Abstract
Purpose
To investigate the effects of male ageing on DNA fragmentation and chromatin packaging in the spermatozoa of oligoasthenoteratozoospermic (OAT) patients.
Methods
Sixty-one OAT patients and 49 men with proven fertility (controls) were included in the present study. DNA fragmentation was detected by terminal deoxynucleotidyl transferase-mediated dUTP-nick end labelling (TUNEL) assay, while chromatin packaging was assessed by chromomycin A3 (CMA3) staining.
Results
In the patient group, semen volume, percentage of normally shaped spermatozoa and sperm motility decreased significantly (P < 0.05) with age, while sperm concentration and the percentage of TUNEL and CMA3 positive spermatozoa showed a statistically significant increase with age (P < 0.05). In the control group, conventional semen parameters as well as DNA fragmentation and chromatin packaging did not show a statistically significant change with age (P > 0.05).
Conclusion
Increased age in OAT patients is associated with an increase in sperm concentration, DNA fragmentation and poor chromatin packaging, as well as a decline in semen volume, sperm morphology and motility.
Keywords: Ageing effect, Male infertility, Semen parameters, DNA Fragmentation, Terminal deoxynucleotidyl transferase-mediated dUTP nick end labelling (TUNEL), Chromomycin A3 (CMA3)
Introduction
In developed countries, it has become socially acceptable to delay fatherhood, but the inherant consequences of this trend remain poorly understood. Although, it is well known that maternal age is a significant contributor to human infertility [1], advanced paternal age has been associated with decreased semen parameters, chromosomal abnormalities and reduced fertility [2]. As reported by Wyrobek et al., it has been associated with diseases of complex etiology such as schizophrenia and with autosomal dominant inherited diseases such as achondroplasia and Apert’s syndrome [1]. Additionally, several studies have shown that older men seem to produce more sperm with DNA damage [1, 3, 4], which derives from three potential sources: oxidative stress, abortive Fas-mediated apoptosis or deficiencies in natural processes such as recombination and chromatin packaging that induce DNA strand breaks [5].
The effects of male age on sperm DNA damage are significant for men attending reproductive clinics, although concerns have been raised about the risk of transmission of damaged DNA to the offspring, particularly at levels that exceed the DNA repair capacity of the oocyte [4–7]. Moreover, negative correlations have been observed between the stability of DNA in the sperm nucleus and the fertilising capacity of spermatozoa in vivo and in vitro [8–11].
The aim of the current study was to determine any possible correlation between male age of oligoasthenoteratozoospermic (OAT) patients and the level of spermatozoal DNA fragmentation by using the terminal deoxynucleotidyl transferase-mediated dUTP-nick end labelling (TUNEL) assay. It is known that fertility decreases in women >35 years old. In addition, it has been reported that men over 35 years are twice more likely to require more than 12 months to impregnate their female partners compared to men that are less than 25 years old [12]. We therefore used 35 year of age as a cut-off point to determine any synergistic effect that may lead to genetic defects in couples >35 years old. The hypothesis that the presence of DNA damage in mature spermatozoa is correlated with poor chromatin packaging due to underprotamination [13, 14] has been studied, by chromomycin A3 (CMA3) staining. The results obtained were compared to normal fertile donors (controls). Finally, the association between male age and conventional semen parameters was analysed and compared to normal fertile donors.
Materials and methods
Patients
Sixty-one patients were referred to the Department of Assisted Reproduction at Elena Venizelou hospital with the view of participating in an ICSI program, after experiencing at least 2 years of infertility. A severe male factor condition was the main cause of infertility due to oligoasthenoteratozoospermia. Furthermore, 49 healthy donors, with proven fertility, were also included in the study in order to evaluate possible differences between fertile and infertile men. In order to eliminate the potential confounding effects of the duration of sexual abstinence [15], all semen samples were collected after 4–5 days of sexual abstinence.
After liquefaction for 30 min at room temperature, all semen samples were first analysed to evaluate volume, concentration, linear progressive motility and morphology, according to WHO guidelines [16] and strict criteria [17]. Semen samples with volume ≥2 ml, sperm concentration ≥20 × 106/ml, morphology >14% and percentage of spermatozoa with total progressive motility ≥50% were regarded as normal. Semen with lower values than aforementioned was considered as abnormal.
Semen preparation/fixation
The sperm samples were washed three times in phosphate-buffered saline (PBS), pH 7.2, centrifuged at 280×g for 10 min and the pellet was then fixed in methanol/acetic acid (3:1). The fixed spermatozoa were spread on poly-L-Lys-coated slides and kept at room temperature for 1–3 days. At least two slides were prepared for each patient.
Decondensation treatment
The fixed sperm was washed in 2X standard saline citrate solution (SSC) with 0.3 mol/l NaCl, incubated for 30 min in 0.1 mol/l Tris–HCl (pH 7.5) with 10 mmol/l dithiothreitol (DTT) and for another 90 min in 0.1 mol/l Tris–HCl (pH 7.5) containing 4 mmol/l 3,5-diiodo salycyclic acid (LIS) at room temperature. After decondensation, the slides were washed once in 2X SSC, once in 1X PBS and finally dehydrated through an ethanol series (70, 85 and 100%) and air-dried. As this treatment allows the maintenance of the sperm structure, including the tail, the differentiation between the spermatozoa and other cells present in the ejaculate was unequivocal and easy to detect.
TUNEL
DNA fragmentation induced in spermatozoa was measured using the TUNEL assay (Roche Biochemicals, Mannheim, Germany). Briefly, the TdT-labelled nucleotide mix was added to each slide and incubated at 37°C for 60 min. The slides were rinsed twice in PBS and then counterstained with 10 mg/ml 4′,6-diamidino-2-phenyl-indole (DAPI, Vysis). Controls were also included in every experiment: for the negative control TdT was omitted in the nucleotide mix, whereas positive controls were generated by incubating the sperm cells for 10 min at room temperature with 50 units/ml DNase I (Boehrinhger Mannheim, Germany).
At least 500 spermatozoa per sample were evaluated using a Zeiss-Axioscope (Athens, Greece) fluorescence microscope equipped with a single band pass filter (DAPI), triple band pass filter (DAPI/Orange/Green), dual band pass filter (Aqua/Orange) and a single band pass filter (Green). The images were collected using a cooled CCD (charged coupled device) camera and captured by using Quips® PathVysion (Smart Capture VP, Athens, Greece). The number of cells per field stained with DAPI (blue) was first counted; the number of cells with Texas red fluorescence (TUNEL positive) was expressed as a percentage of the total sample.
Chromomycin A3 staining
For the CMA3 staining, the slides were treated for 20 min with CMA3 solution (0.25 mg/ml in McIlvane’s buffer, pH 7.0, containing 10 mM MgCl2), rinsed twice in PBS and counterstained with 10 mg/ml 4′, 6-diamidino-2-phenyl-indole (DAPI, Vysis).
The slides were observed using a Zeiss Axioscope fluorescence microscope and a total of 500 spermatozoa were randomly evaluated on each slide. Two types of staining patterns were identified, namely bright yellow fluorescence of the sperm head (chromatin packaging abnormal) and dull yellow staining (normal chromatin packaging).
Statistical analysis
All calculations were performed using SPSS-PC V11.0 Software. Because not all variables are normally distributed (as shown by analysis of variance using the Kolmogorov–Smirnov and Shapiro–Wilk tests), nonparametric analyses were applied for all variables. Differences between the means were analysed using the Mann–Whitney U test for two non-paired data, which is better suited to represent small samples and skewed distributions. For the two group comparisons, age was treated as a categorical variable and 35 years was selected as the cut-off because it corresponds to the 35-year old criterion for prenatal diagnostic testing due to advanced maternal age and was also a very symmetric and representative cut point for our study’s data. For correlation analysis, age was treated as a continuous variable. The Spearman rank correlation coefficient was used to determine the correlation between two variables. A P value of <0.05 was considered statistically significant and the results are presented as means±Standard Deviations.
Results
Age, semen parameters, DNA fragmentation and chromatin packaging of the 110 men included in the study, are presented in Table 1.
Table 1.
Descriptive statistics and comparison between OAT patients and controls
| Group | Age (years) | Duration of sexual abstinence (days) | Volume (ml) | Concentration (X 106/ml) | Morphologically normal sperm (%) | Total progressive motility (%) | DNA fragmentation (% TUNEL positive spermatozoa) | % Chromomycin A3 positive spermatozoa | |
|---|---|---|---|---|---|---|---|---|---|
| Patients | (mean±SD) | 36.8 ± 6.4 | 4.1 ± 0.4 | 2.9 ± 1.1 | 4.2 ± 2.7 | 6.8 ± 2.9 | 21.5 ± 9.1 | 30.0 ± 7.1 | 31.5 ± 7.1 |
| (n = 61) | Range | 24–54 | 4–5 | 0.5–5.0 | 0.8–11.0 | 1.0–13.0 | 0.0–33.0 | 19.4–49.8 | 11.6–47.8 |
| Controls | (mean±SD) | 34.6 ± 5.4 | 4.1 ± 0.3 | 3.4 ± 0.7 | 46.8 ± 19.3 | 29.0 ± 7.4 | 55.6 ± 4.3 | 6.2 ± 1.8 | 6.3 ± 1.8 |
| (n = 49) | Range | 24–45 | 4–5 | 2.0–5.1 | 22.0–106.0 | 15.0–45.0 | 50–70 | 3.4–12.6 | 3.2–11.4 |
| P (Mann–Whitney test) | NS (0.097) | NS (0.479) | P = 0.02 | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 |
NS = not significant
Semen analysis
As summarised in Table 1, the OAT patients obviously showed significantly lower semen parameters in comparison to normal controls. In order to evaluate any possible association between male age and conventional semen parameters, the OAT patients and the controls were split into two age groups, patient group 1 (24–34 years), patient group 2 (35–54 years) and control groups 1 and 2 aged 24–34 and 35–45, respectively.
As it is shown in Table 2, patient group 1 had significantly higher percentage of morphologically normal spermatozoa (P < 0.001) and percentage of progressive motility (P = 0.018) comparing to patient group 2. Conversely, sperm concentration was significantly lower in patient group 1 compared to patient group 2 (P = 0.004), while semen volume showed a non-significant decrease with age (P = 0.028). For the healthy donors (controls), sperm concentration, morphology and progressive motility did not differ significantly between the two age groups.
Table 2.
Descriptive statistics and comparisons between the two age subgroups in both OAT patients and controls
| Group | Age (years) | Duration of sexual abstinence (days) | Volume (ml) | Concentration (X 106/ml) | Morphologically normal sperm (%) | Total progressive motility (%) | DNA fragmentation (% TUNEL positive spermatozoa) | % Chromomycin A3 positive spermatozoa | |
|---|---|---|---|---|---|---|---|---|---|
| Patients Group1 | (mean±SD) | 31.9 ± 2.4 | 4.1 ± 0.2 | 3.2 ± 1.0 | 3.3 ± 2.3 | 7.9 ± 2.8 | 23.9 ± 9.6 | 26.3 ± 5.3 | 27.5 ± 7.9 |
| (n = 30) | Range | 24–34 | 4–5 | 0.5–5.0 | 0.8–9.0 | 2.0–13.0 | 0.0–33.0 | 19.4–40.6 | 11.6–41.6 |
| Patient Group2 | (mean±SD) | 41.6 ± 5.4 | 4.2 ± 0.4 | 2.6 ± 1.0 | 5.0 ± 2.7 | 5.8 ± 2.7 | 19.2 ± 8.0 | 33.7 ± 6.7 | 35.4 ± 8.2 |
| (n = 31) | Range | 35–54 | 4–5 | 0.6–4.7 | 0.8–11.0 | 1.0–13.0 | 5.0–33.0 | 20.6–49.8 | 15.0–47.8 |
| P (Mann–Whitney test) | P < 0.001 | NS (0.082) | P = 0.028 | P = 0.004 | P < 0.001 | P = 0.018 | P < 0.001 | P < 0.001 | |
| Control Group1 | (mean±SD) | 30.3 ± 2.6 | 4.1 ± 0.3 | 3.3 ± 0.7 | 51.0 ± 22.4 | 30.0 ± 8.0 | 56.5 ± 4.4 | 6.5 ± 1.9 | 6.5 ± 1.8 |
| (n = 26) | Range | 24–34 | 4–5 | 2.1–4.6 | 22.0–106.0 | 15.0–45.0 | 50.0–70.0 | 3.4–12.6 | 3.4–11.4 |
| Control Group2 | (mean±SD) | 39.3 ± 3.2 | 4.1 ± 0.3 | 3.4 ± 0.8 | 42.0 ± 14.0 | 27.8 ± 6.7 | 54.7 ± 4.1 | 5.9 ± 1.7 | 6.2 ± 1.8 |
| (n = 23) | Range | 35–45 | 4–5 | 2.0–5.1 | 22.0–81.0 | 20.0–45.0 | 50.0–65.0 | 3.8–9.4 | 3.2–10.6 |
| P (Mann–Whitney test) | P < 0.001 | NS (0.745) | NS (0.880) | NS (0.173) | NS (0.144) | NS (0.301) | NS (0.189) | NS (0.602) |
NS = not significant.
Sperm DNA fragmentation and chromatin packaging
A total of 110,000 spermatozoa were scored in order to evaluate the possible level of DNA fragmentation and chromatin packaging. The results, presented in Table 1, show that the overall DNA fragmentation rate of samples from the OAT patients and the percentage of chromomycin A3 positive spermatozoa was 30.0 ± 7.1% and 31.5 ± 7.1%, respectively, in contrast to the 6.2 ± 1.8% and 6.3 ± 1.8%, P < 0.001 in the controls.
The association analyses between double stranded DNA breaks, poor chromatin packaging and age showed that at the breakpoint of 35 years, patient group 1 exhibited significantly lower percentages of TUNEL (26.3 ± 5.3%, P < 0.001) and CMA3 positive (27.5 ± 7.9%, P < 0.001) spermatozoa in comparison to patient group 2 (33.7 ± 6.7% and 35.4 ± 8.2%, respectively). Conversely, in the controls there was no statistically significant difference between the two age groups in the percentages of cells with fragmented DNA and CMA3 positive spermatozoa (Table 2).
Correlation of age with semen parameters, DNA fragmentation and poor chromatin packaging
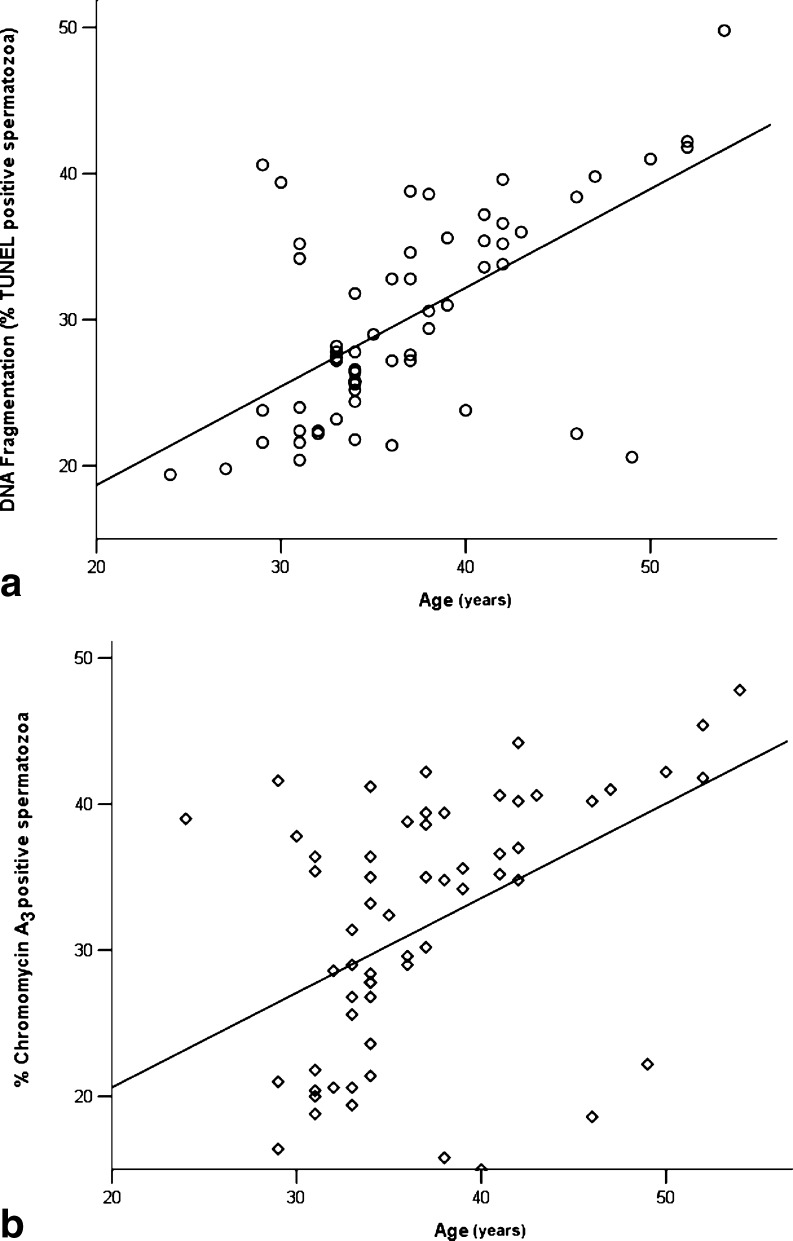
Using the Spearman rank correlation coefficient, statistically significant negative correlations were found between patients’ age and semen volume (r = −0.319, P = 0.012), percentage normal morphology (r = −0.418, P = 0.001) and motility of the ejaculated sperm (r = −0.294, P = 0.022). In contrast, positive and statistically significant correlations were observed between patients’ age and sperm concentration per ml (r = 0.258, P = 0.045), the percentage of sperm with DNA fragmentation (r = 0.558, P < 0.001) and the percentage of CMA3 stained spermatozoa (r = 0.493, P < 0.001; Table 3; Fig. 1).
Table 3.
Correlations of age with conventional semen parameters, DNA fragmentation and poor chromatin packaging in OAT patients and controls
| Volume (ml) | Sperm Concentration (X106/ml) | Morphologically normal sperm (%) | Total progressive motility (%) | DNA fragmentation (% TUNEL positive spermatozoa) | % Chromomycin A3 positive spermatozoa | ||
|---|---|---|---|---|---|---|---|
| Patients | Age (Years) | r = −0.319* | r = 0.258* | r = −0.418* | r = −0.294* | r = 0.558* | r = 0.493* |
| (n = 61) | P = 0.012 | P = 0.045 | P = 0.001 | P = 0.022 | P < 0.001 | P < 0.001 | |
| Controls | Age (Years) | r = 0.060 | r = −0.266 | r = −0.150 | r = −0.206 | r = −0.105 | r = −0.021 |
| (n = 49) | P = 0.682 | P = 0.065 | P = 0.304 | P = 0.155 | P = 0.472 | P = 0.884 |
*P < 0.05
Fig. 1.
Scatter graphs illustrating the associations between age and DNA fragmentation (a; r = 0.558, P < 0.001) as well as age and percentage of Chromomycin A3 positive spermatozoa (b; r = 0.493, P < 0.001)
In the control groups, a positive but not statistically significant correlation was found between age and semen volume (r = 0.060, P = 0.682). In addition, the negative correlations of age with sperm concentration per ml (r = −0.266, P = 0.065), percentage normal morphology (r = −0.150, P = 0.304) and motility of the ejaculated sperm (r = −0.206, P = 0.155) were not statistically significant. Similarly, sperm samples with DNA fragmentation and positively stained with CMA3 had a not statistically significant negative correlation with age (r = −0.105, P = 0.472; r = −0.021, P = 0.884, respectively).
Correlations of DNA fragmentation and poor chromatin packaging
In the patient group, a strong, positive, statistically significant correlation was found between TUNEL and CMA3 positive spermatozoa (r = 0.757, P < 0.001). Similar results were observed in the control group (r = 0.777, P < 0.001).
Table 4 shows the correlation of TUNEL and CMA3 positive spermatozoa with conventional semen parameters.
Table 4.
Correlations of DNA fragmentation (% TUNEL positive spermatozoa) and poor chromatin packaging (% Chromomycin A3 positive spermatozoa) with conventional semen parameters, in OAT patients and controls
| Volume (ml) | Sperm concentration (X106/ml) | Morphologically normal sperm (%) | Total progressive motility (%) | ||
|---|---|---|---|---|---|
| Patients (n = 61) | DNA Fragmentation | r = −0.165 | r = 0.182 | r = −0.349* | r = −0.406* |
| P = 0.205 | P = 0.161 | P = 0.006 | P = 0.001 | ||
| Poor chromatin packaging | r = −0.176 | r = 0.153 | r = −0.295* | r = −0.329* | |
| P = 0.174 | P = 0.240 | P = 0.021 | P = 0.010 | ||
| Controls (n = 49) | DNA Fragmentation | r = 0.105 | r = −0.033 | r = −0.032 | r = −0.112 |
| P = 0.474 | P = 0.821 | P = 0.827 | P = 0.443 | ||
| Poor chromatin packaging | r = 0.080 | r = −0.018 | r = −0.075 | r = −0.211 | |
| P = 0.586 | P = 0.900 | P = 0.608 | P = 0.145 |
*P < 0.05
Discussion
Previous studies have reported that increased male age is significantly associated with a decrease in semen volume, decrease of morphologically normal spermatozoa and decrease of progressive motility while no consistent effect has been reported on sperm concentration [15]. In the present study, OAT patients’ age correlated negatively and significantly with the percentage of morphologically normal spermatozoa and the progressive motility of the ejaculated sperm, while a negative correlation with age was observed in semen volume that was non-significant. In contrast, a statistically significant, positive correlation was observed between OAT patients’ age and sperm concentration, which is supported by several studies [18–21].
These changes in conventional semen parameters of OAT patients, due to increased male age, could be explained by several mechanisms [15]. In the control group, the same trend as in the patients group was observed, although no statistical significance between age and semen parameters was achieved, a finding also observed by Lopes et al. [22], despite the fact that several studies have reported age-related changes in semen quality of fertile men [23–25].
Additionally, a number of studies have demonstrated an age-related increase in sperm cells with double-stranded DNA breaks or poor chromatin packaging [1, 4, 26–28]. In the current study, it was observed that DNA fragmentation and poor chromatin packaging was significantly higher in OAT patients ≥35 years compared to patients younger than 34 years old. While in the control groups, the results that were observed were not statistically significant. This age-related effect may happen as older men may produce more sperm with DNA fragmentation due to higher exposures of oxidative stress in their reproductive tracts [29]. As reported by Aitken et al., oxidative stress can damage both mitochondrial and nuclear membranes as well as sperm DNA [30]. Alternatively, in older men, the apoptotic functions of spermatogenesis are probably less effective, resulting in the production of more spermatozoa with fragmented DNA [31].
These observations of age-related effects on sperm DNA damage were also presented by Wyrobek et al. [1]. Moreover, in the present study, DNA fragmentation and poor chromatin packaging in infertile men appeared to be inversely correlated with conventional semen parameters, a fact also highlighted in several studies [14, 32, 33]. However, this relationship was not found in the spermatozoa of fertile donors, which was also observed by Hughes et al. [34].
The hypothesis that the presence of DNA damage in mature spermatozoa is correlated to poor chromatin packaging [13, 14] has been confirmed as a strong correlation between TUNEL and CMA3 positive spermatozoa was observed. This trend can be attributed to double-stranded DNA breaks that naturally occur in the male germ line both in preparation for recombination and during the process of chromatin packaging [35]. Normally, these DNA breaks are resolved during the spermatid stage of spermatogenesis. It may, therefore, be postulated that abnormal chromatin packaging is due to unresolved DNA breaks in mature human spermatozoa. However, more evidence is required to support this concept.
Although, sperm DNA damage is strongly associated with sperm function and infertility [7], the exact mechanism of sperm DNA damage is still ambiguous. Both intratesticular and post-testicular events have been postulated. Different mechanisms have been proposed to explain the presence of these anomalies in human ejaculated spermatozoa. Sperm DNA damage may be due to oxidative stress, defective sperm chromatin packaging and disordered apoptosis [36–38].
There is also strong clinical evidence that sperm DNA damage in association with increased abnormalities in conventional semen parameters have a pronounced negative effect on reproductive outcome [7, 39–41]. Furthermore, for a threshold value of DNA fragmentation above 10%, a significant negative correlation to the fertilisation rate has been reported by Benchaib et al. [42].
From a clinical point of view, a study by Greco et al. [43] showed that ICSI with testicular spermatozoa is a very efficient assisted reproduction treatment option in men with high levels of sperm DNA fragmentation, because most of the DNA damage observed in ejaculated spermatozoa occurs post-testicularly. Another potential and less invasive approach is the oral administration of the antioxidants vitamin C and E which can reduce the increased incidence of DNA fragmentation in ejaculated spermatozoa [44]. As shown in the current study, OAT patients aged >35 years show greater semen DNA damage compared to younger patients. Therefore, the above treatment options may be considered in such patients based on each patient’s clinical history, previous exams and specific indications.
In conclusion, this study shows an age-related decrease in conventional semen parameters, an increase in human sperm DNA damage and poor chromatin packaging in oligoasthenoteratozoospermic patients, over 35 years. DNA fragmentation, although still controversially discussed, is predictive of pregnancy and it may be associated with de novo genetic abnormalities. Testing sperm chromatin packaging may help in selecting spermatozoa with the least amount of damage for use in assisted conception.
Acknowledgements
The authors are grateful to Mrs. Carol Spanos for the English text editing. The project is co-funded by the European Social Fund and National Resources—(EPEAEK II) PYTHAGORAS (grant “Pythagoras” 70/3/7361).
References
- 1.Wyrobek AJ, Eskenazi B, Young S, Arnheim N, Tiemann-Boege I, Jabs EW, et al. Advancing age has differential effects on DNA damage, chromatin integrity, gene mutations, and aneuploidies in sperm. Proc Natl Acad Sci USA. 2006;103:9601–9606. doi: 10.1073/pnas.0506468103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuhnert B, Nieschlag E. Reproductive functions of the ageing male. Hum Reprod Update. 2004;10:327–339. doi: 10.1093/humupd/dmh030. [DOI] [PubMed] [Google Scholar]
- 3.Morris ID, Ilott S, Dixon L, Brison DR. The spectrum of DNA damage in human sperm assessed by single cell gel electrophoresis (Comet assay) and its relationship to fertilization and embryo development. Hum Reprod. 2002;17:990–998. doi: 10.1093/humrep/17.4.990. [DOI] [PubMed] [Google Scholar]
- 4.Singh NP, Muller CH, Berger RE. Effects of age on DNA double-strand breaks and apoptosis in human sperm. Fertil Steril. 2003;80:1420–1430. doi: 10.1016/j.fertnstert.2003.04.002. [DOI] [PubMed] [Google Scholar]
- 5.Aitken RJ, Krausz C. Oxidative stress, DNA damage and the Y chromosome. Reproduction. 2001;122:497–506. doi: 10.1530/rep.0.1220497. [DOI] [PubMed] [Google Scholar]
- 6.Maher ER, Brueton LA, Bowdin SC, Luharia A, Cooper W, Cole TR, et al. Beckwith-Wiedemann syndrome and assisted reproduction technology (ART) J Med Genet. 2003;40:62–64. doi: 10.1136/jmg.40.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zini A, Libman J. Sperm DNA damage: clinical significance in the era of assisted reproduction. Cmaj. 2006;175:495–500. doi: 10.1503/cmaj.060218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aitken RJ, Gordon E, Harkiss D, Twigg JP, Milne P, Jennings Z, et al. Relative impact of oxidative stress on the functional competence and genomic integrity of human spermatozoa. Biol Reprod. 1998;59:1037–1046. doi: 10.1095/biolreprod59.5.1037. [DOI] [PubMed] [Google Scholar]
- 9.Evenson DP, Jost LK, Marshall D, Zinaman MJ, Clegg E, Purvis K, et al. Utility of the sperm chromatin structure assay as a diagnostic and prognostic tool in the human fertility clinic. Hum Reprod. 1999;14:1039–1049. doi: 10.1093/humrep/14.4.1039. [DOI] [PubMed] [Google Scholar]
- 10.Host E, Lindenberg S, Smidt-Jensen S. The role of DNA strand breaks in human spermatozoa used for IVF and ICSI. Acta Obstet Gynecol Scand. 2000;79:559–563. doi: 10.1034/j.1600-0412.2000.079007559.x. [DOI] [PubMed] [Google Scholar]
- 11.Sun JG, Jurisicova A, Casper RF. Detection of deoxyribonucleic acid fragmentation in human sperm: correlation with fertilization in in vitro. Biol Reprod. 1997;56:602–607. doi: 10.1095/biolreprod56.3.602. [DOI] [PubMed] [Google Scholar]
- 12.Ford WC, North K, Taylor H, Farrow A, Hull MG, Golding J. Increasing paternal age is associated with delayed conception in a large population of fertile couples: evidence for declining fecundity in older men. The ALSPAC Study Team (Avon Longitudinal Study of Pregnancy and Childhood) Hum Reprod. 2000;15:1703–1708. doi: 10.1093/humrep/15.8.1703. [DOI] [PubMed] [Google Scholar]
- 13.Ahmadi A, Ng SC. Developmental capacity of damaged spermatozoa. Hum Reprod. 1999;14:2279–2285. doi: 10.1093/humrep/14.9.2279. [DOI] [PubMed] [Google Scholar]
- 14.Irvine DS, Twigg JP, Gordon EL, Fulton N, Milne PA, Aitken RJ. DNA integrity in human spermatozoa: relationships with semen quality. J Androl. 2000;21:33–44. [PubMed] [Google Scholar]
- 15.Kidd SA, Eskenazi B, Wyrobek AJ. Effects of male age on semen quality and fertility: a review of the literature. Fertil Steril. 2001;75:237–248. doi: 10.1016/S0015-0282(00)01679-4. [DOI] [PubMed] [Google Scholar]
- 16.World Health O. WHO laboratory manual for the examination of human semen and sperm-cervical mucus interaction. 4th ed. Cambridge, UK, Published on behalf of the World Health Organization by Cambridge University Press, 1999.
- 17.Kruger TF, Menkveld R, Stander FS, Lombard CJ, Merwe JP, Zyl JA, et al. Sperm morphologic features as a prognostic factor in in vitro fertilization. Fertil Steril. 1986;46:1118–1123. doi: 10.1016/s0015-0282(16)49891-2. [DOI] [PubMed] [Google Scholar]
- 18.Andolz P, Bielsa MA, Vila J. Evolution of semen quality in North-eastern Spain: a study in 22,759 infertile men over a 36 year period. Hum Reprod. 1999;14:731–735. doi: 10.1093/humrep/14.3.731. [DOI] [PubMed] [Google Scholar]
- 19.Bujan L, Mansat A, Pontonnier F, Mieusset R. Time series analysis of sperm concentration in fertile men in Toulouse, France between 1977 and 1992. Bmj. 1996;312:471–472. doi: 10.1136/bmj.312.7029.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fisch H, Goluboff ET, Olson JH, Feldshuh J, Broder SJ, Barad DH. Semen analyses in 1,283 men from the United States over a 25-year period: no decline in quality. Fertil Steril. 1996;65:1009–1014. doi: 10.1016/s0015-0282(16)58278-8. [DOI] [PubMed] [Google Scholar]
- 21.Irvine S, Cawood E, Richardson D, MacDonald E, Aitken J. Evidence of deteriorating semen quality in the United Kingdom: birth cohort study in 577 men in Scotland over 11 years. Bmj. 1996;312:467–471. doi: 10.1136/bmj.312.7029.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lopes S, Sun JG, Jurisicova A, Meriano J, Casper RF. Sperm deoxyribonucleic acid fragmentation is increased in poor-quality semen samples and correlates with failed fertilization in intracytoplasmic sperm injection. Fertil Steril. 1998;69:528–532. doi: 10.1016/S0015-0282(97)00536-0. [DOI] [PubMed] [Google Scholar]
- 23.Auger J, Kunstmann JM, Czyglik F, Jouannet P. Decline in semen quality among fertile men in Paris during the past 20 years. N Engl J Med. 1995;332:281–285. doi: 10.1056/NEJM199502023320501. [DOI] [PubMed] [Google Scholar]
- 24.Carlsen E, Giwercman A, Keiding N, Skakkebaek NE. Evidence for decreasing quality of semen during past 50 years. Bmj. 1992;305:609–613. doi: 10.1136/bmj.305.6854.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang C, Chan SY, Leung A, Ng RP, Ng M, Tang LC, et al. Cross-sectional study of semen parameters in a large group of normal Chinese men. Int J Androl. 1985;8:257–274. doi: 10.1111/j.1365-2605.1985.tb00840.x. [DOI] [PubMed] [Google Scholar]
- 26.Moskovtsev SI, Willis J, Mullen JB. Age-related decline in sperm deoxyribonucleic acid integrity in patients evaluated for male infertility. Fertil Steril. 2006;85:496–499. doi: 10.1016/j.fertnstert.2005.05.075. [DOI] [PubMed] [Google Scholar]
- 27.Schmid TE, Eskenazi B, Baumgartner A, Marchetti F, Young S, Weldon R, et al. The effects of male age on sperm DNA damage in healthy non-smokers. Hum Reprod. 2007;22:180–187. doi: 10.1093/humrep/del338. [DOI] [PubMed] [Google Scholar]
- 28.Zubkova EV, Wade M, Robaire B. Changes in spermatozoal chromatin packaging and susceptibility to oxidative challenge during aging. Fertil Steril. 2005;84(Suppl 2):1191–1198. doi: 10.1016/j.fertnstert.2005.04.044. [DOI] [PubMed] [Google Scholar]
- 29.Barroso G, Morshedi M, Oehninger S. Analysis of DNA fragmentation, plasma membrane translocation of phosphatidylserine and oxidative stress in human spermatozoa. Hum Reprod. 2000;15:1338–1344. doi: 10.1093/humrep/15.6.1338. [DOI] [PubMed] [Google Scholar]
- 30.Aitken RJ, Baker MA, Sawyer D. Oxidative stress in the male germ line and its role in the aetiology of male infertility and genetic disease. Reprod Biomed Online. 2003;7:65–70. doi: 10.1016/S1472-6483(10)61730-0. [DOI] [PubMed] [Google Scholar]
- 31.Print CG, Loveland KL. Germ cell suicide: new insights into apoptosis during spermatogenesis. Bioessays. 2000;22:423–430. doi: 10.1002/(SICI)1521-1878(200005)22:5<423::AID-BIES4>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 32.Moskovtsev SI, Willis J, Azad A, Mullen JB. Sperm DNA integrity: correlation with sperm plasma membrane integrity in semen evaluated for male infertility. Arch Androl. 2005;51:33–40. doi: 10.1080/014850190512770. [DOI] [PubMed] [Google Scholar]
- 33.Shen H, Ong C. Detection of oxidative DNA damage in human sperm and its association with sperm function and male infertility. Free Radic Biol Med. 2000;28:529–536. doi: 10.1016/S0891-5849(99)00234-8. [DOI] [PubMed] [Google Scholar]
- 34.Hughes CM, Lewis SE, McKelvey-Martin VJ, Thompson W. A comparison of baseline and induced DNA damage in human spermatozoa from fertile and infertile men, using a modified comet assay. Mol Hum Reprod. 1996;2:613–619. doi: 10.1093/molehr/2.8.613. [DOI] [PubMed] [Google Scholar]
- 35.Sakkas D, Mariethoz E, St John JC. Abnormal sperm parameters in humans are indicative of an abortive apoptotic mechanism linked to the Fas-mediated pathway. Exp Cell Res. 1999;251:350–355. doi: 10.1006/excr.1999.4586. [DOI] [PubMed] [Google Scholar]
- 36.Agarwal A, Said TM. Role of sperm chromatin abnormalities and DNA damage in male infertility. Hum Reprod Updat. 2003;9:331–345. doi: 10.1093/humupd/dmg027. [DOI] [PubMed] [Google Scholar]
- 37.Sakkas D, Seli E, Bizzaro D, Tarozzi N, Manicardi GC. Abnormal spermatozoa in the ejaculate: abortive apoptosis and faulty nuclear remodelling during spermatogenesis. Reprod Biomed Online. 2003;7:428–432. doi: 10.1016/S1472-6483(10)61886-X. [DOI] [PubMed] [Google Scholar]
- 38.Sakkas D, Urner F, Bizzaro D, Manicardi G, Bianchi PG, Shoukir Y, et al. Sperm nuclear DNA damage and altered chromatin structure: effect on fertilization and embryo development. Hum Reprod. 1998;13(Suppl 4):11–19. doi: 10.1093/humrep/13.suppl_4.11. [DOI] [PubMed] [Google Scholar]
- 39.Henkel R, Hajimohammad M, Stalf T, Hoogendijk C, Mehnert C, Menkveld R, et al. Influence of deoxyribonucleic acid damage on fertilization and pregnancy. Fertil Steril. 2004;81:965–972. doi: 10.1016/j.fertnstert.2003.09.044. [DOI] [PubMed] [Google Scholar]
- 40.Li Z, Wang L, Cai J, Huang H. Correlation of sperm DNA damage with IVF and ICSI outcomes: a systematic review and meta-analysis. J Assist Reprod Genet. 2006;23:367–376. doi: 10.1007/s10815-006-9066-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Virro MR, Larson-Cook KL, Evenson DP. Sperm chromatin structure assay (SCSA) parameters are related to fertilization, blastocyst development, and ongoing pregnancy in in vitro fertilization and intracytoplasmic sperm injection cycles. Fertil Steril. 2004;81:1289–1295. doi: 10.1016/j.fertnstert.2003.09.063. [DOI] [PubMed] [Google Scholar]
- 42.Benchaib M, Braun V, Lornage J, Hadj S, Salle B, Lejeune H, et al. Sperm DNA fragmentation decreases the pregnancy rate in an assisted reproductive technique. Hum Reprod. 2003;18:1023–1028. doi: 10.1093/humrep/deg228. [DOI] [PubMed] [Google Scholar]
- 43.Greco E, Scarselli F, Iacobelli M, Rienzi L, Ubaldi F, Ferrero S, et al. Efficient treatment of infertility due to sperm DNA damage by ICSI with testicular spermatozoa. Hum Reprod. 2005;20:226–230. doi: 10.1093/humrep/deh590. [DOI] [PubMed] [Google Scholar]
- 44.Greco E, Iacobelli M, Rienzi L, Ubaldi F, Ferrero S, Tesarik J. Reduction of the incidence of sperm DNA fragmentation by oral antioxidant treatment. J Androl. 2005;26:349–353. doi: 10.2164/jandrol.04146. [DOI] [PubMed] [Google Scholar]