Abstract
Purpose
The aim of the study was to measure circulating BDNF levels, a neurotrophin recently identified in the ovary, in parallel with estradiol, to verify if assessing this factor could add any predictive value to the outcome of in vitro fertilization.
Methods
Blood sampling for BDNF and estradiol was performed in 23 subjects undergoing IVF on day 1 (D1), day 8 (D8), day of HCG administration (DHCG) and day of oocyte retrieval.(DOR).
Results
There was a positive correlation between BDNF and estradiol throughout the stimulation cycle in all subjects. In both pregnant and nonpregnant patients, the values of BDNF grew significantly only between D8 and DHCG and remained constant until DOR. Between-group comparisons showed no statistically significant differences in both BDNF and estradiol values throughout the IVF cycle.
Conclusion
Although BDNF plasma concentrations are not seemingly predictive of IVF outcome, this neurotrophin is highly correlated to estradiol levels and seems to be an important factor especially in the periovulatory period.
Keywords: Brain derived neurotrophic factor, IVF/ICSI outcome, Assisted reproduction, Estradiol
Introduction
For years, clinical practitioners in the field of in vitro fertilization (IVF) have relied on circulating estradiol levels for assessing a patient’s response to controlled ovarian stimulation. It was believed that estradiol levels represented undoubtedly the best marker for the quality and maturity of oocytes [1]. However, recent studies have clearly demonstrated that levels of estradiol following hCG administration, whether increasing, unchanged or decreasing, are not in any way correlated with achievement of pregnancy [2, 3]. Moreover, it is ever so evident the ovarian follicular development and oocyte maturation are regulated by a complex steroid hormone–growth factor interactions. more than just hypothalamic–pituitary–ovarian steroids long feedback loops. Follicular microenvironment plays a very important role in the selection of oocytes with the best fertilization and developmental potential. Very recently, it was demonstrated that the human ovary is capable of expressing neurotrophins, such as brain-derived growth factor (BDNF) and neurotrophin-4/5 (NT-4/5), neurotrophin 3 (NT-3), nerve growth factor (NGF), which were once thought to be implied solely in the survival and differentiation of neurons within the central and peripheral nervous systems [4]. In particular, BDNF was isolated, along with the other neurotrophins, within mural and cumulus granulosa cells of human preovulatory follicles [4]. The fact that BDNF is present in follicular fluid of normally cycling women suggests that this neurotrophin could be a physiologic regulator of normal follicle maturation [5]. Furthermore, the Trk B receptor, principal target of BDNF action, was identified in preovulatory human unfertilized oocytes indicating that BDNF may have a role in supporting granulosa cell oocyte communication within the follicle [4]. Indeed BDNF seems to mediate LH and HCG actions in promoting preovulatory oocyte meiotic maturation [6] In vitro mouse studies have provided a direct demonstration that BDNF, like neurotrophin-4/5, has a role in the maturation of the oocyte, promoting extrusion of first polar body [7, 8]. In light of the very particular role of this neurotrophin and of the fact that BDNF easily crosses the blood–brain barrier [9] and can be measured in plasma [10], we thought it would be insightful to perform serial measurements of this neurotrophin in women undergoing ovarian stimulation for IVF. The aim of the study was to measure circulating BDNF levels before and during the IVF cycle, in parallel with estradiol, in order to verify if assessing this neurotrophin could add any predictive value regarding IVF outcome.
Materials and methods
Subjects
In a prospective observational study, we enrolled 23 consecutive patients undergoing their first IVF cycle between January and November 2006 at the Centre of Reproductive Physiopathology of the Pisa University Hospital. The study was performed in accordance with the guidelines in The Declaration of Helsinki and has been formally approved by the local ethical committee.Informed consent was obtained from all subjects. Mean age of women was 34.8 ± 4.4 years and average body mass index (BMI) was 21.74 ± 2.71. Mean basal FSH levels were 7.92 ± 2.77. Indications for IVF included tubal factor (n = 14) severe male factor (n = 9). IVF or IVF with intracytoplasmic sperm injection and embryo transfer were performed as appropriate.
Methods
Controlled ovarian stimulation was carried out with two to six ampoules/day, according to basal FSH levels and age, of recombinant FSH (Puregon®, Organon, Italy or Gonal F®, Serono, Italy) after a pretreatment with oral contraceptives. All patients were administered cetrorelix, a GnRH antagonist, according to a personalized regimen, i.e. when the lead follicle reached 14 mm in diameter, to prevent premature ovulation. Recombinant HCG (Ovitrelle®, Serono) was administered when at least 2 follicles reached a mean diameter of 18 mm. After approximately 36 hours, transvaginal follicular aspiration was performed for oocyte retrieval. Blood sampling for BDNF and estradiol was performed for all subjects on day 1 (D1), day 8 (D8), day of HCG administration (DHCG) and day of oocyte retrieval.(DOR) after overnight fasting, between 8 am and 9 am in order to minimize the effects of a possible circadian variation of plasma BDNF concentrations, as previously suggested in the literature [11]. After blood sampling, test tubes were kept on ice and immediately centrifuged at 4°C (2500 g for 15 minutes). Plasma was aliquoted and stored at −80° C until assay.
BDNF assay
Plasma levels of BDNF were determined with a previously described [10] enzyme-linked immunosorbent assay (ELISA) method (BDNF Emax Immunoassay System, Promega, USA), after appropriate dilution of samples (1:4) using Block & Sample Buffer, according to manufacturer instructions. The sensitivity of the assay, expressed as a minimal amount of BDNF distinguishable from the zero sample with 95% probability was 15.6 pg/ml, and the intra- and inter-assay coefficients of variation were 6.0% and 8.5%, respectively. The antiserum employed was highly specific, showing less than 3% cross-reactivity with other related neurotrophic factors (nerve growth factor, NT-3 and NT-4) at 100 ng/ml, as specified by the manufacturer.
Estradiol assay
Plasma concentrations of estradiol were determined by specific commercially available radioimmunoassay kits (Radim, Pomezia, Italy). The sensitivity of the assay was 10 pg/ml The intra- and inter-assay coefficients of variation were 2.1% and 4.2%, respectively.
Statistical analysis
Plasma BDNF and estradiol levels are expressed in pg/ml. All data are reported as mean±SD. Data obtained were analyzed by one-way analysis of variance, as appropriate. Statistical significance between IVF cycle days (D1, D8, DHCG, DOR) or between pregnant and nonpregnant patients was calculated by t test. Correlations between BDNF and estradiol values were performed by Pearson’s method. A P value of < 0.05 was considered statistically significant.
Results
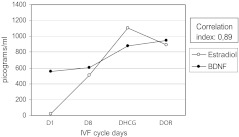
All 23 patients completed the ovarian stimulation cycle, underwent oocyte retrieval and subsequent embryo transfer. Pregnancy rate (i.e. cardiac activity on transvaginal ultrasound per retrieval). was 39,1% in this population. Mean BDNF and estradiol values for D1, D8, DHCG and DOR are represented in Fig. 1. There was a positive correlation between BDNF and estradiol throughout the stimulation cycle in the group of 23 subjects. Patients were then subdivided in two groups, pregnant (n = 9) and nonpregnant (n = 14). Mean age was statistically different in the group of pregnant patients with respect to nonpregnant patients. However, comparisons for BMI, number of total rFSH ampoules used, basal FSH levels, number of total retrieved oocytes, number of mature oocytes, number of embryos available for transfer, taking into account that the Italian law allows insemination of a maximum of 3 oocytes, revealed no differences between the two groups (Table 1). Percentage of ICSI cycles was 55.5% in the pregnant group and 35.6% in the nonpregnant group. Fertilization rates were 76.3% in the pregnant group and 74.1% in the nonpregnant group.
Fig. 1.
BDNF and estradiol levels in all patients (n = 23) throughout the IVF cycle
Table 1.
Patient characteristics and IVF results; between-group comparisons (pregnant vs nonpregnant patients)
| Characteristics | Pregnant women (n = 9) | Nonpregnant women (n = 14) | P value |
|---|---|---|---|
| Age | 32,11 ± 4,17 | 36,57 ± 3,78 | 0.01 |
| BMI | 21.43 ± 2.18 | 21.95 ± 2.47 | 0.33 |
| Basal FSH levels | 7.50 ± 2.39 | 8.19 ± 3.03 | 0.28 |
| Number of total rFSH ampoules | 40.89 ± 18.78 | 53.86 ± 21.43 | 0.08 |
| Number of total retrieved oocytes | 7.11 ± 5.84 | 6.36 ± 5.84 | 0.38 |
| Number of retrieved mature oocytes | 5, 0 ± 4.15 | 3.93 ± 2.76 | 0.23 |
| Number of embryos available for transfer | 2.11 ± 0.93 | 1.86 ± 1.03 | 0.28 |
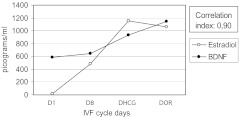
A strong positive correlation between BDNF and estradiol values persisted even after the subdivision in pregnant and nonpregnant patients (Figs. 2 and 3). In pregnant patients and nonpregnant patients, the values of BDNF, unlike those of estradiol, grew significantly only between D8 and DHCG and remained constant until DOR (Table 2). No statistical significance was found between DHCG and DOR values for estradiol as well within each of the studied groups. (Table 2). Between-group comparisons showed no statistically significant differences in both BDNF and estradiol values at D1, D8, DHCG and DOR the IVF cycle.
Fig. 2.
BDNF and estradiol levels in pregnant patients (n = 9) throughout the IVF cycle
Fig. 3.
BDNF and estradiol levels in nonpregnant patients (n = 14) throughout the IVF cycle
Table 2.
Within-group comparisons of mean ± SD BDNF and estradiol concentrations in pg/ml at D1, D8, DHCG and DOR of the IVF cycle
| Parameters | Subgroup | D1 | D8 | DHCG | DOR |
|---|---|---|---|---|---|
| BDNF | Pregnant | 589.36 ± 243.42 | 644.2 ± 246.50 | 933.36 ± 363.30*/** | 1,142.93 ± 499.03*/** |
| Nonpregnant | 543.09 ± 219.65 | 588.93 ± 262.04 | 838.39 ± 240.80*/** | 886.21 ± 518.51*/** | |
| Estradiol | Pregnant | 16.00 ± 8.35 | 404.67 ± 377.04* | 1,150 ± 656.48*/** | 1,063.20 ± 518.45*/** |
| Nonpregnant | 18.64 ± 10.04 | 526.00 ± 408.25 * | 1,078 ± 652.55*/** | 883.90 ± 419.63*/** |
*p < 0.05 with respect to D1
**p < 0.05 with respect to D8
Discussion
This is a first report of a serial plasma quantification of BDNF throughout an IVF cycle. Our results indicate that plasma BDNF, like estradiol, exhibits dynamic changes during controlled ovarian stimulation for IVF. In fact these two circulating factors showed a positive correlation throughout the controlled ovarian stimulation in both women who became pregnant and those who did not. These data are in line with a very recent report [10] on a positive correlation between circulating BDNF and estradiol levels throughout the menstrual cycle in normally cycling fertile women. Studies carried out on hippocampal neurons have shown that there is a strong link between estrogen synthesis and BDNF synthesis [12]. Indeed, it is likely that estrogens can induce the synthesis of BDNF by acting at an estrogen-responsive element on the BDNF gene [13]. This kind of mechanism may apply to the granulosa cells, the main source of BDNF within the ovary, as well. In our series, BDNF increased more slowly with respect to estradiol in the first half of the IVF cycle, reaching significantly higher levels, with respect to baseline, only at DHCG. This may be due to the timing required for estrogen to induce BDNF gene expression [14]. Both estradiol and BDNF plasma concentrations remained constant between DHCG and DOR in both study groups. Therefore, in the second half of the IVF cycle, the patterns of circulating BDNF and estradiol were very similar. In our opinion, it seems that the increase in BDNF plasma concentrations is an exquisitely periovulatory phenomenon, as already evidenced in physiological menstrual cycles [10].
Between-group comparisons showed no statistically significant differences in both BDNF and estradiol values at D1, D8, DHCG and DOR of the IVF cycle For this reason, based on our results, we cannot say that BDNF values have any predictive value in IVF outcome. There are some drawbacks to our study. Firstly, the small number of patients may not allow significant differences in number of total retrieved oocytes, number of mature oocytes, number of embryos available for transfer, to emerge between pregnant and nonpregnant patients. Secondly, pregnant patients were on average 3 years younger and this may explain their better chance in achieving a pregnancy. Finally, it is known that BDNF expression increases under conditions of stress [15] and this may represent a confounding factor in a population of women undergoing IVF that notoriously exhibit higher levels of anxiety due to the treatment itself [16].
In conclusion, although, based on our findings, BDNF plasma concentrations are not seemingly predictive of IVF outcome, this neurotrophin is obviously highly correlated to estradiol levels and seems to be an important factor especially in the periovulatory period.
Footnotes
Capsule
BDNF plasma concentrations are not predictive of IVF outcome, however they are highly correlated to estradiol levels and seem to be implicated in periovulatory processes.
References
- 1.Laufer N, DeCherney AH, Tarlatzis BC, Naftolin F. The association between preovulatory serum 17 beta-estradiol pattern and conception in human menopausal gonadotropin–human chorionic gonadotropin stimulation. Fertil Steril. 1986;46:73–76. doi: 10.1016/s0015-0282(16)49460-4. [DOI] [PubMed] [Google Scholar]
- 2.Meyer WR, Beyler SA, Baker ST, Somkuti SG, Lowden DA, Grainger DA. Value of estradiol response after human chorionic gonadotropin administration in predicting in vitro fertilization success. Fertil Steril. 1999;72:542–545. doi: 10.1016/S0015-0282(99)00281-2. [DOI] [PubMed] [Google Scholar]
- 3.Chiasson MD, Bates GW, Robinson RD, Arthur NJ, Propst AM. Measuring estradiol levels after human chorionic gonadotropin administration for in vitro fertilization is not clinically useful. Fertil Steril. 2007;87:448–450. doi: 10.1016/j.fertnstert.2006.06.024. [DOI] [PubMed] [Google Scholar]
- 4.Seifer DB, Feng B, Shelden RM. Immunocytochemical evidence for the presence and location of the neurotrophin-Trk receptor family in adult human preovulatory ovarian follicles. Am J Obstet Gynecol. 2006;194:1129–1134. doi: 10.1016/j.ajog.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 5.Seifer DB, Lambert-Messerlian G, Schneyer AL. Ovarian brain-derived neurotrophic factor (BDNF) is present in follicular fluid from normally cycling women. Fertil Steril. 2003;79:451–452. doi: 10.1016/S0015-0282(02)04669-1. [DOI] [PubMed] [Google Scholar]
- 6.Feng B, Chen S, Shelden RM, Seifer DB. Effect of gonadotropins on brain-derived neurotrophic factor secretion by human follicular cumulus cells. Fertil Steril. 2003;80:658–659. doi: 10.1016/S0015-0282(03)00742-8. [DOI] [PubMed] [Google Scholar]
- 7.Seifer DB, Feng B, Shelden RM, Chen S, Dreyfus CF. Neurotrophin-4/5 and neurotrophin-3 are present within the human ovarian follicle but appear to have different paracrine/autocrine functions. J Clin Endocrinol Metab. 2002;87:4569–4571. doi: 10.1210/jc.2002-020499. [DOI] [PubMed] [Google Scholar]
- 8.Seifer DB, Feng B, Shelden RM, Chen S, Dreyfus CF. Brain-derived neurotrophic factor: a novel human ovarian follicular protein. J Clin Endocrinol Metab. 2002;87:655–659. doi: 10.1210/jc.87.2.655. [DOI] [PubMed] [Google Scholar]
- 9.Pan W, Banks WA, Fasold MB, Bluth J, Kastin AJ. Transport of brain-derived neurotrophic factor across the blood–brain barrier. Neuropharmacology. 1998;37:1553–1561. doi: 10.1016/S0028-3908(98)00141-5. [DOI] [PubMed] [Google Scholar]
- 10.Begliuomini S, Casarosa E, Pluchino N, Lenzi E, Centofanti M, Freschi L, Pieri M, Genazzani AD, Luisi S, Genazzani AR 2007 Influence of endogenous and exogenous sex hormones on plasma brain-derived neurotrophic factor. Hum Reprod (in press). [DOI] [PubMed]
- 11.Solum DT, Handa RJ. Estrogen regulates the development of brain-derived neurotrophic factor mRNA and protein in the rat hippocampus. J Neurosci. 2002;22:2650–2659. doi: 10.1523/JNEUROSCI.22-07-02650.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scharfman HE, MacLusky NJ. Estrogen and brain-derived neurotrophic factor (BDNF) in hippocampus: complexity of steroid hormone–growth factor interactions in the adult CNS Front. Neuroendocrinol. 2006;27:415–435. doi: 10.1016/j.yfrne.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sohrabji F, Miranda RC, Toran-Allerand CD. Estrogen response element in the gene encoding brain-derived neurotrophic factor. Proc Natl Acad Sci U S A. 1995;92:11110–11114. doi: 10.1073/pnas.92.24.11110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gibbs RB. Levels of trkA and BDNF mRNA, but not NGF mRNA, fluctuate across the estrous cycle and increase in response to acute hormone replacement. Brain Res. 1998;787:259–268. doi: 10.1016/S0006-8993(97)01511-4. [DOI] [PubMed] [Google Scholar]
- 15.Franklin TB, Perrot-Sinal TS. Sex and ovarian steroids modulate brain-derived neurotrophic factor (BDNF) protein levels in rat hippocampus under stressful and non-stressful conditions. Psychoneuroendocrinology. 2006;31:38–48. doi: 10.1016/j.psyneuen.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 16.Campagne DM. Should fertilization treatment start with reducing stress? Hum Reprod. 2006;21:1651–1658. doi: 10.1093/humrep/del078. [DOI] [PubMed] [Google Scholar]