Abstract
Purpose
To investigate the clinical application of fluorescence in situ hybridization (FISH) for assessing chromosome disorders of embryos in preimplantation diagnosis of carriers with der(15)t(Y;15)(q12;p11) translocations.
Methods
Multicolor FISH was performed using directly-labelled DNA probes, chromosome X with one (DXZ1, Xp11.1-q11.1), but Y with two (DYZ3, Yp11.1-q11.1 and DYZ1, Yq12). Normal embryos were transferred on day 6 at blastocyst stage.
Results
Couple A: Three of 6 biopsied embryos were normal. Two normal blastocysts were transferred, but no pregnancy was achieved. Couple B: Three of 6 biopsied embryos were normal. Two normal blastocysts were transferred. A normal male infant weighing 3,230 g was born by cesarean section on the 39th week of gestation. All of the remaining nonreplaced embryos showed mosaic or der(15).
Conclusion
Embryos from carries of der(15)t(Y;15)(q12;p11) translocation showed a high frequency of chromosome abnormalities. PGD is a valuable screen tool for those couples to treat their infertility and break the transmission of der(15) chromosome for their offspring.
Keywords: Preimplantation genetic diagnosis, der(15)t(Y;15)(q12;p11) translocation, FISH
Introduction
Translocations involving the Y and an autosome are very rare. The incidence is about 1/2,000 in general population. But translocation between the heterochromatin of the long arm of chromosomes Y (breakpoint at q12) and the short arm of chromosome 15 (breakpoint at p11-13) is the most frequent one [1, 2]. This may be the consequence of a frequent association at the pachytene stage of male meiosis of the 15p and Yq heterochromatin based on sequence homology [3].
Yq12 and 15p belong to heterochromatic region of human chromosome. In traditional genetic view, some change of heterochromatic region used to be considered as polymorphic chromosomal variants of population. These carriers of der(15)t(Y;15)(q12; p11) translocations usually have normal phenotype with unaffected fertility, therefore usually used to called variants [4]. However, some reports found that der(15)t(Y;15)(q12; p11) carriers could have abnormal phenotype like infertility, repeated spontaneous abortion, stillbirths, liveborn children with defects [5, 6]. These researches showed genetic counseling and early prenatal prevention were very important when these patients hoped for obtaining normal children.
Preimplantation genetic diagnosis (PGD) has been well established for carriers of reciprocal and Robertsonian translocations [7, 8]. However, to our knowledge, PGD for carriers of der(15) t(Y;15) translocation has not yet been reported. To assess the feasibility of PGD in determination of this type translocation, here we apply the technique to two patients with der(15) t(Y;15) translocation in analysis of embryo karyotypes by FISH.
Materials and methods
Patients
Two carriers with der(15) translocation attended our reproductive center for preimplantation genetic diagnosis. The patients and their respective partners were seen by a genetic counselor prior to IVF treatment and signed a consent form approved by ethical board of our hospital.
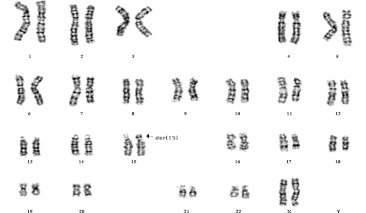
Couple A had twice spontaneous abortions, karyotyping of the 33-year-old wife was performed by G-banded metaphase spreads of cultured peripheral blood lymphocytes using conventional methods. In all observed metaphases, GTG banding showed a 15P+ chromosome (Fig. 1), and further confirmed the additional short arm of this der(15) to be mainly heterochromatic by CBG-banding, suggesting a Y chromosome origin. Probe for Yq12 was applied by FISH, the translocation between Yq12 and 15p11 was identified. Finally the patient’s karyotype was interpreted as 46, XX,der(15)t(Y;15)( q12;p11) (see Fig. 2).
Fig. 1.
G banded karyotype showing the der(15) (arrow) in the female carrier of couple A
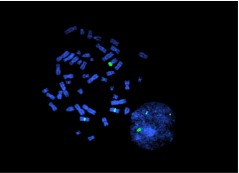
Fig. 2.
FISH result showed that there were two chromosomes X and one derived chromosome 15 contained Yq12 in peripheral blood lymphocyte from the female carrier of couple A. The specific probe for chromosome X (DXZ1, Xp11.1-q11.1) was labelled in aqua, for chromosome Yq12 (DYZ1, q12) in green
Couple B complained primary infertility for 3 years, caused by husband asthenozoospermia and obstructive fallopian tubes of the 27-year-old wife. The husband also had abnormal karyotype interpreted as 46, XY,der(15)t(Y;15)( q12;p11) determined by same procedures as the first one (Figs. 3 and 4).
Fig. 3.
G (left) and C (right) banded karyotype showing the der(15) (arrow) in the male carrier of couple B
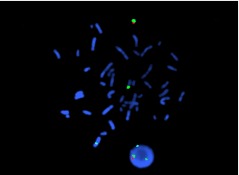
Fig. 4.
FISH result showed that there were one chromosome X, one chromosome Y and one derived chromosome 15 contained Yq12 in peripheral blood lymphocyte from the male patient of couple B. The specific probe for chromosome X (DXZ1, Xp11.1-q11.1) was labelled in aqua, for chromosome Yq12 (DYZ1, q12) in green, for chromosome Y (DYZ1, DYZ3, Yp11.1-q11.1) in orange
Embryos and biopsy
Ovarian stimulation was performed using protocol with GnRHa, recombinant FSH and hCG. Oocytes and embryos were cultured in GIII series culture media (Vitrolife). The embryos biopsy were carried out by mechanical method at 6–8 cell stage on day 3. One or two blastomeres were removed from each embryo. The nuclei of all blastomeres were spread with 1%Tween-20/0.01N HCl following Dozortsev methods [9], and then were analyzed by FISH procedure. Abnormal and nonreplaced embryos were also reanalyzed with all or most of their blastomeres on day 6.
FISH procedure
Three commercial α-satellite (DNA) probes were ordered from Vysis (Vysis, Downers Grove, IL, USA), special for chromosomes X (DXZ1, Xp11.1-q11.1, Spectrum Aqua), Y (DYZ3, Yp11.1-q11.1, Spectrum Orange), Y12 (DYZ1, q12, Spectrum Green). The probes were prepared according to the manufacturer’s instructions. Briefly, three probes were mixed equally and then added to hybridization buffer (50% formamide/50% dextran sulphate) to a final volume of 10 μl. Probes and slides were denatured separately for 5 min at 75°C in a water bath. Each probe mix was applied to the denatured slides, and slides were covered with coverslips, sealed with rubber cement and hybridized overnight in a dark, moist chamber at 37°C, Coverslips were gently removed, and the slides were washed three times in 50% formamide/2X SSC solution, then once in 2X SSC and once in 2X SSC/0.1%NP-40 at 45°C. Finally mounted with 10 μl DAPI (4′,6-diamidino-2-phenylindole) in antifade solution. The slides were analyzed by two independent observers using a Nikon fluorescence microscope equipped with a filter set for FITC, Texas Red, Aqua and DAPI/Texas Red/FITC.
Classification of embryo chromosome patterns
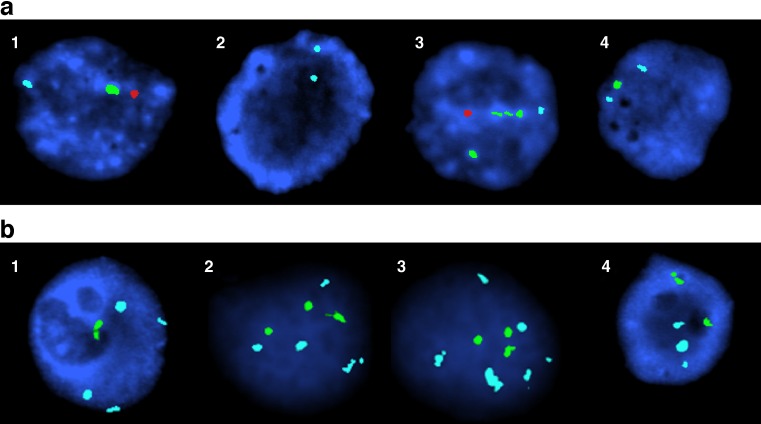
Two aqua signals indicated normal female embryo (XX); two aqua signals and one green signal indicated abnormal female embryo with derivation chromosome 15 (XX,der(15)); one aqua , one green and one orange signal indicated normal male embryo(XY); but one aqua signal, two green signals and one orange signal indicated abnormal male embryo with derivation chromosome 15 (XY,der(15))(see Fig. 5a). Some abnormal Karyotypes were also illustrated as Fig. 5b according to the FISH method.
Fig. 5.
a FISH results obtained in part of embryos from couple A on D3: 1 XY, 2 XX, 3 XY,der(15), 4 XX,der(15). The specific probe for chromosome X (DXZ1, Xp11.1-q11.1) was labelled in aqua, for chromosome Yq12 (DYZ1, q12) in green, for chromosome Y (DYZ1, DYZ3, Yp11.1-q11.1) in orange. b FISH results obtained in part of spare embryos from couple B on D6: 1 XXXX,der(15), 2 XXXX,der(15),der(15),der(15), 3 XXXXXX,der(15),der(15),der(15), 4 XXX,der(15), der(15). The specific probe for chromosome X (DXZ1, Xp11.1-q11.1) was labelled in aqua, for chromosome Yq12 (DYZ1, q12) in green, for chromosome Y (DYZ1, DYZ3, Yp11.1-q11.1) in orange
Transfer and follow-up
Normal karyotype embryos with the higher score were selected for transfer on day 6. Luteal phase supports were given routinely using hCG and progesterone. Serum β-hCG levels were measured at 14 days after ET. Clinical pregnancy was defined by the presence of a gestational sac and fetal heart beat by ultrasound at 5 weeks after ET. Amniocentesis was performed at 18 weeks of gestation.
Results
Clinical results and embryo FISH analysis
Couple A
A total 8 oocytes were collected and inseminated by ICSI technique. Six oocytes were fertilized, and fortunately all reached 6–8 cell stage at 72 h post ICSI. They were all biopsied. After FISH detection, three normal embryos without der(15) were found, the other three abnormalities including one embryo had two nuclei and the other one showed XY,der(15), while the third one is XX,der(15) (see Table 1; Fig. 5a). Two normal embryos developed to blastocyst stage at day 6, and were transferred, but no pregnancy was achieved. Abnormal and nonreplaced embryos were reanalyzed, and interestingly, three abnormal embryos all showed mosaic karyotypes (Table 1).
Table 1.
FISH analysis and clinic results obtained in embryos from couple A
| Embryo no. | Cell numbers of biopsied embryos on D3 | FISH results | Embryo morphology and treatment on D6 | FISH results of spare embryos on D6 | Genetic constitution |
|---|---|---|---|---|---|
| 1 | 11 | Including two nuclei, one was XY, one had no signal | Degenerated, discarded | XY[12]/XXY[1]/X[2] | Mosaic |
| 2 | 8 | XX | Morula, transferred | Normal | |
| 3 | 11 | XX | Degenerated, discarded | XX[6] | Normal |
| 4 | 8 | XY,der(15) | Morula, discarded | XY,der15[8]/XXY,der15[4]/XXX[2] | Mosaic |
| 5 | 8 | XX,der(15) | Blastocyst, discarded | XX,der15[28]/X[3]/XX[2]/XXXXder15[4] | Mosaic |
| 6 | 8 | XY | Blastocyst, Transferred | Normal |
Couple B
A total of 11 oocytes were collected and inseminated by ICSI technique. Seven were fertilized, six embryos reached 6–8 cell stage at 72 h post ICSI, and six embryos all were biopsied at this time. Three normal embryos without der(15) were found, the other three were abnormal (Table 2). Abnormal and nonreplaced embryos were reanalyzed on day 6. Two abnormal embryos were found as mosaic karyotypes (see Table 2; Fig. 5b). Two of three normal embryos developed to blastocyst stage and were transferred on day 6. A successful pregnancy was achieved and one sac with fetal heart beat observed on 34 days after the embryo replacement. Cytogenetic analysis of cultured amnion cells showed a normal karyotype with 46, XY. A normal male infant weighting 3,230 g was born at the 39th week of gestation by cesarean section.
Table 2.
FISH analysis and clinic results obtained in embryos from couple B
| Embryo no. | Cell numbers of biopsied embryos on D3 | FISH results | Embryo morphology and treatment on D6 | FISH results of spare embryos on D6 | Genetic constitution |
|---|---|---|---|---|---|
| 1 | 5 | XXXXXX | Degenerated, discarded | XX[3] | Mosaic |
| 2 | 11 | XX | Blastocyst, transferred | Normal | |
| 3 | 6 | XX,der(15) | Blastocyst, discarded | XX,der(15)[30] | Carrier |
| 4 | 9 | XX,der(15) | Blastocyst, discarded | XX,der(15)[14]/XXX,der (15)[2]/XXXXXX,der(15),der(15),der(15)[3]/XXX,der(15),der(15)[2]/XXXX,der(15),der(15),der(15)[1]/XXXX,der(15)[1] | Mosaic |
| 5 | 10 | XX | Degenerated, discarded | XX[6] | Normal |
| 6 | 10 | XY | Blastocyst, transferred | Normal |
Discussion
To our knowledge, this is the first report of a normal birth following PGD to select embryos for transfer in carriers with der(15)t(Y;15) (q12; p11) by now.
This association of t(Y;15)(q12;p11) translocations with reproductive abnormalities were still controversial. Most of carriers are fertile, so this karyotype used to be considered as variants of populations. However, some carriers had history of infertility, abortion or abnormal phenotypes. It was reported that one azoospermia male patient had been performed cytogenetic analysis, the karyotype showed 46,XY,t(Y;1)(cen-q11; cen-p11),(Y;15)(q12;p11), and further it was found his mother carried 46,XX,t(Y;15)(q12;p11) [10]. Recurrent spontaneous abortion was also reported in a male partner carried a der(15)t(Y;15) (q12;p12) [5]. In the present report, couple A had twice abortions, while the male carrier of couple B is asthenozoospermia. These suggested that translocation between chromosome Yq12 and 15p might relate to some infertility problems.
Recently, two studies further hypothesized relationship between the t(Y;15)(q12;p11) and some clinic syndromes, such as Prader–Willi (PWS), Angelman syndromes (AS) and uniparental disomy (UPD). One report found a female carrier of der(15)t(Y;15)(q12;p13) who had two pregnancy losses with trisomy 15 and one with tetraploidy. They suggested that there may be an increased risk for trisomy 15 in some carriers of unbalanced t(Y;15) [6]. Another report described a child with PWS due to 15q11-q13 microdeletion. The deletion occurred on a paternally derived der(15)t(Y;15) (q12;p11). Alternatively, the association may have been fortuitous too [11]. It remains to be proven and further investigated.
To provided more comprehensive genetic information about der(15)t(Y;15) translocations, the present work applied PGD technique to analyze preimplantation embryo karyotype in two carriers of der(15)t(Y;15) translocations. Six of 12 embryos showed abnormality. The higher frequency of mosaic embryos were observed in the abnormal embryos (5/6), including sex chromosome mosaic, sex chromosome/der(15) abnormal mosaic embryos, and der(15) carrier. The results suggested the occurrence of high proportion of unbalanced and mosaic embryos from carriers of der(15)t(Y;15) translocations may account for the repeated abortions and sterility. It really reminded us these were possibly related to the absence of the normal cell cycle check-points during early cleavage described by some investigators [12–14]. In our work the normal birth suggested that PGD is a worthy screen method for these carriers, not only to treat their infertility, but also to break these translocation to inherit to their offspring.
References
- 1.Alitalo T, Tiihonen J, Hakola P, Chapelle A. Molecular characterization of a Y;15 translocation segregating in a family. Hum Genet. 1988;79:29–35. doi: 10.1007/BF00291705. [DOI] [PubMed] [Google Scholar]
- 2.Powell C. Sex chromosomes and sex abnormalities. In: Gersen GL, Keagle MB, editors. The principles of clinical cytogenetics. Totowa, NJ: Humana Press; 1999. pp. 229–258. [Google Scholar]
- 3.Metzler-Guillemain C, Mignon C, Depetris D, Guichaoua MR, Mattei MG. Bivalent 15 regularly associates with the sex vesicle in normal male meiosis. Chromosome Res. 1999;7:369–378. doi: 10.1023/A:1009268014387. [DOI] [PubMed] [Google Scholar]
- 4.Neumann A, Robson L, Smith A. A 15p variant shown to be a t(Y;15) with fluorescence in situ hybridization. Ann Genet. 1992;35:227–230. [PubMed] [Google Scholar]
- 5.Fukada Y, Yasumizu T, Amemiya A, Kohno K, Takizawa M, Hoshi K. Prenatal confirmation of the translocation between chromosome 15 and Y-chromosome by fluorescence in situ hybridization. Tohoku J Exp Med. 1999;187:285–289. doi: 10.1620/tjem.187.285. [DOI] [PubMed] [Google Scholar]
- 6.Rajcan-Separovic E, Robinson WP, Stephenson M. Recurrent trisomy 15 in a female carrier of der(15)t(Y;15)(q12;p13) Am J Med Genet. 2001;99:320–324. doi: 10.1002/1096-8628(2001)9999:9999<::AID-AJMG1173>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 7.Kyu Lim C, Hyun Jun J, Mi Min D, Lee HS, Young Kim J, Koong MK, et al. Efficacy and clinical outcome of preimplantation genetic diagnosis using FISH for couples of reciprocal and Robertsonian translocations: the Korean experience. Prenat Diagn. 2004;24:556–561. doi: 10.1002/pd.923. [DOI] [PubMed] [Google Scholar]
- 8.Verlinsky Y, Cohen J, Munne S, Gianaroli L, Simpson JL, Ferraretti AP, et al. Over a decade of experience with preimplantation genetic diagnosis: a multicenter report. Fertil Steril. 2004;82:292–294. doi: 10.1016/j.fertnstert.2003.09.082. [DOI] [PubMed] [Google Scholar]
- 9.Dozortsev DI, McGinnis KT. An improved fixation technique for fluorescence in situ hybridization for preimplantation genetic diagnosis. Fertil Steril. 2001;76:186–188. doi: 10.1016/S0015-0282(01)01836-2. [DOI] [PubMed] [Google Scholar]
- 10.Gregori-Romero M, Lopez-Gines C, Gil R, Galan Sanchez F, Pellin-Perez A. 2 new cases of Y-autosome translocation associated with azoospermia. Rev Clin Esp. 1990;187:71–73. [PubMed] [Google Scholar]
- 11.Eliez S, Morris MA, Dahoun-Hadorn S, DeLozier-Blanchet CD, Gos A, Sizonenko P, et al. Familial translocation t(Y;15)(q12;p11) and de novo deletion of the Prader–Willi syndrome (PWS) critical region on 15q11-q13. Am J Med Genet. 1997;70:222–228. doi: 10.1002/(SICI)1096-8628(19970613)70:3<222::AID-AJMG3>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 12.Delhanty JD, Handyside AH. The origin of genetic defects in the human and their detection in the preimplantation embryo. Hum Reprod Update. 1995;1:201–215. doi: 10.1093/humupd/1.3.201. [DOI] [PubMed] [Google Scholar]
- 13.Delhanty JD, Harper JC, Ao A, Handyside AH, Winston RM. Multicolour FISH detects frequent chromosomal mosaicism and chaotic division in normal preimplantation embryos from fertile patients. Hum Genet. 1997;99:755–760. doi: 10.1007/s004390050443. [DOI] [PubMed] [Google Scholar]
- 14.Iwarsson E, Malmgren H, Inzunza J, Ahrlund-Richter L, Sjoblom P, Rosenlund B, et al. Highly abnormal cleavage divisions in preimplantation embryos from translocation carriers. Prenat Diagn. 2000;20:1038–1047. doi: 10.1002/1097-0223(200012)20:13<1038::AID-PD976>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]