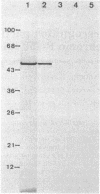
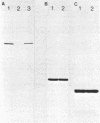
Abstract
Insertion of newly synthesized P-450(1), the major phenobarbital-inducible form of rabbit liver microsomal cytochrome P-450, into microsomal membranes was studied in a wheat germ cell-free translation system programed with total RNA from the liver of a phenobarbital-treated rabbit. P-450(1) synthesized in vitro had the same molecular weight as the mature molecule and was co-translationally inserted into dog pancreas rough microsomal membranes. In the presence of salt-washed microsomes, instead of unwashed ones, the insertion was greatly diminished. It could, however, be restored by supplementation of the system with purified signal recognition particle (SRP), a known component of the membrane translocation machinery for secretory proteins. In the absence of microsomes, SRP inhibited the translation of mRNA encoding P-450(1) and this translation arrest was released by the addition of salt-washed microsomes. On the other hand, SRP did not affect the translation of mRNAs encoding yeast porin and reticulocyte globin, which are mitochondrial and cytosolic proteins, respectively. We conclude that co-translational insertion of P-450(1) into microsomal membranes requires SRP and postulate that P-450(1) possesses an uncleavable signal sequence that can be recognized by SRP.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. J., Walter P., Blobel G. Signal recognition protein is required for the integration of acetylcholine receptor delta subunit, a transmembrane glycoprotein, into the endoplasmic reticulum membrane. J Cell Biol. 1982 May;93(2):501–506. doi: 10.1083/jcb.93.2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Nun S., Kreibich G., Adesnik M., Alterman L., Negishi M., Sabatini D. D. Synthesis and insertion of cytochrome P-450 into endoplasmic reticulum membranes. Proc Natl Acad Sci U S A. 1980 Feb;77(2):965–969. doi: 10.1073/pnas.77.2.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Botelho L. H., Ryan D. E., Levin W. Amino acid compositions and partial amino acid sequences of three highly purified forms of liver microsomal cytochrome P-450 from rats treated with polychlorinated biphenyls, phenobarbital, or 3-methylcholanthrene. J Biol Chem. 1979 Jul 10;254(13):5635–5640. [PubMed] [Google Scholar]
- Erickson A. H., Walter P., Blobel G. Translocation of a lysosomal enzyme across the microsomal membrane requires signal recognition particle. Biochem Biophys Res Commun. 1983 Aug 30;115(1):275–280. doi: 10.1016/0006-291x(83)91000-8. [DOI] [PubMed] [Google Scholar]
- Fisher P. A., Berrios M., Blobel G. Isolation and characterization of a proteinaceous subnuclear fraction composed of nuclear matrix, peripheral lamina, and nuclear pore complexes from embryos of Drosophila melanogaster. J Cell Biol. 1982 Mar;92(3):674–686. doi: 10.1083/jcb.92.3.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii-Kuriyama Y., Mizukami Y., Kawajiri K., Sogawa K., Muramatsu M. Primary structure of a cytochrome P-450: coding nucleotide sequence of phenobarbital-inducible cytochrome P-450 cDNA from rat liver. Proc Natl Acad Sci U S A. 1982 May;79(9):2793–2797. doi: 10.1073/pnas.79.9.2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman B. M., Blobel G. Biogenesis of peroxisomes: intracellular site of synthesis of catalase and uricase. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5066–5070. doi: 10.1073/pnas.75.10.5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haugen D. A., Armes L. G., Yasunobu K. T., Coon M. J. Amino-terminal sequence of phenobarbital-inducible cytochrome P-450 from rabbit liver microsomes: similarity to hydrophobic amino-terminal segments of preproteins. Biochem Biophys Res Commun. 1977 Aug 8;77(3):967–973. doi: 10.1016/s0006-291x(77)80072-7. [DOI] [PubMed] [Google Scholar]
- Haugen D. A., Coon M. J. Properties of electrophoretically homogeneous phenobarbital-inducible and beta-naphthoflavone-inducible forms of liver microsomal cytochrome P-450. J Biol Chem. 1976 Dec 25;251(24):7929–7939. [PubMed] [Google Scholar]
- Heinemann F. S., Ozols J. The complete amino acid sequence of rabbit phenobarbital-induced liver microsomal cytochrome P-450. J Biol Chem. 1983 Apr 10;258(7):4195–4201. [PubMed] [Google Scholar]
- Howe J. G., Hershey J. W. A sensitive immunoblotting method for measuring protein synthesis initiation factor levels in lysates of Escherichia coli. J Biol Chem. 1981 Dec 25;256(24):12836–12839. [PubMed] [Google Scholar]
- Imai Y., Hashimoto-Yutsudo C., Satake H., Girardin A., Sato R. Multiple forms of cytochrome P-450 purified from liver microsomes of phenobarbital- and 3-methylcholanthrene-pretreated rabbits. I. Resolution, purificaton, and molecular properties. J Biochem. 1980 Aug;88(2):489–503. doi: 10.1093/oxfordjournals.jbchem.a132996. [DOI] [PubMed] [Google Scholar]
- Johnson E. F., Schwab G. E., Muller-Eberhard U. Multiple forms of cytochrome P-450: catalytic differences exhibited by two homogeneous forms of rabbit cytochrome P-450. Mol Pharmacol. 1979 May;15(3):708–718. [PubMed] [Google Scholar]
- Lu A. Y., West S. B. Multiplicity of mammalian microsomal cytochromes P-45. Pharmacol Rev. 1979 Dec;31(4):277–295. [PubMed] [Google Scholar]
- Meyer D. I., Krause E., Dobberstein B. Secretory protein translocation across membranes-the role of the "docking protein'. Nature. 1982 Jun 24;297(5868):647–650. doi: 10.1038/297647a0. [DOI] [PubMed] [Google Scholar]
- Mihara K., Blobel G., Sato R. In vitro synthesis and integration into mitochondria of porin, a major protein of the outer mitochondrial membrane of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7102–7106. doi: 10.1073/pnas.79.23.7102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihara K., Blobel G. The four cytoplasmically made subunits of yeast mitochondrial cytochrome c oxidase are synthesized individually and not as a polyprotein. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4160–4164. doi: 10.1073/pnas.77.7.4160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller M., Ibrahimi I., Chang C. N., Walter P., Blobel G. A bacterial secretory protein requires signal recognition particle for translocation across mammalian endoplasmic reticulum. J Biol Chem. 1982 Oct 25;257(20):11860–11863. [PubMed] [Google Scholar]
- Negishi M., Fujii-Kuriyama Y., Tashiro Y., Imai Y. Site of biosynthesis of cytochrome P450 in hepatocytes of phenobarbital treated rats. Biochem Biophys Res Commun. 1976 Aug 23;71(4):1153–1160. doi: 10.1016/0006-291x(76)90774-9. [DOI] [PubMed] [Google Scholar]
- Okada Y., Frey A. B., Guenthner T. M., Oesch F., Sabatini D. D., Kreibich G. Studies on the biosynthesis of microsomal membrane proteins. Site of synthesis and mode of insertion of cytochrome b5, cytochrome b5 reductase, cytochrome P-450 reductase and epoxide hydrolase. Eur J Biochem. 1982 Feb;122(2):393–402. doi: 10.1111/j.1432-1033.1982.tb05894.x. [DOI] [PubMed] [Google Scholar]
- Palade G. Intracellular aspects of the process of protein synthesis. Science. 1975 Aug 1;189(4200):347–358. doi: 10.1126/science.1096303. [DOI] [PubMed] [Google Scholar]
- Patrick J., McMillan J., Wolfson H., O'Brien J. C. Acetylcholine receptor metabolism in a nonfusing muscle cell line. J Biol Chem. 1977 Mar 25;252(6):2143–2153. [PubMed] [Google Scholar]
- Raymond Y., Shore G. C. The precursor for carbamyl phosphate synthetase is transported to mitochondria via a cytosolic route. J Biol Chem. 1979 Oct 10;254(19):9335–9338. [PubMed] [Google Scholar]
- Sabatini D. D., Kreibich G., Morimoto T., Adesnik M. Mechanisms for the incorporation of proteins in membranes and organelles. J Cell Biol. 1982 Jan;92(1):1–22. doi: 10.1083/jcb.92.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields D., Blobel G. Cell-free synthesis of fish preproinsulin, and processing by heterologous mammalian microsomal membranes. Proc Natl Acad Sci U S A. 1977 May;74(5):2059–2063. doi: 10.1073/pnas.74.5.2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoffel W., Blobel G., Walter P. Synthesis in vitro and translocation of apolipoprotein AI across microsomal vesicles. Eur J Biochem. 1981 Dec;120(3):519–522. doi: 10.1111/j.1432-1033.1981.tb05730.x. [DOI] [PubMed] [Google Scholar]
- Walter P., Blobel G. Purification of a membrane-associated protein complex required for protein translocation across the endoplasmic reticulum. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7112–7116. doi: 10.1073/pnas.77.12.7112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P., Blobel G. Signal recognition particle contains a 7S RNA essential for protein translocation across the endoplasmic reticulum. Nature. 1982 Oct 21;299(5885):691–698. doi: 10.1038/299691a0. [DOI] [PubMed] [Google Scholar]
- Walter P., Blobel G. Signal recognition particle: a ribonucleoprotein required for cotranslational translocation of proteins, isolation and properties. Methods Enzymol. 1983;96:682–691. doi: 10.1016/s0076-6879(83)96057-3. [DOI] [PubMed] [Google Scholar]
- Walter P., Blobel G. Translocation of proteins across the endoplasmic reticulum III. Signal recognition protein (SRP) causes signal sequence-dependent and site-specific arrest of chain elongation that is released by microsomal membranes. J Cell Biol. 1981 Nov;91(2 Pt 1):557–561. doi: 10.1083/jcb.91.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P., Blobel G. Translocation of proteins across the endoplasmic reticulum. II. Signal recognition protein (SRP) mediates the selective binding to microsomal membranes of in-vitro-assembled polysomes synthesizing secretory protein. J Cell Biol. 1981 Nov;91(2 Pt 1):551–556. doi: 10.1083/jcb.91.2.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P., Ibrahimi I., Blobel G. Translocation of proteins across the endoplasmic reticulum. I. Signal recognition protein (SRP) binds to in-vitro-assembled polysomes synthesizing secretory protein. J Cell Biol. 1981 Nov;91(2 Pt 1):545–550. doi: 10.1083/jcb.91.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]