Abstract
Purpose: To investigate the effect of microenvironment maintenance on embryo culture and clinical results using two types of incubators.
Methods: Temperature and oxygen concentration in a mini-incubator and a conventional incubator were compared following a 5-s door opening/closing procedure. Embryos of 30 in-vitro fertilization embryo transfer (IVF-ET) cases were randomly allocated to either one of the incubator, cultured, and the early-stage good embryo formation rate and the good blastocyst formation rate were compared, as indicators for micro-environment maintenance ability.
Results: Temperature recovery after a 5-s door opening/closing procedure was approximately 5 min for the mini-incubator and 30 min for the conventional incubator. The oxygen concentration return was significantly improved in the mini-incubator (3.0 ± 0 min) compared with the conventional incubator (7.8 ± 0.9 min). Both the early-stage good embryo formation rate and the good blastocyst formation rate were significantly higher in the mini-incubator (39.5% and 15.1%) than the conventional incubator (28.4% and 7.8%).
Conclusion: The microenvironment maintenance ability of incubators appears to significantly influence the formation of good embryos.
Keywords: IVF-ET, Incubator, Micro-environment maintenance, Embryo formation rate
Introduction
One of the reasons for the improvement in IVF-ET results is believed due to commercially-available standard materials required for embryo culture. As for culture media, although the usefulness of various media has been compared by researchers, no great differences have been found [1, 2]. The standard micro-environment for culture is generally held to be a temperature of 37°C, with gas concentrations of O2 5%, CO2 5%, and N2 90% [3]. Also, to maintain an optimum environment, reports note the use of various types of heaters during embryo culture management [4] and the adjustment of incubator temperature referencing actual media temperature, aiming for more precise temperature control [5]. In this study, we compared the influence of micro-environment maintenance ability on embryo culture outcome, using two types of incubators: a top-load mini-incubator (K-MINC-1000, COOK, Co., Australia), which is considered to provide high maintenance ability of temperature and gas concentration phase, and a conventional front-load incubator with a water-jacket heating system (Personal Multi Gas CO2 incubator, APM-30D, ASTEC, Co. Japan).
Materials and methods
Assessment of micro-environment maintenance ability for embryo culture
A small-chamber, bench-top, mini-incubator (K-MINC-1000, COOK, Co., Australia) is based on a dish method, having heaters both above and beneath the dish in order to warm the dishes directly. This top-load four-well dish type incubator can accommodate a maximum of 8 dishes (Fig. 1A). Whereas, the front-load conventional incubator (Personal Multi Gas CO2 incubator, APM-30D, ASTEC, Co. Japan) used in our study accommodates a maximum of 48 plates (60 mm Petri dishes), permitting greater storage area for each patient as the chamber of the front-load incubator is relatively large. However, this incubator is based on a water jacket heating method, which heats the chamber wall using hot water and takes time to restore the gas concentration phase in the chamber (Fig. 1B).
Fig. 1.
Photograph of top-load mini-incubator and front-load conventional incubator: (A) top-load mini-incubator; (B) front-load conventional incubator
In this study, we investigated and compared the environmental maintenance ability associated with a timed opening of the two types of incubators. Assuming a general average open-door duration of 5 sec, we determined the elapsed time between the door-closing (after remaining open for 5 sec) and the point in time when oxygen concentration recovered to standard levels. The standard incubator temperature was set at 37°C and the room temperature of the laboratory was set at 25°C. A thermistor-thermometer was used to measure temperature inside the incubator chamber and in the dish, and the gas indicator of the incubator was used to determine oxygen concentration. This procedure was repeated 10 times and the average values were recorded as results.
Assessment of embryo culture outcome
Our subjects were 30 infertile patients (average 34.8 years old) from whom 8 or more oocytes were retrieved at our institution between June 2005 and April 2006. The average number of retrieved oocytes per subject was 17.4. All inseminations were done after oocyte retrieval using Universal IVF mediumTM (MediCult, Co., Denmark). After incubating the fertilized eggs for 17 h using a conventional incubator and confirming that the embryo was in the 2-PN stage, the embryos from one patient were randomly allocated to either one of the two incubators, and cultured using commercially available culture media (BlastAssist System mediumTM; MediCult, Co., Denmark). Then, the early-stage good embryo formation rate and the good blastocyst formation rate were compared. Embryo culture was done by the open method (each embyo was transferred separately in a 0.5 ml droplet of culture medium overlaid with nothing), culturing each embryo in one independent well, and the growth of each embryo was observed.
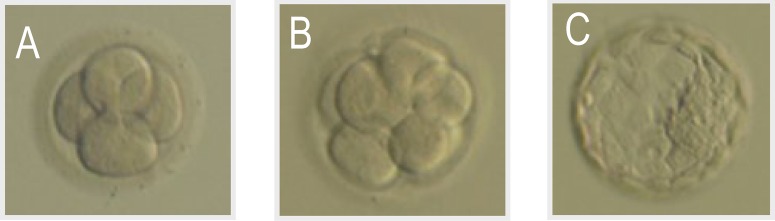
The Bolton classification [6] was used to define a good early-stage embryo when it had developed to 4 cells at day 2 (in Grade 3 or higher) and 8 cells at day 3 (in Grade 3 or higher). The Gardner classification [7] was used to define a good blastocyst as 3AB or higher at day 5 (Fig. 2).
Fig. 2.
Embryo and blastcyst of good condition: (A) Early-stage embryo, 4 cells, G3; (B) Early-stage embryo, 8 cells, G3; (C) Blastocyst, 3AB
Statistical analysis
Values were expressed as the mean ± SD Differences between groups were statistically analyzed using the Kolmogorov-Sminov two-sample test or the chi-square test. A p-value < 0.05 was considered to be statistically significant.
Results
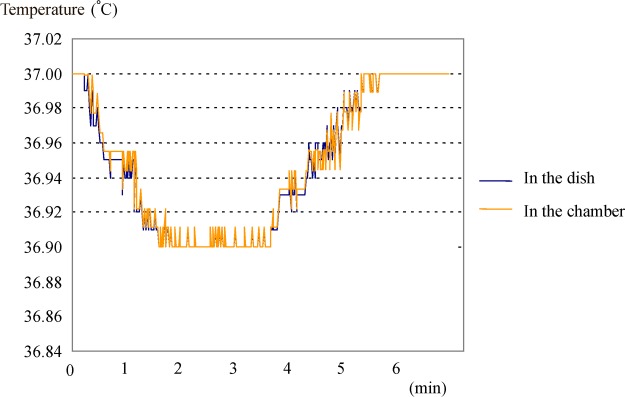
With the top-load mini-incubator, the time necessary to for the temperature to recover after the 5-s opening of the incubator door was approximately 5 min for both inside the chamber and in the dish (36.9–37.0°C) (Fig. 3).
Fig. 3.
Temperature changes of top-load mini-incubator (after opening the door for 5 s)
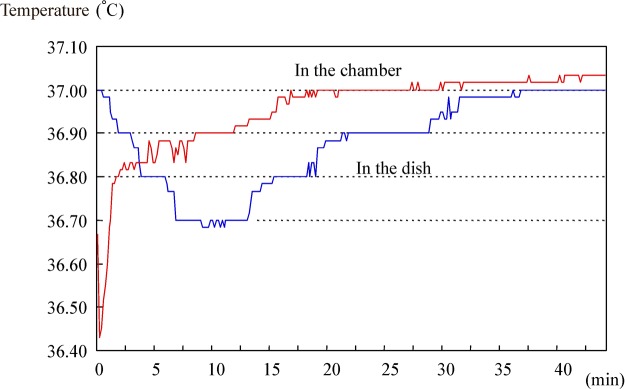
With the conventional front-load incubator, time-lapse for temperature recovery after the 5-s opening of the door was approximately 20 min for the chamber; the temperature in the chamber rapidly and greatly decreased to 36.4°C before recovery to 37°C. However, the temperature in the dish decreased more gradually, reaching its lowest of 36.7°C at 10 min after closing the door, and took approximately 30 min to recover to 37°C (Fig. 4).
Fig. 4.
Temperature changes of conventional front-load incubator (after opening the door for 5 s)
Regarding the oxygen concentration recovery, the mini-incubator took a significantly shorter time (3.0 ± 0 min) compared with the conventional incubator (7.8 ± 0.9 min) (Table 1).
Table 1.
Comparison of micro-environment recovery
| Front-load | ||
|---|---|---|
| Top-load | conventional | |
| mini-incubator | incubator | |
| Temperature | 4.9 ± 0.5 min* | 31.5 ± 2.9 min |
| O2 | 3.0 ± 0 min* | 7.8 ± 0.9 min |
Note. Time-lapse for recovery after opening the door for 5 s.
*P<0.01 (mini-incubator vs. conventional incubator).
Following the incubator culturing process, we found that the early-stage good embryo formation rate was significantly higher in the mini-incubator (75 of 190 fertilized eggs, 39.5%) compared with the conventional incubator (42 of 158 fertilized eggs, 28.4%). The good blastocyst formation rate after the continuing culture process was also significantly higher in the mini-incubator (25 of 166 fertilized eggs, 15.1%) compared with the conventional incubator (10 of 139 fertilized eggs, 7.8%) (Table 2).
Table 2.
Embryo formation rate and blastocyst formation rate
| Top-load mini-incubator | Front-load conventional incubator | |
|---|---|---|
| Early-stage embryo formation rate (%) (good embryos/Fertilized eggs) | 40.3* (75/186) | 28.4 (42/148) |
| Blastocyst formation rate (%) (good blastocysts/total embryos) | 15.1* (25/166) | 7.2 (10/139) |
*P<0.05 (mini-incubator vs. conventional incubator).
Although we could not evaluate the pregnancy rate, as we used multiple oocytes from each patient, the eventual pregnancy rate was 37.8%.
Discussion
After the opening/closing of the door, the environment recovery ability of the mini incubator proved to be superior to that of the conventional incubator, showing a great difference in the temperature maintenance ability. Important factors in the maintenance of embryo culture temperature, other than the type of incubator, have been reported to include the use of the stage warmer during fertilization management, the amount of media, and the use of dishes [8]. The open method used at our institution is considered better than the drop method in managing the appropriate temperature, due to the large amount of culture medium. We evaluated the benefit of the open method for the maintenance ability of the temperature, considering the importance of temperature maintenance and recovery [3]. We understand the benefit of the drop method and we anticipate increased use of the drop method in the future because of the cost effectiveness. Another reason that we evaluated the open method is that we have been accumulating the established clinical data using the open method.
The reason for relatively large drop in temperature in the conventional incubator (to 36.4°C) was likely due to the large air-exchange volume when the door was opened with additional effect for the cold gas infusion from the gas cylinder necessary to restore gas concentration. Temperature recovery in the incubator dishes lagged behind that of the gradual recovery of the chamber. The maximum difference of the temperature was 0.6°C in our study, but temperature differences in ordinary clinical settings are anticipated to be greater, with the lowered temperature continuing for larger periods than our results, because the door is opened more frequently for observation and replacement of culture media. When there is a large number of patients, or many oocytes are retrieved from one patient, the maintenance of the micro-environment in the incubator is believed to affect the culture outcome. Our clinical results show the good embryo formation rate to be significantly higher in a top-load mini-incubator culture.
We need to study a larger number of cases for a more in depth analysis. However, our patient number was limited to a certain period of time. One beneficial aspect of our study is that we could compare the embryo culture environment with less bias, as we allocated multiple samples of the same patient randomly to one or the other of the incubators. We hope this explains the limitations and parameters of our study. Furthermore, we defined the good embryo formation rate as a comparative scale of the microenvironment for embryos, not the successful formation rate of blastocysts. In this way, the aim of our study was different from other similar studies.
Mini-incubators have been reported to provide significantly better pregnancy rates and lower miscarriage rates in IVF-ET outcome, however, the protocols of these comparative studies and the subject patient characteristics were varied [5, 9]. In an evaluation of external fertilization, especially when assessing the pregnancy rate, it is difficult to eliminate the bias in patients. Although our study was not designed to evaluate pregnancy rate, we believe our random allocation of embryos of the same patient served to reduce such bias.
Although the larger chamber is made with a water-jacket system, we think that its capability of retaining heat is better than the one with air-jacket system. On the other hand, an air-jacket system is considered to provide quick recovery of various parameters.
Regarding the CO2 concentration and pH level, the larger chamber incubator has a TC sensor, which displays CO2 concentration also (simultaneously measured). However, we did not include the CO2 analysis in our study. The CO2 concentration does not fluctuate by opening and closing the door, because the concentration of CO2 in the ambient air is small. The mini incubator we used did not have a function to measure CO2 concentration, because it directly sends mixed air. In our study, we could compare ability to gas recovery of the two incubators based on the time of temperature recovery only.
Our results confirm that the micro-environment maintenance ability of an incubator influences the rate of successful formation of good embryos, and that improvement of this micro-environment can be easily achieving by replacing the culture equipment.
References
- 1.Van Langendonckt A, Demylle D, Wyns C, Nisolle M, Donnez J. Comparison of G1.2/G2.2 and Sydney IVF cleavage/blastocyst sequential media for the culture of human embryos: a prospective, randomized, comparative study. Fertil Steril. 2001;76:1023–1031. doi: 10.1016/S0015-0282(01)02854-0. [DOI] [PubMed] [Google Scholar]
- 2.Zollner KP, Zollner U, Schneider M, Dietl J, Steck T. Comparison of two media for sequential culture after IVF and ICSI shows no differences in pregnancy rate: a randomized trial. Med Sci Monit. 2004;10:1–7. [PubMed] [Google Scholar]
- 3.Gardner DK, Lane M. Culture systems for the human embryo. In: Gardner DK, Weissman A, Howles CM, Shoham Z, editors. Textbook of Assisted Reproductive Techniques. 2. London: Taylor & Francis; 2004. pp. 211–234. [Google Scholar]
- 4.Yeung QS, Briton-Jones CM, Tjer GC, Chiu TT, Haines C. The efficacy of test tube warming devices used during oocyte retrieval for IVF. J Assist Reprod Genet. 2004;21:355–360. doi: 10.1023/B:JARG.0000046203.44045.0e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hadachi Y, Kato Y, Genji Y, Ueno Y, Nishikori K, Mio Y. Effect of optimal temperature in culture medium on embryo quality and ART result in couples with elder maternal age. J Fertil Implant (Tokyo) 2003;20:114–117. [Google Scholar]
- 6.Bolton VN, Hawes SM, Taylor CT, Parsons JH. Development of spare human preimplantation embryos in vitro: an analysis of the correlations among gross morphology, cleavage rates, and development to the blastcyst. J In Vitro Fert Embryo Transf. 1989;6:30–35. doi: 10.1007/BF01134578. [DOI] [PubMed] [Google Scholar]
- 7.Gardner DK, Schoolcraft WB. In vitro culture of human blastcysts. In: Jansen R, Mortimer D, editors. Towards Reproductive Certainty: Fertility and Genetics Beyond 1999. Carnforth, UK: Parthenon Publishing; 1999. pp. 378–388. [Google Scholar]
- 8.Cooke S, Tyler JP, Driscoll G. Objective assessments of temperature maintenance using in vitro culture techniques. J Assist Reprod Genet. 2002;19:368–375. doi: 10.1023/A:1016394304339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mortimer D, Henman M, Catt JW, Bowman MC, Jansen RPS. Development of an improved IVF culture system. SYDNEY IVF MEDIA ABSTRACTS, pp. 8–9, 2006