Abstract
Purpose
To compare effectiveness of two different chemical zona thinning techniques.
Method
We studied 163 patients who had experienced IVF or ICSI failures in two or more cycles. Patients were assigned to one of three groups: zona intact (n = 72), partial thinning (n = 59), or circumferential thinning (n = 73). Before transfer, the zonae pellucidae of embryos were thinned partially or circumferentially using acidified Tyrode’s solution.
Results
Implantation rates were 8.9% in the intact zona group, 17.6% in the partial thinning group, and 11.9% in the circumferential thinning group: respective clinical pregnancy rates were 16.7% (12/72), 32.2% (19/59), and 27.4% (20/73). Both rates were significantly higher in the partial thinning group than the intact zona group. For circumferential thinning versus zona intact groups, differences fell short of significance.
Conclusions
Following embryo transfer failure, partial thinning would be recommended over circumferential thinning for successful assisted hatching.
Keywords: Assisted hatching, Acidified Tyrode’s solution, Partial zona thinning, Circumferential zona thinning
Introduction
Assisted reproductive technology has continued to advance during recent decades. However, even with proper care that embryos of good quality are chosen for transfer to the uterus, the implantation rate remains low. As part of the natural hatching process in vivo, the zona pellucida (zona) is thinned and softened by a protease-like enzyme [1, 2]. In contrast, embryo culture conditions in vitro are believed to induce abnormal hardening of the zona [3], which may impair the hatching process and thus contribute to low implantation rates after embryo transfer [4].
Embryo transfer following assisted hatching involving mechanical dissection or chemical opening of the zona was first reported to promote the hatching process at the blastocyst stage [4, 5]. Partial thinning and circumferential thinning of the zona, both using acidified Tyrode’s solution, respectively were reported in 1992 [6] and 1993 [7]. In experiments using mouse embryos, where they proved particularly effective in promoting blastocyst hatching [6, 7].
These studies led us to initiate human clinical investigation of assisted hatching. The present randomized trial compares the effectiveness of partial and circumferential chemical thinning of the zona in patients with previous implantation failure after embryo transfer.
Materials and methods
Patients
Between January 1999 and July 2002, 204 IVF or ICSI cycles in 163 patients were studied prospectively in the Yano maternity clinic IVF center in Ehime, Japan. Patients were selected for IVF or ICSI according to standard accepted indications. All patients had experienced previous IVF or ICSI failures in two or more cycles, and were less than 40 years old. Cycles were assigned randomly to one of three groups: group A, intact zona (n = 72); group B, partial zona thinning (n = 59); or group C, circumferential zona thinning (n = 73). Chemical zona thinning was performed after obtaining the couple’s informed consent.
Ovarian stimulation and oocyte retrieval
Generally, controlled ovarian hyperstimulation (COH) was carried out using a long GnRH analogue protocol. Buserelin acetate (Suprecur; Hoechst Japan, Tokyo) was administered as a nasal spray in the mid-luteal phase of the cycle until the day of hCG injection. In some cycles, a short (“flare”) protocol was used. All patients were administered gonadotropins in combination with pure FSH (Fertinorm P; Serono Japan, Tokyo) and hMG (Humegon; Organon Japan, Tokyo) beginning on day 3 of the cycle at a dose of 150 to 300 IU daily. The dose and duration of COH were modified according to the patient’s ovarian response. Follicular growth was monitored by vaginal ultrasound on day 10 of the cycle. When two or more follicles reached 18 mm in diameter, oocyte retrieval was performed with trans vaginal ultrasound guidance 35 h after injection of 10,000 IU of hCG (HCG; Mochida, Osaka, Japan).
Laboratory procedures
IVF procedure
After retrieval, all mature cumulus–oocyte complexes (COC) were washed and pre-incubated for 5 h in Human Tubal Fluid Media (Irvine Scientific, Santa Ana, CA) or Quinn’s Advantage Fertilization Medium (SAGE In-Vitro Fertilization, Pasadena, CA) in 5% CO2, 5% O2, and 90% N2 at 37°C. For insemination, the oocytes were introduced into 1 ml sperm droplets containing 100,000 motile sperm under mineral oil (Squibb, Princeton, NJ) in a 60 mm plastic dish (Falcon 1007, Becton Dickinson, NJ) and incubated overnight.
ICSI procedure
After being denuded of their surrounding cumulus cells using hyaluronidase, oocytes were transferred to medium in which corona cells were removed by repeated aspiration. Then the oocytes were incubated in a 50 mm plastic dish (Falcon 1006, Becton Dickinson, NJ) under mineral oil until the time of injection. Microinjection (ICSI) was performed only into oocytes that had extruded their first polar bodies.
Embryo culture
At 16 to 20 h post insemination, fertilization was determined by the presence of two pronuclei and two polar bodies. Embryos were cultured in Human Tubal Fluid Media (Irvine Scientific, Santa Ana, CA) or Quinn’s Advantage Cleavage Medium (SAGE In-Vitro Fertilization, Pasadena, CA) in a 4-well plastic dish (Multidish, Nunclon, Roskilde, Denmark). Culture media were refreshed daily.
Prior to embryo transfer, on the morning of day 2 or 3 following oocyte retrieval, embryos were graded as follows: grade 1, no fragmentation with equal-sized homogenous blastomeres; grade 2, less than 20% fragmentation with equal-sized homogenous blastomeres; grade 3, 20 to 50% fragmentation with unequal-sized blastomeres; grade 4, over 50% fragmentation with unequal-sized blastomeres. Only grade 1 or 2 embryos were used for the trial of assisted hatching.
Chemical thinning of the zona pellucida
Partial zona thinning
All micromanipulations were performed in micro droplets in a 50 mm plastic dish (Falcon 1006, Becton Dickinson, NJ) under mineral oil (Squibb, Princeton, NJ). Sucrose was not used for these micromanipulation procedures. The actual procedure for partial zona thinning using acidified Tyrode’s solution (pH 2.5 ± 0.3, SIGMA T 1788) has been described in detail [6]. Micromanipulation was performed using an inverted microscope equipped with Nomarski interference contrast optics (Olympus IMT-2; Tokyo, Japan) and fitted with a heated stage. A holding pipette and micropipettes were attached to micromanipulators (Narishige, Tokyo, Japan). An embryo was kept at the tip of the holding pipette, at the 9 o’clock position, while a micropipette containing acidified Tyrode’s solution was positioned at the 3 o’clock position.
The zona pellucida of a human embryo is bilayered; the outer layer is thick and easily digested by acidic solutions, while the inner layer is more compact, resilient, and difficult to dissolve. Zona thinning was performed with these characteristics in mind. The outer layer of the zona was dissolved gently by expelling acidified Tyrode’s solution using mouth-controlled suction. When the outer layer of the zona was dissolved completely, expulsion of solution was terminated immediately to avoid damage to the inner layer. Slightly more than one-third of the zona circumference was thinned.
With the couple’s informed consent, some low quality embryos (grade 3 or 4) not used for transfer were subjected to partial zona thinning and then rinsed in phosphate buffer (0.1 M, pH 7.4) and fixed in 2% glutaraldehyde in phosphate buffer for 2 h. After fixation, this specimen was rinsed in phosphate buffer, dehydrated in a grated series of ethanol–water solutions, dried using the critical point method, and spatter-coated with gold. The surface then was examined using a scanning electron microscope (S-450DX; HITACHI, Tokyo, Japan).
Circumferential zona thinning
The embryo was incubated up to 30 s in a 1 ml micro droplet containing the acidified Tyrode’s solution (pH 2.5 ± 0.3) in a 50 mm plastic dish under mineral oil. This procedure was performed rapidly and carefully to dissolve only the outer layer of the zona and avoid unnecessary exposure of the embryo to the acidic solution. The embryo was then quickly examined on the heated stage of an inverted microscope to observe the zona. If the zona was not thinned sufficiently, the embryo was further incubated for an additional 15–30 sec.
Embryo transfer
Only good quality (grade 1 or 2) embryos were selected for embryo transfer. Embryos that had undergone zona thinning were washed carefully several times to remove the acidic solution and then cultured for an additional 1 h prior to embryo transfer. Embryo transfer was performed on day 2 or day 3 after oocyte retrieval. From one to three embryos were transferred using the ϕ con IVF catheter (Fuji Systems, Tokyo, Japan), if the ϕ con IVF catheter could not be introduced, a Wallace catheter (HG. Wallace, West Sussex, UK) was used. Each embryo transfer was performed under trans abdominal or trans vaginal ultrasound guidance. After embryo transfer, luteal support was provided by administration of hCG (5000 IU) on day 7 and progesterone (100 mg/day; Progehormon, Mochida, Osaka, Japan) starting on day 2. A pregnancy test was performed using a urine specimen obtained 14 days after embryo transfer. Clinical pregnancy was defined as the presence of an intrauterine gestational sac demonstrated by trans vaginal ultrasound.
Statistical analysis
The Bonferroni/Dunn test was used to test differences among embryos with intact zona (Group A), those with partial zona thinning (Group B), and those with circumferential zona thinning (Group C). The Fisher’s exact probability test was used to test between-group differences. A P value below 0.05 was considered indicative of statistical significance.
Results
Patient characteristics for each group are shown in Table 1. Mean patient age, number of previous embryo transfers and duration of infertility were similar between the three groups. Outcomes for each group are shown in Table 2. Number of oocytes retrieved, 2PN fertilization rate, cleavage rate and number of embryos transferred were similar between three groups.
Table 1.
Clinical profiles of groups with intact zona and zona thinning
| Intact zona | Zona thinning | ||
|---|---|---|---|
| Group A | Group B | Group C | |
| No. of patients | 60 | 50 | 53 |
| No. of embryo transfer cycle | 72 | 59 | 73 |
| Patient age(M±SD) | 36.4±2.1 | 34.5±3.3 | 35.0±3.7 |
| No. of previous embryo transfer(M±SD) | 4.5±1.3 | 3.4±1.2 | 3.3±1.9 |
| Duration of infertility (y)(M±SD) | 8.5±4.5 | 7.8±3.3 | 8.3±4.1 |
Group A: Embryos with intact zona.
Group B: Embryos with partial thinning of the zona.
Group C: Embryos with circumferential thinning of the zona.
Table 2.
Outcomes in groups with intact zona and zona thinning
| Intact zona | Zona thinning | ||
|---|---|---|---|
| Group A | Group B | Group C | |
| No. of oocytes retrieved(M±SD) | 7.5±2.7 | 8.8±2.3 | 8.1±2.8 |
| 2-PN fertilization rate (%) | 64.6 | 69.5 | 71.8 |
| Cleavage rate (%) | 97.4 | 98.8 | 94.4 |
| No. of embryos transferred(M±SD) | 2.1±1.1 | 2.7±0.5 | 2.7±1.1 |
| Implantation rate (%) | 14/157(8.9)* | 28/159(17.6)* | 23/194(11.9) |
| Clinical pregnancy rate (%) | 12/72(16.7)** | 19/59(32.2)** | 20/73(27.4) |
| Abortion rate (%) | 1/12(8.3) | 3/19(15.0) | 2/20(10.0) |
| Multiple pregnancy rate (%) | 1/11(9.0) | 4/16(25.0) | 4/18(22.2) |
| Monozygotic twin | 0 | 0 | 0 |
* P=0.02 by Bonferroni/Dunn test and P=0.03 by Fisher’s exact probability test.
**P=0.04 by Bonferroni/Dunn and Fisher’s exact probability tests.
Respective implantation rates in the intact zona group (A), the partial zona thinning group (B), and the circumferential zona thinning group(C) were 8.9, 17.6, and 11.9, while clinical pregnancy rates were 16.7 (12/72), 32.2 (19/59), and 27.4% (20/73). Both implantation rate and clinical pregnancy rate were significantly higher in the partial zona thinning group (B) than in the intact zona group (A; P < 0.05). In the circumferential zona thinning group (C), outcomes appeared somewhat better than in the intact zona group (A), but no significant difference was detected. Abortion rate and multiple pregnancy rates were similar between the three groups, and no pregnancy included monozygotic twins.
When the surface of the zona was examined by scanning electron micrograph (SEM) following partial thinning, the outer layer was dissolved extensively while the inner layer was intact (Fig. 1).
Fig. 1.
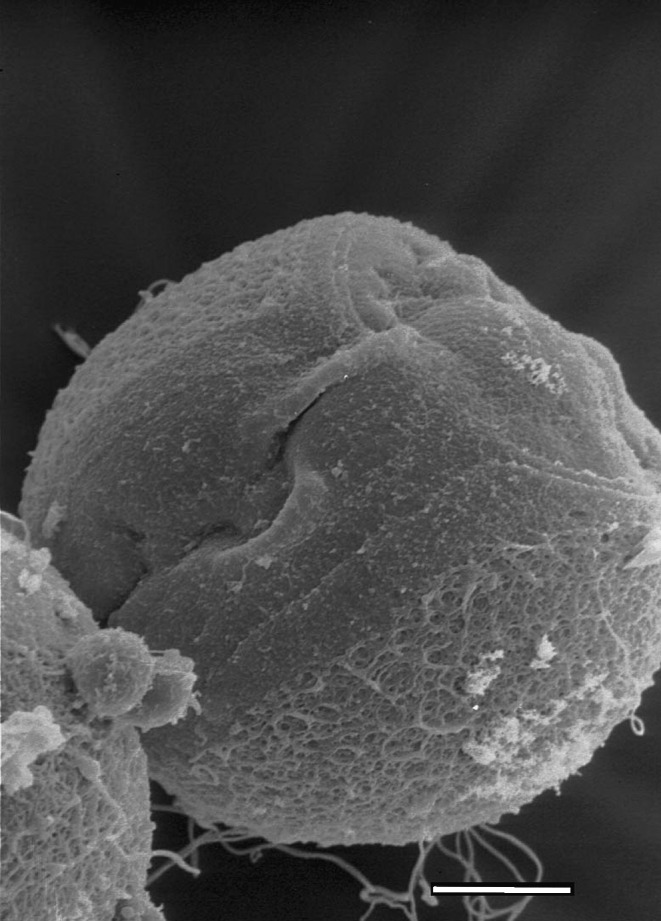
Scanning electron micrograph (SEM) of a chemically partial thinned region of the zona pellucida. The outer layer is disrupted widely, while the inner layer appears intact. Original magnifications; ×4,000
Discussion
The zona consists of the muco-polysaccharide and has a thickness of about 10 μm. The human zona is bilayered, with a less dense, thick, easily digestible outer layer and a more compact but resilient inner layer [8]. The zona may protect the embryo against infection by bacterial agents and other harmful interactions with the environment. The zona also, keeps blastomeres from separating as the embryo undergoes cleavage [8].
In vitro embryo culture conditions may induce qualitative changes in the zona that would impair hatching at the blastocyst stage [4]. To promote hatching despite the abnormal zona, chemical opening of the zona first was performed in 1992 [5]. Effectiveness has been confirmed by several clinical IVF centers [9–12]. Among several assisted hatching methods, chemical opening of the zona pellucida has been performed particularly widely because of technical ease and relative cost-effectiveness.
Recently, high implantation rates resulting in successful pregnancies have been reported after transfer of zona-free embryos [13]. Blastocysts or day 3 embryos were exposed to pronase or acidic solution that completely dissolved the zona [13, 14]. However, such techniques may risk adverse effects. Embryos could be damaged by acidic or enzymatic solutions, blastomeres could separate from embryos during cleavage, and infection by bacterial agents within the uterine cavity could occur [15].
Zona thinning techniques could avoid these disadvantages by retaining the resilient inner layer of the zona [8]. Both partial and circumferential zona thinning have been described in animal experiments [6, 7]. Both these chemical thinning techniques proved significantly more effective than zona-free methods in promoting blastocyst hatching in mouse embryos. However, Tucker reported that partial zona thinning did not improve implantation rates in human embryos [16], and no investigators have reported circumferential thinning applied to human embryos.
In our present study, both the implantation rate and the clinical pregnancy rate in the partial thinning group were significantly higher than in the intact zona group (P < 0.05). On the other hand, the circumferential thinning group appeared to show some improvement in outcomes compared with the intact zona group, but without reaching statistical significance. Thus, we found the partial thinning technique to be more effective than circumferential thinning in our patients with previous implantation failure after embryo transfer.
We suspect that while the use of micromanipulators, an inverted microscope, and meticulous performance of micro techniques by a skilled laboratory staff are required to achieve partial thinning, the method permits precise adjustment of the extent and depth of zona dissolution. While circumferential thinning can be performed conveniently with relatively little special equipment, time, and skilled manipulation, a consistent degree of thinning may be difficult to achieve given individual variation in zona hardness or thickening induced by culture conditions. Insufficient or excessive zona dissolution by the circumferential technique may result.
In conclusion, partial zona thinning using acidified Tyrode’s solution in patients with previous failure of embryo transfer improved implantation and the clinical pregnancy rates, while circumferential zona thinning could not attain a significant difference from results with an intact zona. Partial thinning, then, would be recommended over circumferential thinning for success in assisted hatching. However, further studies of greater numbers of patients are needed.
Footnotes
In patients with previous failure of embryo transfer, partial zona pellucida thinning for assisted hatching improved implantation and clinical pregnancy rates.
References
- 1.Perona RM, Wasserman PM. Mouse blastocysts hatch in vitro by using a trypsin-like proteinase associated with cells of mural trophectoderm. Dev Biol. 1986;114:42–52. doi: 10.1016/0012-1606(86)90382-9. [DOI] [PubMed] [Google Scholar]
- 2.Sawada H, Yamasaki K, Hoshi M. Trypsin-like protease from mouse embryos: evidence for the presence in culture medium and its enzymatic properties. J Exp Zool. 1990;254:83–87. doi: 10.1002/jez.1402540112. [DOI] [PubMed] [Google Scholar]
- 3.De Felici M, Siracusa G. “Spontaneous” hardening of the zona pellucida of mouse oocytes during in vitro culture. Gamete Res. 1982;6:107–113. doi: 10.1002/mrd.1120060203. [DOI] [Google Scholar]
- 4.Cohen J, Elsner C, Kort H, Massey J, Mayer MP, Wiemer K. Impairment of the hatching process following IVF in the human and improvement of implantation by assisted hatching using micromanipulation. Hum Reprod. 1990;5:7–13. doi: 10.1093/oxfordjournals.humrep.a137044. [DOI] [PubMed] [Google Scholar]
- 5.Cohen J, Alikani M, Trowbridge J, Rosenwaks Z. Implantation enhancement by selective assisted hatching using zona drilling of human embryos with poor prognosis. Hum Reprod. 1992;7:685–691. doi: 10.1093/oxfordjournals.humrep.a137720. [DOI] [PubMed] [Google Scholar]
- 6.Khalifa EA, Tucker MJ, Hunt P. Cruciate thinning of the zona pellucida for more successful enhancement of blastocyst hatching in the mouse. Hum Reprod. 1992;7:532–536. doi: 10.1093/oxfordjournals.humrep.a137685. [DOI] [PubMed] [Google Scholar]
- 7.Gordon JW, Dapunt U. Restoration of normal implantation rates in mouse embryos with a hatching impairment by use of a new method of assisted hatching. Fertil Steril. 1993;59:1302–1307. doi: 10.1016/s0015-0282(16)55994-9. [DOI] [PubMed] [Google Scholar]
- 8.Tucker MJ, Biol MI, Wiker SR, Kort HI. Embryonal zona pellucida thinning and uterine transfer. Assist Reprod Rev. 1993;3:168–171. [Google Scholar]
- 9.Schoolcraft WB, Jones GS, Schlenker T. Assisted hatching in the treatment of poor prognosis in vitro fertilization candidates. Fertil Steril. 1994;62:551–554. doi: 10.1016/s0015-0282(16)56944-1. [DOI] [PubMed] [Google Scholar]
- 10.Bider D, Livshits A, Yonish M. Assisted hatching by zona drilling of human embryos in women of advanced age. Hum Reprod. 1997;12:317–320. doi: 10.1093/humrep/12.2.317. [DOI] [PubMed] [Google Scholar]
- 11.Magli MC, Gianaroli L, Ferraretti AP, Fortini D, Aicardi G. Rescue of implantation potential in embryos with poor prognosis by assisted zona hatching. Hum Reprod. 1998;13:1331–1335. doi: 10.1093/humrep/13.5.1331. [DOI] [PubMed] [Google Scholar]
- 12.Graham MC, Hoeger KM, Phipps WR. Initial IVF-ET experience with assisted hatching performed 3 days after retrieval followed by day 5 embryo transfer. Fertil Steril. 2000;74:668–671. doi: 10.1016/S0015-0282(00)01528-4. [DOI] [PubMed] [Google Scholar]
- 13.Fong C-Y, Bongso A, Ng S, Anandakumar C, Trounson A, Ratnam S. On going normal pregnancy after transfer of zona-free blastocysts; Implications for embryo transfer in the human. Hum Reprod. 1997;3:557–560. doi: 10.1093/humrep/12.3.557. [DOI] [PubMed] [Google Scholar]
- 14.Mansour RT, Rhodes CA, Aboulghar MA, Serour GI, Kamal A. Transfer of zona-free embryos improves outcome in poor prognosis patients: a prospective randomized controlled study. Hum Reprod. 2000;15:1061–1064. doi: 10.1093/humrep/15.5.1061. [DOI] [PubMed] [Google Scholar]
- 15.Nichols J, Gardner RL. Effect of damage to zona pellucida on development of preimplantation embryos in the mouse. Hum Reprod. 1989;4:180–187. doi: 10.1093/oxfordjournals.humrep.a136868. [DOI] [PubMed] [Google Scholar]
- 16.Tucker MJ, Luecke N, Wilker S, Wright G. Chemical removal of the outside of the zona pellucida of day 3 human embryos has no impact on implantation rate. J Assist Reprod Genet. 1993;10:187–191. doi: 10.1007/BF01239219. [DOI] [PubMed] [Google Scholar]


