Abstract
Purpose: To evaluate the percentages of macrophages present in granulosa cells (GC) cultures from patients with different responses to the hyperstimulation, in relation to the percentages of apoptotic cells (ApC), as well as to the release of cytokines.
Methods: We studied 42 patients: 12 Hyporesponders, (with ≤4 follicles), 15 Normoresponders, (5–14 follicles), and 15 Hyperresponders, (≥15 follicles). In GC cultures percentages of macrophages and ApC were counted and, in the conditioned media, cytokines were measured
Results: Percentages of macrophages were significantly higher in GC cultures from Hyporesponders compared with Hyperresponders patients. Also, the percentages of ApC cells were the highest in Hyporesponders. On the contrary, cytokines concentrations were the lowest in this group.
Conclusions: The low ovarian response is probably due to the decreased angiogenesis, which in turn produces increased apoptosis and decreased production of cytokines. The increased percentage of macrophages could be related to increased frequency of apoptotic cells.
Keywords: Macrophages, Ovary response, Apoptosis, Cytokines, Angiogenesis
Introduction
In female reproduction, macrophages contribute to the regulation of the pituitary – gonadal axis and are found throughout the female reproductive tract [1]. In the ovary, macrophages have been found during the different phases of the menstrual cycle [2], localized primarily in the vascular connective tissue and theca – lutein areas of the corpus luteum, while some are found in the granulosa - lutein cell layer [3, 4]. Recently, Balasch et al. [4] showed that the concentration of vascular endothelial growth factor (VEGF) in the culture medium of macrophages from follicular fluid, was significantly higher than secreted into the culture medium of luteinized granulosa cells, emphasizing the importance of macrophages in the regulation of angiogenesis. Growth factors, like epidermal growth factor (EGF) and insulin-like growth factors (IGF), as well as cytokines like interleukins 1, 6, 10, 12 (IL-1, IL-6, IL-10, IL-12), interferon α (IFNα), tumor necrosis factor α (TNFα), etc., secreted by macrophages, are known to impact on many important aspects of the ovarian function [5–8], including follicle growth and differentiation, ovulation and corpus luteum formation and regression.
Also, ovarian macrophages use phagocytosis to remove apoptotic granulosa cells and apoptotic luteal cells [9, 10], thereby contributing to the processes of follicular atresia and luteolysis.
The presence of macrophages in the ovary has been known for many years. Their specific localization and temporal variations during the cycle suggest that macrophages play multiple roles in intraovarian events. Therefore, the purpose of our study is to determine the concentrations of macrophages in the follicular aspirates of the larger follicles in Assisted Reproductive Technology (ART) patients, with different types of controlled ovarian hyperstimulation (COH) response, and then correlate these concentrations of macrophages with the percentages of granulosa cells- apoptosis as well as to the concentrations of IL-1β, IL-6 and VEGF in the culture supernatants.
Materials and methods
Patients
We studied in a prospective manner 42 patients who had regular menstrual cycles, all younger than 38 years of age and with a normal day 3 serum FSH (≤10 mIU/ml) and estradiol (≤75 pg/ml), who underwent follicular aspiration for their first cycle of in vitro fertilization (IVF) procedure. The indications for IVF were variable, male factor, unexplained infertility, tubal factor etc, but none of the patients was treated under the diagnosis of ovarian dysfunction or history of anovulation. Patients were selected in a consecutive way until groups described as follows were completed.
All patients were treated with leuprolide acetate (Lupron®, Abbot Laboratories, Buenos Aires, Argentina) for pituitary desensitization. Beginning on day 21 of the cycle leuprolide acetate was given daily for a minimum of 10 days at 0.5 mg/day s.c. Serum estradiol was evaluated to assess the adequacy of suppression, when serum estradiol concentration was <50 pg/ml and a basal ultrasound showed the absence of cystic ovarian structures over 12 mm in diameter. A total of 3 ampoules of rFSH -75 IU, (Gonal-F®, Serono. Laboratory. Buenos Aires, Argentina) was administered for 3 days s.c. in addition to the daily dose of leuprolide acetate at 0.25 mg/day. Subsequently, the stimulation was followed by using HMG IM at 150 to 300 IU per day based on the assessment of the patient’s gonadal response by serum estradiol and ovarian ultrasound. The group of patients that had a lower ovarian response used a higher amount of HMG compared to those patients that showed a hyperresponse, yet the number of units between groups did not show statistical difference.
Follicular development was monitored by vaginal ultrasound using a 5 MHz transvaginal probe and serum estradiol concentrations. When the serum estradiol was ≥450 pg/ml and at least two follicles measured ≥17 mm in mean diameter, 10,000 IU of hCG (Profasi, Serono Laboratory., Buenos Aires, Argentina) was injected IM. Transvaginal follicular aspiration was performed 34–36 h after hCG administration.
Patients were divided as follows: Hyporesponders n = 12 (Hypo): which showed ≤4 follicles, ≥ than 14 mm on hCG day (n = 12) Normoresponders n = 15 (Normo): which had ≥5 ≤14 follicles, ≥ than 14 mm on hCG day (n = 15), and Hyperresponders n = 15 (Hyper): which had ≥15 follicles, ≥ than 14 mm on hCG day (n = 15).
The Institutional Review Committee at our institution approved the study, and we obtained an informed consent from each patient that participated.
Culture of granulosa cells (GC)
GC from each patient were isolated from pooled follicular fluid (only from follicles over 14 mm in diameter during aspiration) by centrifugation at 400 × g per 10 min and suspended in RPMI 1640 (GIBCO BRL. Life Technologies, Rockville, MD, USA) media supplemented with 1% of Fetal Bovine Serum (GIBCO BRL.) and 50 ug/ml of antibiotic-antimycotic (SIGMA Chemical CO., St. Louis, MO, USA). RPMI medium was described by Moore et al. (1967) Then, GC were purified by a centrifugation gradient of Histopaque (SIGMA Chemical CO.) whose density was adjusted to 1.065 g/l.
We seeded between 3 and 8 wells, according to the amount of granulosa cells retrieved from each patient. To assure ourselves that we would have a significant number of adhered cells, we placed 106 cells/well in Labteck of 8 wells. (Permanox Lab-Teck, Nalge Nunc International, Naperville, MD, USA), during 48 h at 37°C and 5% CO2. Viability of GC was assessed using Trypan -blue stain in an isolated well.
After 48 h of incubation the culture media were collected, but in all cases we left a drop of medium in each well (about 20 μl) to prevent the surfaces from becoming dry. The culture media were centrifuged and stored at −20°C until use for cytokine determinations. In all cases cytokine determination was done in a pool of conditioned media from 3 wells per patient.
Percentage of macrophages
In two wells of each culture the proportion of macrophages was determined by establishing the number of macrophages identified for every 200 cells. Macrophages were recognized by an indirect immunofluorescence technique using as a first antibody a polyclonal antihuman macrophages (Zymed Laboratories Inc, CA., USA) and as a second antibody an anti-mouse total immunoglobulin F(ab)2 fragment FITC conjugated (Dako Corp, CA, USA). We counted the cells in a clockwise fashion over the borders of the wells to avoid counting the same cells twice and only report on 200 cells because we read the fluorescence at 400 X.
Apoptosis
In other well of the same culture, the percentage of apoptotic cells were counted. We added 10 ul of acridine orange - ethidium bromide mixture, and immediately examined the cultures as described by Abrams et al. [11]. In accordance with this technique the viable cells were entirely stained by green, while early apoptotic cells were observed with the nucleus stained by orange and the cytoplasm green and later apoptotic cells were totally stained by orange respectively.
Likewise as we counted macrophages, apoptotic cells were counted in a clockwise fashion over the borders of the wells, to avoid counting the same cells twice. As we may have live cells, cells that were starting apoptosis and cells that were completely apoptotic, we read at 400 X magnification to secure the highest accuracy possible. Furthermore, as the reading was done in fresh samples (but after almost completely removing the culture medium), if we were to delay the reading, most cells would begin the process of apoptosis spontaneously. The percentage of apoptotic cells was calculated as the number of cells with fluorescent orange nucleus/total cells counted.
Quantification of IL-1β, IL-6 and VEGF
The IL-1β, IL-6 and VEGF concentrations in the GC conditioned media and in serum samples, were quantified using ELISA commercial kits (Cytimmune Sciences Inc., USA). The sensitivity for IL-1β ELISA was 0.87 pg/ml. For IL-6 ELISA kit the sensitivity was 3.4 pg/ml. The VEGF ELISA kit had a sensitivity of 18.6 pg/ml. The intra-assay variability for IL-1β, IL-6 and VEGF were ±7.9%, ±8.1% and ±8.9% respectively and the inter-assay variability were ±11.4%, ±10.4% and 11.1% for IL-1β, IL-6 and VEGF respectively. All samples were assessed by triplicate.
Statistical analysis
Values are expressed as mean (X) ± Standard Error (S.E.). Comparisons among the different groups were assessed by the Kruskal -Wallis nonparametric ANOVA test and Dunn’s Multiple Comparisons test. A p value ≤0.05 was considered statistically significant.
Results
Number of follicles, oocytes and concentrations of serum estradiol
The average number of follicles and oocytes retrieved as well as the serum concentrations of estradiol, present in patients from groups Hypo, Normo and Hyper, are shown on Table 1. We assumed that the difference encountered between the groups was reflecting the total number of follicles and not the size, since all follicles were within the same size range.
Table 1.
Number of follicles and oocytes retrieved, average age and serum concentrations of estradiol, present in patients from groups Hypo, Normo and Hyper
| Hypo (n = 12) | Normo (n = 15) | Hyper (n = 15) | |
|---|---|---|---|
| # of Follicles | 3.09 ± 0.27a,b | 10.57 ± 0.58a | 19.28 ± 0.68a,b |
| # of Oocytes | 2.36 ± 0.36a,b | 9.39 ± 0.58a | 16.07 ± 0.60b |
| Age (years) | 35.45 ± 1.01 | 34.07 ± 0.96 | 33.57 ± 0.88 |
| Estradiol (pg/ml) | 1,053 ± 232a,c | 3,716 ± 651a | 5,917 ± 2,231c |
ap < 0.001 Hypo vs Normo, bp < 0.001 and cp < 0.01 Hypo vs. Hyper by Dunn’s Multiple comparisons Test.
Patients from each group had similar age range, however the number of follicles as well as the number of oocytes retrieved were significantly different (p < 0.001 Hypo vs. Normo and vs. Hyper for both parameters).
In addition, concentrations of serum estradiol were also statistically different (p < 0.001 Hypo vs. Normo and p < 0.01 Normo vs. Hyper).
Percentages of macrophages and apoptotic cells
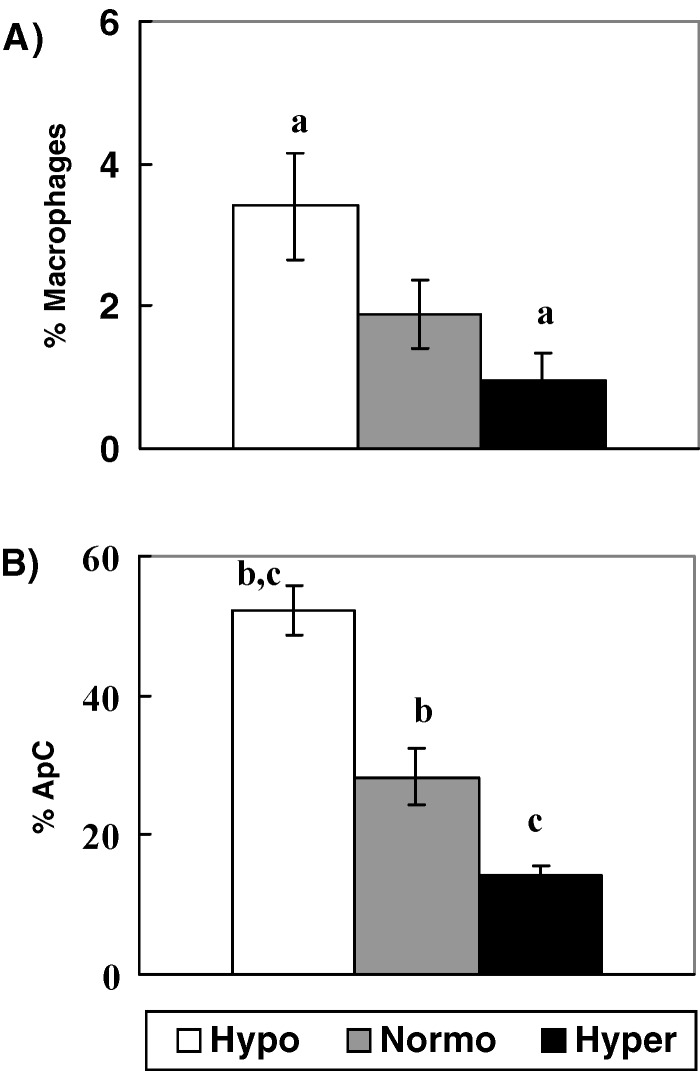
The absolute number of granulosa cells and macrophages recovered per follicle in each group of patients, are shown on Table 2. The percentages of macrophages were counted by an indirect immunofluorescence technique. Percentages obtained were 3.4 ± 0.7, 1.9 ± 0.4 and 0.9 ± 0.4% in Groups Hypo, Normo and Hyper respectively, (Group Hypo vs. Hyper, p < 0.03), (Fig. 1A)
Table 2.
Number of follicles retrieved, average of Granulosa Cells and macrophages recovered per follicle from groups Hypo, Normo and Hyper
| Hypo (n = 12) | Normo (n = 15) | Hyper (n = 15) | |
|---|---|---|---|
| # of Follicles | 3.09 ± 0.27a,b | 10.57 ± 0.58a | 19.28 ± 0.68b |
| # of GC /follicle (×103) | 1024.0 ± 211.1 | 980.6 ± 65.74 | 914.5 ± 212.5 |
| # of Macrophages/follicle (×103) | 33.8 ± 121.3c | 19.6 ± 4.8 | 8.7 ± 3.9c |
ap < 0.001 Hypo vs Normo, bp < 0.001 and cp < 0.05 Hypo vs. Hyper by Dunn’s Multiple comparisons Test.
Fig. 1.
Percentages of (A) Macrophages and (B) ApC in GC cultures from Hypo, Normo and Hyperresponder patients. Results are expressed as X ± S.E. (a) p < 0.03 (b) p< 0.05 and (c) p < 0.001 by Dunn’s Multiple comparisons Test
In other wells of the same cultures, percentages of apoptotic cells (ApC) were counted, and the values obtained were significantly higher in Group Hypo in comparison to Group Normo (52.2 ± 3.6% vs. 28.3±4.0%) (p < 0.05) and also vs. Group Hyper (14.2 ± 1.2%) (p < 0.001), (Fig. 1B).
Concentrations of IL-1β, IL-6 and VEGF in conditioned media of GC cultures
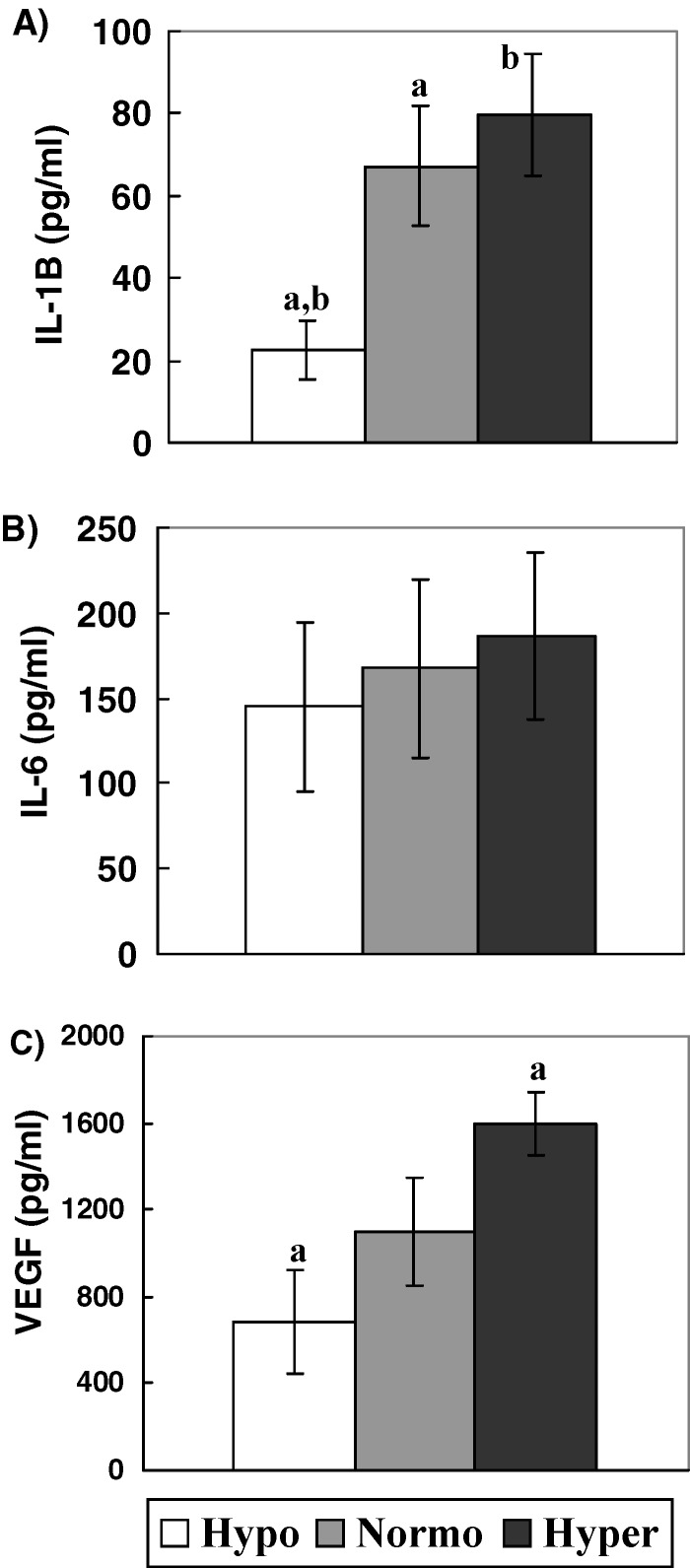
We measured the concentration of IL-1β, IL-6 and VEGF in 48 h GC cultures conditioned media from Groups Hypo, Normo and Hyper and found that IL-1β concentrations were significantly decreased in GC cultures from Group Hypo with respect to Group Normo (p < 0.05) and Group Hyper (p < 0.01). Values obtained were: 22.3 ± 7.1 pg/ml, 67.2 ± 14.7 pg/ml and 79.6 ± 14.8 pg/ml respectively (Fig. 2A).
Fig. 2.
Cytokine concentrations in conditioned media of GC cultures from Hypo, Normo and Hyper responder patients. Results are expressed as X ± S.E. (A) IL-1 β concentrations, (B) IL-6 concentrations and (C) VEGF concentrations. (a) p < 0.05 and (b) p < 0.01 by Dunn’s Multiple comparisons Test
No significant differences were found in IL-6 concentrations between the groups studied. Values obtained were: 145.1 ± 49.5 pg/ml, 167.7 ± 52 pg/ml and 186.1 ± 48.9 pg/ml for Hypo, Normo and Hyper respectively, (Fig. 2B).
We observed at the same time, that VEGF concentrations were diminished in GC cultures from Hypo vs. Hyper (p < 0.05). Values obtained were: 684 ± 238 pg/ml, 1,101 ± 249 pg/ml and 1,596 ± 145 pg/ml for Groups Hypo, Normo and Hyper respectively (Fig. 2C).
We also measured cytokine concentrations in serum from the same groups of patients and we did not find differences in the values obtained: IL-1β: 16.4 ± 7.8 pg/ml, 5.8 ± 1.9 pg/ml and 9.7 ± 4.2 pg/ml in sera from Groups Hypo, Normo and Hyper respectively (N.S.), while IL-6 values were: 91.2 ± 43.3 pg/ml in Group Hypo, 60.4 ± 33.0 pg/ml in Normo and 49.3 ± 24.0 pg/ml in Hyper (N. S.). Serum VEGF values were: 191.0 ± 70.0 pg/ml, 86.8 ± 27.0 pg/ml and 106.2 ± 76.0 pg/ml from Groups Hypo, Normo and Hyper respectively, which also were not statistically significant.
Discussion
The ovarian response to a standard COH protocol, even in young patients with normal day 3 serum FSH and estradiol, is variable. Unexpectedly, despite adequate amounts of gonadotropin administration, sometimes few follicles are identified during monitoring, and these patients are then labelled as having a hyporesponse.
The outcome of these cycles in terms of pregnancy rate per ART cycle, in general is not as good as in patients with a normal or a hyper-response. In our study, patients that had a decreased response to the COH protocol used, had a significantly higher concentration of macrophages in follicular fluid; considering that this same group of patients also showed a higher degree of apoptosis in granulosa cells in culture, it can be speculated, that the phagocytosis of apoptotic bodies is responsible for the increased macrophage population found in hyporesponders.
From each patient, using the same methodology, the cells were isolated from pooled follicular fluids from all aspirated follicles ≥14 mm in diameter. Then, according to the amount of recovered cells, we seeded between 3 and 8 wells with 106 cells/well, from each patient. This number of seeded cells is sufficiently large to obtain a good visualization after 48 h of culture in Lab-Teck. Therefore, if the proportion of macrophages to granulosa cells was constant, this would have to be similar in all groups studied. Nevertheless, we have observed variations in the proportion of macrophages encountered that correlated with the proportion of apoptotic cells, and similarly with the type of ovarian response clinically observed.
Hyporesponders, also exhibited a lower concentration of IL-1β and VEGF in the culture supernatants, compared to hyper-responder patients, emphasizing that in hypo-responders the primary function of macrophages is to eliminate the apoptotic bodies, as was previously reported [9, 10]. In a recent article by Balasch et al. [4] it was shown that macrophages do release VEGF, but whether these macrophages are able to release cytokines while they are phagocytosing is still a matter of controversy.
Weiss, first reported that phagocytes taking up apoptotic cells do not release cytokines, a similar finding was described by Savill et al. [12]. In a more recent report, the same author suggested that in some situations the phagocytosing cell may release cytokines [13]. We do not rule out the possibility that macrophages may have released cytokines in our cultures, but given the fact that the concentration of macrophages present in our cultures was inversely proportional to the concentration of cytokines measured in the supernatants, it is tempting to speculate that granulosa cells were most likely responsible for the cytokine production.
Angiogenesis is a prominent histological feature that occurs during luteinization, and VEGF appears to be the principal factor involved in this phenomenon [14]. VEGF is a highly mitogenic factor for vascular endothelial cells that also promotes cell migration and inhibits apoptosis [15]. Additionally, IL-1β and IL-6 have been shown to induce the expression of VEGF in different kind of cells [16–18].
Van Blerkom [19] reported that the perifollicular angiogenesis is correlated with the degree of O2 concentration within the follicle, as well as with the degree of apoptosis and VEGF concentration; our group, recently published data showing that the direct intra-ovarian injection of VEGF in mice enhanced the degree of neo-vascularization and ovarian response [20]. In these animals, we observed that a direct VEGF ovarian injection increased vascularization, the number of antral and luteinized follicles, and showed diminished apoptosis compared to controls.
Furthermore, in a previous report [21], we have demonstrated that the concentration of cytokines in follicular fluid does not correlate with the concentration in the supernatant of granulosa cell cultures. We found that the concentrations of VEGF in follicular fluid (FF) and in the supernatant of GC cultures were inversely proportional. Different publications evaluated concentrations of cytokines in both, follicular fluid and supernatants of GC cultures separately, but we could not find any reports comparing both.
Most authors agree that low concentrations of VEGF in follicular fluid are found in good prognosis ART patients (as determined by peak serum estradiol concentrations, number of gonadotropin ampoules administered per cycle, number of oocytes retrieved, embryo quality and pregnancy rate) [22, 23]. In contrast, high levels of VEGF production by GC cultures are found in patients with elevated concentrations of serum estradiol, higher number of follicles and oocytes retrieved, fertilization and pregnancy rates [24, 25]. These opposite findings in prognosis between concentrations of VEGF in follicular fluid and in supernatants of GC cultures, remain to be elucidated.
Levitas et al. [17], working with rats, observed that an increment in ovarian VEGF, is preceded by a rise in IL-1 beta, and in this context, VEGF may be joined by other IL-1-dependent angiogenesis-promoters, such as IL-6 or transforming growth factor beta-1.
One possible hypothesis to explain these interactions, could be that GC exposed to hypoxia in vivo, produce a reactive secretion of IL-1 and VEGF, while GC cultured in vitro and exposed to different oxygen conditions, inhibits the production of these cytokines.
In summary, we conclude that the low response to gonadotropin stimulation as seen in hyporesponders, is likely the result of decreased angiogenesis in the gonad, and specifically in the perifollicular areas, resulting in an increased granulosa cell apoptosis and decreased production of IL-1β and VEGF [17, 21]. The concentration of macrophages in hyporesponders is increased within the follicular aspirates, primarily in order to eliminate through phagocytosis the increased apoptotic bodies among the granulosa cells. This enhanced concentration of macrophages, showed an inverse relationship with the production of IL-1β and VEGF in the culture supernatants, therefore it is possible to speculate, that the cytokines encountered in the culture supernatant, is most likely produced by granulosa cells present in the follicular aspirates and not from the present population of macrophages.
Acknowledgements
This study was supported in part by the National Research Council of Argentina (CONICET).
References
- 1.Takaya, Fukaya, Sasano, Suzuki, Tamura, Yajima Macrophages in normal cycling human ovaries; immunohistochemical localization and characterization. Hum Reprod. 1997;12:1508–12. doi: 10.1093/humrep/12.7.1508. [DOI] [PubMed] [Google Scholar]
- 2.Kasuya, Kawabuchi Macrophages are present not only in atretic mature follicles but also in the growing follicles of the guinea pig ovary. Ital J Anat Embryol. 1998;103:183–9. [PubMed] [Google Scholar]
- 3.Wu, Van Der Hoek, Ryan, Norman, Robker Macrophage contributions to ovarian function. Hum Reprod Update. 2004;10:119–33. doi: 10.1093/humupd/dmh011. [DOI] [PubMed] [Google Scholar]
- 4.Balasch, Guimera, Martinez-Pasarell, Ros, Vanrell, Jimenez Adrenomedullin and vascular endothelial growth factor production by follicular fluid macrophages and granulosa cells. Hum Reprod. 2004;19:808–14. doi: 10.1093/humrep/deh204. [DOI] [PubMed] [Google Scholar]
- 5.Brannstrom, Wang, Norman Ovulatory effect of interleukin-1 beta on the perfused rat ovary. Endocrinology. 1993;132:399–404. doi: 10.1210/en.132.1.399. [DOI] [PubMed] [Google Scholar]
- 6.Vinatier, Dufour, Tordjeman-Rizzi, Prolongeau, Depret-Moser, Monnier Immunological aspects of ovarian function: role of the cytokines. Eur J Obstet Gynecol Reprod Biol. 1995;63:155–68. doi: 10.1016/0301-2115(95)02227-9. [DOI] [PubMed] [Google Scholar]
- 7.Terranova, Rice Review: cytokine involvement in ovarian processes. Am J Reprod Immunol. 1997;37:50–63. doi: 10.1111/j.1600-0897.1997.tb00192.x. [DOI] [PubMed] [Google Scholar]
- 8.Chung, Ando, Adashi Periovulatory and interleukin (IL)-1-dependent regulation of IL-6 in the immature rat ovary: a specific IL-1 receptor-mediated eicosanoid-dependent effect. J Soc Gynecol Investig. 2000;7:301–8. doi: 10.1016/S1071-5576(00)00072-1. [DOI] [PubMed] [Google Scholar]
- 9.Gaytan, Morales, Garcia-Pardo, Reymundo, Bellido, Sanchez-Criado Macrophages, cell proliferation, and cell death in the human menstrual corpus luteum. Biol Reprod. 1998;59:417–25. doi: 10.1095/biolreprod59.2.417. [DOI] [PubMed] [Google Scholar]
- 10.Kasuya [Elimination of apoptotic granulosa cells in atretic follicles: the role of macrophages and intact granulosa cells] Kaibogaku Zasshi. 2002;77:23–30. [PubMed] [Google Scholar]
- 11.Abrams, White, Fessler, Steller Programmed cell death during Drosophila embryogenesis. Development. 1993;117:29–43. doi: 10.1242/dev.117.1.29. [DOI] [PubMed] [Google Scholar]
- 12.Savill, Fadok, Henson, Haslett Phagocyte recognition of cells undergoing apoptosis. Immunol Today. 1993;14:131–6. doi: 10.1016/0167-5699(93)90215-7. [DOI] [PubMed] [Google Scholar]
- 13.Savill, Dransfield, Gregory, Haslett A blast from the past: clearance of apoptotic cells regulates immune responses. Nat Rev Immunol. 2002;2:965–75. doi: 10.1038/nri957. [DOI] [PubMed] [Google Scholar]
- 14.Davis JS, Rueda BR, Spanel-Borowski K. Microvascular endothelial cells of the corpus luteum. Reprod Biol Endocrinol 10-11-2003;1:89-. [DOI] [PMC free article] [PubMed]
- 15.Lee, Christenson, Patton, Burry, Stouffer Vascular endothelial growth factor production by human luteinized granulosa cells in vitro. Hum Reprod. 1997;12:2756–61. doi: 10.1093/humrep/12.12.2756. [DOI] [PubMed] [Google Scholar]
- 16.Neufeld, Cohen, Gengrinovitch, Poltorak Vascular endothelial growth factor (VEGF) and its receptors. FASEB J. 1999;13:9–22. [PubMed] [Google Scholar]
- 17.Levitas, Chamoun, Udoff, Ando, Resnick, Adashi Periovulatory and interleukin-1 beta-dependent up-regulation of intraovarian vascular endothelial growth factor (VEGF) in the rat: potential role for VEGF in the promotion of periovulatory angiogenesis and vascular permeability. J Soc Gynecol Investig. 2000;7:51–60. doi: 10.1016/S1071-5576(99)00066-0. [DOI] [PubMed] [Google Scholar]
- 18.Lebovic, Bentzien, Chao, Garrett, Meng, Taylor Induction of an angiogenic phenotype in endometriotic stromal cell cultures by interleukin-1beta. Mol Hum Reprod. 2000;6:269–75. doi: 10.1093/molehr/6.3.269. [DOI] [PubMed] [Google Scholar]
- 19.Van Blerkom, Antczak, Schrader The developmental potential of the human oocyte is related to the dissolved oxygen content of follicular fluid: association with vascular endothelial growth factor levels and perifollicular blood flow characteristics. Hum Reprod. 1997;12:1047–55. doi: 10.1093/humrep/12.5.1047. [DOI] [PubMed] [Google Scholar]
- 20.Quintana, Kopcow, Sueldo, Marconi, Rueda, Baranao Direct injection of vascular endothelial growth factor into the ovary of mice promotes follicular development. Fertil Steril. 2004;82(Suppl 3):1101–1105. doi: 10.1016/j.fertnstert.2004.03.036. [DOI] [PubMed] [Google Scholar]
- 21.Quintana, Kopcow, Marconi, Sueldo, Speranza, Baranao Relationship of ovarian stimulation response with vascular endothelial growth factor and degree of granulosa cell apoptosis. Hum Reprod. 2001;16:1814–8. doi: 10.1093/humrep/16.9.1814. [DOI] [PubMed] [Google Scholar]
- 22.Friedman, Danforth, Herbosa-Encarnacion, Arbogast, Alak, Seifer Follicular fluid vascular endothelial growth factor concentrations are elevated in women of advanced reproductive age undergoing ovulation induction. Fertil Steril. 1997;68:607–12. doi: 10.1016/S0015-0282(97)00278-1. [DOI] [PubMed] [Google Scholar]
- 23.Barroso, Barrionuevo, Rao, Graham, Danforth, Huey, Abuhamad, Oehninger Vascular endothelial growth factor, nitric oxide, and leptin follicular fluid levels correlate negatively with embryo quality in IVF patients. Fertil Steril. 1999;72:1024–6. doi: 10.1016/S0015-0282(99)00442-2. [DOI] [PubMed] [Google Scholar]
- 24.Doldi, Bassan, Fusi, Ferrari In controlled ovarian hyperstimulation, steroid production, oocyte retrieval, and pregnancy rate correlate with gene expression of vascular endothelial growth factor. J Assist Reprod Genet. 1997;14:589–92. doi: 10.1023/A:1022580601803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doldi, Destefani, Gessi, Grossi, Ferrari Human albumin enhances expression of vascular endothelial growth factor in cultured human luteinizing granulosa cells: importance in ovarian hyperstimulation syndrome. Hum Reprod. 1999;14:1157–9. doi: 10.1093/humrep/14.5.1157. [DOI] [PubMed] [Google Scholar]