations were assessed relative to the secondary outcome measures of IVF stimulation characteristics and IVF outcomes (pregnancy, cancellation, live birth, and pregnancy loss). BMI was calculated on the patients’ baseline evaluation day after pituitary suppression with oral contraceptives and prior to the start of gonadotropin stimulation. Transvaginal ultrasound to assess basal antral follicle number and ovarian volume was performed on day 3 of the patient’s menstrual cycle the month prior to IVF. All procedures (ultrasounds, IVF stimulations, oocyte retrievals, and embryo transfers) were performed by the senior author (JLF).
Leptin concentrations were compared with respect to patient’s age, BMI, diagnosis, number of follicles, number of oocytes retrieved, number of embryos, basal serum labs (FSH, LH, and estradiol (E2)), peak estradiol level (defined as the level of estradiol on the day of human chorionic gonadotropin administration), ampules of gonadotropins administered, days of stimulation, and outcome rates (implantation, pregnancy, cancellation, miscarriage, and live birth). All basal serum hormone measurements were done during a non-medicated cycle within 3 months of the IVF cycle initiation.
Pregnancy was confirmed by a rising serum quantative β-hCG level at 4 weeks of gestation followed by sonographic confirmation of cardiac activity at 5 to 6 weeks gestation. Patients were canceled for failing to produce ≥3 expanding follicles or failure to respond to gonadotropins with an adequate estradiol response of at least 300 pg/mL. The FSH, LH, and E2 assays were all done with the Advia Centaur chemiluminescence system (Bayer Diagnostics, Tarrytown, New York). The inter- and intra-assay coefficients of variation (CV) were 2.0 to 2.9% and 0.3 to 2.7% for FSH, 3.2 to 3.0% and 1.5 to 2.9% for LH, and 5.3 to 11.3% and 4.6 to 6.7% for E2.
Progesterone was assayed using ACE™ Enzyme Immunoassay (EIA) kits (Cat. No. 582601, Cayman Chemical Company, Ann Arbor, MI) based on the competitive binding of free progesterone and a progesterone tracer (progesterone linked to an acetylcholinesterase molecule) for limited progesterone-specific antiserum binding sites. Limit of detection for the assay was 0.3 pg/mL, and inter- and intra-assay CV were less than 10%.
Serum and follicular fluid leptin levels were determined by leptin immunometric EIA kits (Cat. No. 500600, Cayman Chemical, Ann Arbor, MI). The leptin assay employed a double-antibody sandwich technique and horseradish peroxidase-antibody conjugate. The limit of detection was 1.0 ng/mL, and inter- and intra-assay CV were less than 9%. Each well of the 96-well plate supplied with the kit was coated with a monoclonal antibody specific for human leptin (leptin capture antibody). This antibody binds any human leptin introduced into the well. All samples and standards were assayed in triplicate.
This was a prospective observational pilot study; therefore, no power analysis for sample size was performed. The Kolmogorov-Smirnov test was used to test for normality. For normally distributed data, a t-test was used to compare the mean values between two groups. For data that were not normally distributed, a Mann-Whitney rank sum test was used to compare the mean values between two groups.
A Kruskal-Wallis one-way analysis of variance on ranks was used to compare outcomes among multiple groups whose data were not normally distributed. Dunn’s method was used for pair-wise multiple comparison. A Friedman repeated measures one-way analysis of variance on ranks was used to compare the paired changes of leptin levels among the patients. For pairwise multiple comparison, Ducan’s method was utilized.
Differences in outcome rates were analyzed using a Chi-square or two-tailed Fisher exact test where appropriate. Univariate analysis included: regression and correlation coefficients examining the association of leptin levels with parameters of IVF outcome and response.
An alpha error of 0.05 was considered significant for all comparisons. Relative risk and 95% confidence intervals are displayed where appropriate. All data are reported as means with their associated standard deviations.
Results
A total of 10 consecutive women were counseled, consented, and enrolled into the study during November and December of 2005 at Tripler Army Medical IVF Center, Honolulu, HI. All patients were followed for a single IVF cycle. There were no patient drop-outs and no cycle cancellations. Five patients had no prior conceptions. Of the five with previous conceptions, three had successful prior pregnancies. Infertility diagnosis included male factor (n = 8), previous tubal ligation (n = 1), and unexplained (n = 1).
The demographic data are shown in Table 1. Out of the 10 women enrolled, 5 became pregnant. Of the five who became pregnant, one had an early spontaneous loss. No differences were noted between the pregnant and non-pregnant women with regards to age, weight, BMI, years of infertility, gravidity, or parity (Table 1).
Table 1.
Population demographics of patients undergoing IVF. The patients were subsequently subdivided into pregnant patients and non-pregnant patients.
| All patients (N = 10) | Pregnant (N = 5) | Not pregnant (N = 5) | *P value | |
|---|---|---|---|---|
| Age (years) | 33.7 ± 2.8 | 34.6 ± 2.6 | 32.8 ± 2.7 | 0.36 |
| Weight (lbs) | 162.7 ± 37.5 | 193.0 ± 36.5 | 145.0 ± 20.6 | 0.07 |
| BMI (kg/m2) | 27.2 ± 5.2 | 30.3 ± 5.4 | 24.6 ± 3.2 | 0.12 |
| Years of infertility | 3.1 ± 2.5 | 2.4 ± 0.8 | 3.8 ± 3.3 | 0.44 |
| Gravidity | 1.3 ± 1.9 | 1.2 ± 1.8 | 1.0 ± 1.8 | 0.89 |
| Parity | 0.5 ± 0.9 | 0.4 ± 0.5 | 0.6 ± 1.2 | 0.77 |
Note: Values are means ± SD.*Significant p-values (≤0.05).
Although mean leptin levels increased during gonadotropin stimulation the levels remained clustered for the individual patient. Using a Friedman repeated measures one-way analysis of variance, mean leptin levels significantly increased throughout the IVF cycle, from the time of pituitary suppression until the day after hCG administration (Table 2).
Table 2.
Comparison of serum and follicular fluid leptin levels throughout the IVF stimulation cycle between patients who became pregnant and those not pregnant
| Total population leptin | Leptin levels (ng/mL) in | Leptin levels (ng/mL) in | ||
|---|---|---|---|---|
| Sample | levels (ng/mL) | pregnancy cycles | non-pregnancy cycles | *P value |
| Baseline | 19.3 ± 13.0a | 18.3 ± 7.5a | 20.3 ± 17.9a | 0.83 |
| Day 6 | 23.0 ± 14.6b | 24.6 ± 10.6b | 21.3 ± 18.9a | 0.75 |
| Day of hCG | 29.8 ± 22.4c | 29.0 ± 16.4c | 30.6 ± 29.3b | 0.92 |
| Day of hCG +1 | 35.2 ± 27.2d | 39.1 ± 23.2d | 31.2 ± 33.0b | 0.68 |
| Follicular fluid | 31.0 ± 23.8c | 30.9 ± 30.8c | 31.1 ± 18.0b | 0.99 |
| Leptin day 7 after oocyte retrieval | 32.9 ± 29.1c | 30.8 ± 19.0c | 35.56 ± 42.0c | 0.83 |
| Leptin day 14 after oocyte retrieval | 26.4 ± 17.8c | 26.8 ± 8.6c | 25.9 ± 27.2b | 0.95 |
Note: Values are means ± SD (ng/mL).a,b,c,dValues in each column with different letters are significantly different based on Friedman repeated measures one-way analysis of variance on ranks with pairwise multiple comparison by Duncan’s method (p<0.05).*Reflects the comparison of the pregnant cycles to the non-pregnant cycles. Significant p-values (≤0.05).
No difference in serum leptin levels was noted at any point in the cycle between those patients who became pregnant and those who did not (Table 2). Although leptin levels did not vary based on pregnancy outcome for the individual patient, there was a significant increase in leptin levels during the IVF stimulation in both the pregnant and non pregnant patients (Table 2). Using a Friedman repeated measures one-way analysis of variance with pairwise multiple comparison by Duncan’s method, a significant increase in leptin levels was noted for the non pregnant patients (p < 0.01) and the pregnant patients (p < 0.05) (Table 2).
Baseline serum and follicular fluid leptin levels for each patient correlated significantly with their leptin levels throughout the cycle (day six, day of hCG, day of hCG+1, day of oocyte retrieval, and post retrieval days 7 and 14) (p < 0.01 for all values) (Table 3). The baseline serum and follicular fluid leptin also correlated significantly with the patients BMI (p < 0.01) (Table 3). With the exception of BMI, no significant correlations were found between baseline serum leptin levels and patient demographics, IVF pre-stimulation parameters, or IVF stimulation parameters. Linear regression analysis for all other serum leptin levels revealed similar findings with significant correlations with all leptin levels and BMI.
Table 3.
Linear regression analysis of baseline serum and follicular fluid leptin with serum leptin levels throughout the IVF cycle, patient characteristics, and cycle characteristics
| Leptin serum baseline | Follicular fluid leptin | |
|---|---|---|
| Leptin day 6 | r = 0.95, p < 0.01* | r = 0.98, p < 0.01* |
| Leptin day of hCG | r = 0.91, p < 0.01* | r = 0.99, p < 0.01* |
| Leptin day of hCG + 1 | r = 0.92, p < 0.01* | r = 0.98, p < 0.01* |
| Leptin day 7 after retrieval | r = 0.95, p < 0.01* | r = 0.96, p < 0.01* |
| Leptin day 14 after retrieval | r = 0.93, p < 0.01* | r = 0.94, p < 0.01* |
| Leptin follicular fluid | r = 0.96, p < 0.01* | NA |
| Progesterone follicular fluid | r = 0.08, p = 0.79 | r = –0.03, p = 0.94 |
| Estradiol follicular fluid | r = –0.43, p = 0.21 | r = –0.41, p = 0.23 |
| Estradiol baseline | r = –0.02, p = 0.96 | r = –0.13, p = 0.75 |
| Estradiol day 6 | r = –0.16, p = 0.66 | r = –0.25, p = 0.48 |
| Peak Estradiol | r = –0.08, p = 0.84 | r = –0.13, p = 0.71 |
| Day 3 FSH | r = –0.18, p = 0.62 | r = –0.04, p = 0.91 |
| Day 3 LH | r = 0.16, p = 0.66 | r = 0.24, p = 0.50 |
| Age | r = 0.15, p = 0.69 | r = 0.02, p = 0.95 |
| Follicles | r = 0.04, p = 0.91 | r = –0.01, p = 0.98 |
| Oocytes | r = 0.07, p = 0.84 | r = 0.04, p = 0.92 |
| Mature oocytes | r = 0.16, p = 0.65 | r = 0.08, p = 0.82 |
| Days of stimulation | r = 0.08, p = 0.82 | r = 0.19, p = 0.61 |
| BMI | r = 0.81, p < 0.01* | r = 0.81, p < 0.01* |
*Significant p-values (<0.05).
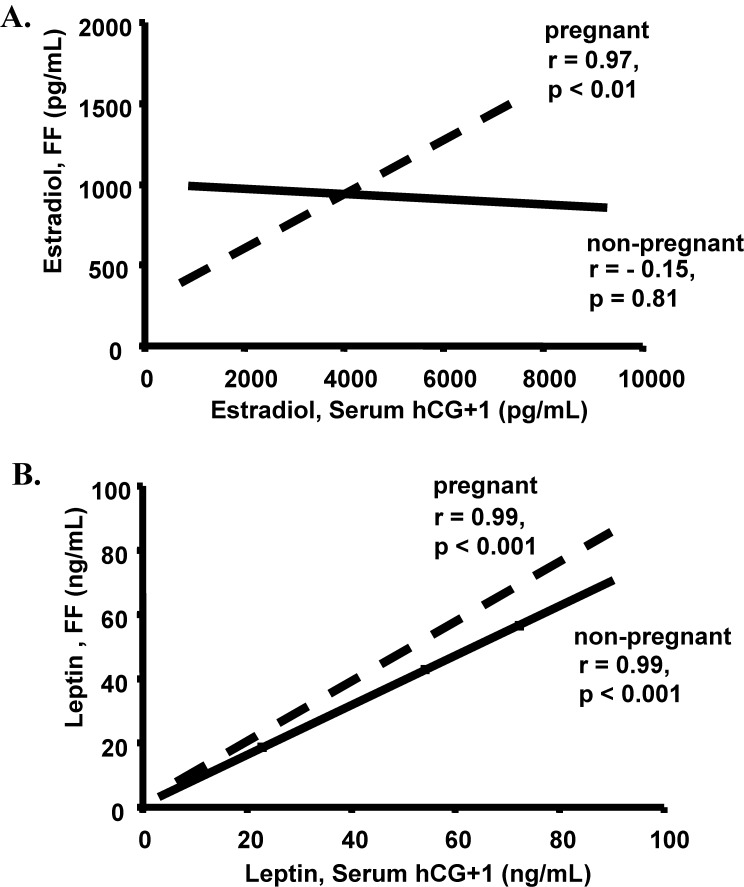
With linear regression analysis we evaluated the correlation between follicular fluid and serum hormone concentrations in the pregnant and non-pregnant patients. All follicular fluid leptin levels significantly correlated with serum leptin levels in both the pregnant and non-pregnant patients (r ≥ 0.89, p < 0.01). When compared to the follicular fluid estradiol levels, the peak estradiol levels on the day after hCG injection showed a significant positive correlation in the pregnant patients (r = 0.97, p < 0.01) and a non-significant negative correlation in the non-pregnant patients (r = −0.15, p = 0.81) (Fig. 1).
Fig. 1.
Bivariate fit of (A) follicular fluid estradiol and serum estradiol on day after hCG administration (hCG + 1) demonstrated a significant positive correlation in the pregnant (dashed line) patients (r = 0.97, p < 0.01) but no correlation in the non-pregnant (solid line) patients (r = −0.15, p = 0.81) and (B) follicular fluid leptin and serum leptin on day after hCG administration (hCG + 1) demonstrated a significant positive correlation in the pregnant (dashed line) patients (r = 0.99, p < 0.001) and in the non-pregnant (solid line) patients (r = 0.99, p < 0.001)
Discussion
Consistent with many previous reports, we found that BMI significantly correlated with leptin levels in both the serum and follicular fluid. While we could find no correlation with either serum or follicular fluid leptin levels and IVF outcomes, we did note that leptin significantly increased in each patient throughout the IVF stimulation cycle and remained elevated up to two weeks after the IVF cycle. This is consistant with a prior study that measured leptin at three points during ovarian hyperstimulation and gives further insight into leptin levels at multiple treatment points during an IVF cycle [14].
As with prior studies, we found no relationship between serum or follicular fluid leptin levels on IVF pregnancy rates [8, 9]. Some prior studies have found a negative correlation with leptin levels and IVF outcomes [10–13]. The primary difference with our study compared to previous studies was the number of data points for collected leptin samples. While previous studies typically measured leptin at two points during the IVF cycle, we looked at six different data points.
Since several recent studies have measured follicular fluid leptin levels, we also measured follicular fluid estradiol and progesterone concentrations. No correlations were found between follicular fluid leptin, estradiol, or progesterone concentrations. This in contrast to one prior study, which showed follicular fluid leptin to be positively correlated to follicular fluid estradiol and progesterone [15]. Importantly, follicular fluid and serum estradiol levels had a significant positive correlation in pregnant patients but not in the non-pregnant patients (Fig. 1). This seems to suggest that the oocytes destined to make good embryos and ultimately an ongoing pregnancy have an intrafollicular estradiol environment that is similar to the serum estradiol levels. Likewise, in the oocytes that are destined to make embryos that do not implant have a mismatch with the serum and follicular fluid estradiol concentrations.
The primary weakness of our study was the small sample size. With ten patients enrolled, we lacked the power to find significant differences in IVF cycle outcomes. Strengths of our study included its prospective design and multiple data points for leptin measurement. A single board certified REI provider (JLF) ran the entire stimulation cycle, retrieval, and transfer procedures and a single laboratory director provided post-retrieval oocyte and embryo management, in effect negating multiple provider variables. All laboratory samples were batched, frozen, and ran together in triplicate to minimize error.
In summary, serum and follicular fluid leptin levels correlated with BMI and significantly increased throughout the IVF cycle. Our findings did not confirm a predictive value of leptin on IVF success; however, pregnancy success was reflected by a strong correlation between follicular fluid and serum estradiol concentrations. No relationship between leptin, estradiol and progesterone levels in the follicular fluid was noted. Whether leptin plays an active role in fertility or is simply a reflection of BMI and ovarian stimulation remains to be seen. Further research is needed to understand the complete role leptin has in human fertility and its value as a predictor of IVF success. Likewise, future research should focus on the correlation between follicular fluid estradiol production and serum estradiol levels predicting pregnancy outcomes.
Acknowledgment
This study was supported by the Department of Clinical Investigation at Tripler Army Medical Center, Honolulu, HI.
Footnotes
The views expressed in this manuscript are those of the authors and do not reflect the official policy or position of the Department of the Army, Department of Defense, or the U.S. Government.
References
- 1.Garcia-Mayor RV, Andrade MA, Rios M, Lage M, Dieguez C, Casanueva FF. Serum leptin levels in normal children: Relationship to age, gender, body mass index, pituitary-gonadal hormones, and pubertal stage. J Clin Endocrinol Metab. 1997;82:2849–55. doi: 10.1210/jc.82.9.2849. [DOI] [PubMed] [Google Scholar]
- 2.Henson MC, Castracane VD, O’Neil JS, Gimpel T, Swan KF, Green AE, Shi W. Serum leptin concentrations and expression of leptin transcripts in placental trophoblast with advancing baboon pregnancy. J Clin Endocrinol Metab. 1999;84:2543–9. doi: 10.1210/jc.84.7.2543. [DOI] [PubMed] [Google Scholar]
- 3.Sivan E, Whittaker PG, Sinha D, Homko CJ, Lin M, Reece EA, Boden G. Leptin in human pregnancy: The relationship with gestational hormones. Am J Obstet Gynecol. 1998;179:1128–32. doi: 10.1016/S0002-9378(98)70118-8. [DOI] [PubMed] [Google Scholar]
- 4.Kitawaki J, Koshiba H, Koshiba H, Tsukamoto K, Honjo H. Expression of leptin receptor in human endometrium and fluctuation during the menstrual cycle. J Clin Endocrinol Metab. 2000;89:1946–50. doi: 10.1210/jc.85.5.1946. [DOI] [PubMed] [Google Scholar]
- 5.Gonzalez RR, Simon C, Caballero-Campo P, Norman R, Chardonnens D, Devoto L, Bischof P. Leptin and reproduction. Human Reproduction Update. 2000;6:290–300. doi: 10.1093/humupd/6.3.290. [DOI] [PubMed] [Google Scholar]
- 6.Imani B, Eijkemans MJ, de Jong FH, Payne NN, Bouchard P, Giudice LC, Fauser BC. Free androgen index and leptin are the most prominent endocrine predictors of ovarian response during clomiphene citrate induction of ovulation in normogonadotropic oligoamenorrheic infertility. J Clin Endocrinol Metab. 2000;85:676–82. doi: 10.1210/jc.85.2.676. [DOI] [PubMed] [Google Scholar]
- 7.Laughlin Ga, Morales AJ, Yen SS. Serum leptin levels in women with polycystic ovary syndrome: the role of insulin resistance/hyperinsulinemia. J Clin Endocrinol Metab. 1997;82:1692–6. doi: 10.1210/jc.82.6.1692. [DOI] [PubMed] [Google Scholar]
- 8.Chen R, Fisch B, Ben-Haroush A, Kaplan B, Hod M, Orvieto R. Serum and follicular fluid leptin levels in patients undergoing controlled ovarian hyperstimulation for in vitro fertilization. Clin Exper Obstet Gynecol. 2004;31:103–6. [PubMed] [Google Scholar]
- 9.Robins JC, Srivastava R, Mershon JL, Thomas MA. Association of leptin with poor ovarian hyperstimulation during in vitro fertilization. J Reprod Med. 2005;50:356–60. [PubMed] [Google Scholar]
- 10.Anifandis G, Koutselini E, Louridas K, Liakopoulos V, Leivaditis K, Mantzavinos T, Siotopoulou D, Vamvakopoulos N. Estradiol and leptin as conditional prognostic IVF markers. Reproduction. 2005;129:531–4. doi: 10.1530/rep.1.00567. [DOI] [PubMed] [Google Scholar]
- 11.Asimakopoulos B, Nikolettos N, Papachristou DN, Simopoulou M, Al-Hasani S, Diedrich K. Follicular fluid levels of vascular endothelial growth factor and leptin are associated with pregnancy outcome or normal women participating in intracytoplasmic sperm injection cycles. Physiological Research. 2005;54:263–70. [PubMed] [Google Scholar]
- 12.Butzow T, Moilanen J, Lehtovitra M, Tuomi T, Hovatta O, Siegber R, Nilson CG, Apter D. Serum and follicular fluid leptin during in vitro fertilization: Relationship among leptin increase, body fat mass, and reduced ovarian response. J Clin Endocrinol Metab. 1999;84:3135–9. doi: 10.1210/jc.84.9.3135. [DOI] [PubMed] [Google Scholar]
- 13.Gurbuz B, Yalti S, Ficicioglu C, Tasdemir S. The relationship of serum and follicular fluid leptin and ovarian steroid levels in response to induction of ovulation in in vitro fertilization cycles. Eur J Obstet Gynecol Reprod Biol. 2005;118:214–8. doi: 10.1016/j.ejogrb.2004.04.038. [DOI] [PubMed] [Google Scholar]
- 14.Wunder DM, Kretschmer R, Bersinger NA. Concentrations of leptin and C-reactive protein in serum and follicular fluid during assisted reproductive cycles. Hum Reprod. 2005;20:1266–71. doi: 10.1093/humrep/deh767. [DOI] [PubMed] [Google Scholar]
- 15.Welt CK, Heist K, Mantzoros CS. Leptin and soluble leptin receptor in follicular fluid. J Assist Reprod Genet. 2003;20:495–501. doi: 10.1023/B:JARG.0000013649.38415.2a. [DOI] [PMC free article] [PubMed] [Google Scholar]