Abstract
A 29 year old woman with difficult to control acromegaly and a pituitary macroadenoma responded to pegvisomant therapy and subsequently conceived with her first cycle of in-vitro fertilization and intra-cytoplasmic sperm injection. Pregnancy was complicated by gestational diabetes, pituitary gland enlargement and deteriorating visual fields. Conservative management with elective cesarean section was performed at 32 weeks gestation. A healthy boy was delivered who remains developmentally normal at 1 year. This complex case required intricate care by a multi-disciplinary team and is likely to represent the first in many cases of assisted conception on pegvisomant therapy for active acromegaly.
Keywords: Acromegaly, ICSI, IVF, Pegvisomant, Pregnancy
Case report
A 29 year old Caucasian woman was diagnosed with acromegaly following a two year history of secondary amenorrhea, change in physical appearance and sweating. The diagnosis was confirmed with an oral glucose tolerance test and radiological imaging that demonstrated a 2.2 cm pituitary tumor compressing the optic chiasm. A bi-temporal visual field defect was confirmed using binocular Goldman perimetry.
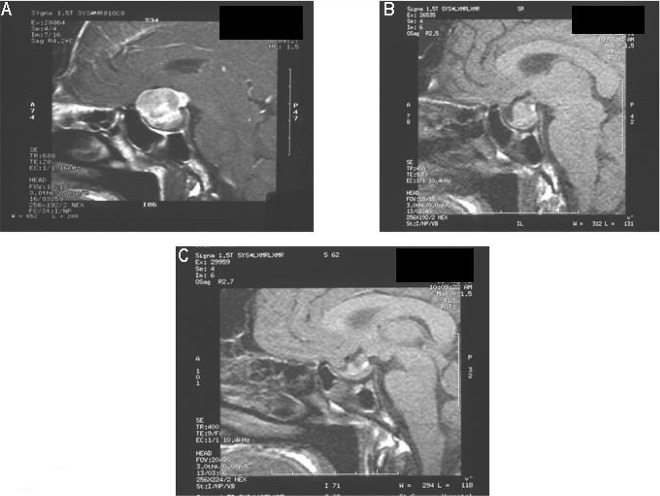
Over a four-year period, she underwent four transphenoidal hypophysectomies and received 53 Gy radiotherapy. Despite a reduction in tumor size (Fig. 1) and the use of somatostatin analogues, she failed to achieve biochemical cure and her insulin-like growth factor-I (IGF-I) level remained persistently high (96–120 nmol/L; normal range 16–52 nmol/L). Hydrocortisone and thyroxine replacement was required for pituitary deficiency.
Fig. 1.
MRI of pituitary gland showing reduction in tumor size (arrows) following transphenoidal hypophysectomies and radiotherapy: (A) MRI at presentation; (B) MRI after two surgical procedures and radiotherapy; (C) MRI after third surgical procedure.
She was desperate to conceive and was counseled on the effect of pregnancy on acromegaly and the risks of pituitary tumor enlargement. After failing to conceive with clomifene therapy she was referred to the assisted conception unit. Her husband was discovered to be oligoasthenozoospermic and they were counseled for in-vitro fertilization (IVF) and intra-cytoplasmic sperm injection (ICSI).
To achieve control of her acromegaly, she was started on daily subcutaneous injections of 20 mg pegvisomant, a highly selective growth hormone receptor (GHR) antagonist, and octreotide was discontinued. Her IGF-I subsequently normalized for the first time in 5 years.
Pituitary down regulation was achieved with a GnRH analogue and she required four days of 300 IU recombinant FSH which was increased to 450 IU on day five of stimulation for a further 11 days for super-ovulation. She produced a total of 14 follicles and transvaginal ultrasound-guided oocyte retrieval was uneventful under general anesthesia on day 14. 13 oocytes were retrieved and 12 were suitable for ICSI. Seven of these fertilized normally and two 4-cell grade 2 embryos were transferred. Cyclogest pessaries 400 mg daily for 14 days were used to provide luteal support. She had a positive pregnancy test two weeks later with a serum β-HCG level of 142 mIU/ml. Pegvisomant was then discontinued.
She was monitored on a weekly basis at our joint obstetric-endocrine clinic and developed gestational diabetes requiring insulin therapy at 15 weeks of pregnancy. Fetal growth was normal as demonstrated by regular ultrasonographic assessments. Goldman perimetry was performed monthly and although a deterioration of her visual fields was noted at 30 weeks of pregnancy, magnetic resonance imaging (MRI) showed no pituitary enlargement; there was however, prolapse of the optic nerve inferiorly.
She underwent a comprehensive anesthetic assessment including an echocardiogram, which was normal. An elective caesarean section was performed at 38 weeks under spinal anesthesia with glucocorticoid cover perioperatively. A healthy boy was delivered weighing 3.5 kg who remains physically and developmentally normal at 1 year.
Three months postpartum, her visual fields had improved, a repeat pituitary MRI demonstrated no further increase in pituitary size and her IGF-I level was 85.2 nmol/L (normal range: 16–52 nmol/L). She was restarted on a combination of weekly pegvisomant and monthly octreotide therapy.
Discussion
This case illustrates the first human pregnancy in a woman with active acromegaly treated with pegvisomant and conceived following IVF/ICSI. This case also illustrates the importance of a functional multi-disciplinary team approach in the intricate management of a complex disorder. In this case, clinicians from over seven medical specialties as well as nurse specialists from various fields were involved: the general practitioner who initiated investigations; the endocrinologist, neurosurgeons and clinical oncologists who co-ordinated treatment and provided continuous surveillance; the obstetricians, pediatricians and anesthetists who performed the IVF/ICSI and closely monitored the pregnancy and planned delivery. The advent of novel treatments is likely to result in an increase in the number of women with acromegaly choosing to conceive and it is therefore vital to establish the safety of pharmaceutical agents such as pegvisomant in pregnancy.
Reports of pregnancy in women with acromegaly are uncommon as fertility is reduced due to several interplaying mechanisms. The expanding tumor mass may cause hypopituitarism and a reduced gonadotrophin reserve. Hyperprolactinemia that occurs in 30–40% of acromegalic patients [1] results in dysfunction of the hypothalamic-pituitary-ovarian axis at several levels including reduction in pulsatile GnRH secretion as well as hypoestrogenism [2]. Growth hormone (GH) and IGF-I are also involved in the regulation of ovarian function. Most commonly however, it is disruption to the hypothalamic-pituitary-ovarian axis following pituitary surgery and or radiotherapy that leads to sub-fertility. Pregnancy in patients with acromegaly has been reported after surgical intervention alone [3] and following treatment with bromocriptine [4], octreotide and lanreotide. Although the number of cases is small, no adverse effects on fetal development have been documented with use of these pharmaceutical agents.
Effect of pregnancy on acromegaly
The pituitary gland enlarges in volume by up to 45% during the first trimester of pregnancy and may thus be associated with compression of the optic chiasm and visual field deterioration in subjects with pituitary macroadenomas. Similar complications are unlikely with microadenomas [4].
Pregnancy has been associated with the aggravation of symptoms of acromegaly. In a review of 24 patients, pregnancy was associated with a recurrence of symptoms, increasing intra-cranial pressure or pituitary tumor enlargement in 4 (17%) patients [5]. For a number of reasons it has been difficult to establish biochemically that pregnancy aggravates GH excess; prolactin and human placental lactogen cross-react with earlier GH immunoassays and IGF–I concentrations increase in normal pregnancy.
Effects of acromegaly on pregnancy
The prevalence of carbohydrate intolerance and diabetes mellitus in acromegaly is 60% and 13–32% respectively [6]. The increased insulin resistance associated with pregnancy further enhances the risk of a hyperglycemia in a patient with acromegaly. It is our opinion that an active search for hyperglycemia should thus be made at booking and again at 28 weeks gestation. Similarly the prevalence of hypertension is high in these patients and blood pressure should be monitored closely in pregnancy.
From the fetal perspective, elevated maternal circulating levels of GH are unlikely to adversely effect fetal development since the placenta is impermeable to this hormone. We were unable to identify any case reports of either growth retardation or acceleration in a pregnant acromegalic patient. The majority of pregnancies in acromegaly thus appear to have a normal outcome.
Pegvisomant in pregnancy
Pegvisomant is a potent genetically engineered GHR antagonist that appears to normalize IGF-I levels in virtually all treated patients. It is thus recommended that IGF-I levels are monitored whilst on therapy to ensure GH deficiency does not develop. It is unlikely however, to replace pituitary surgery or radiotherapy in the management of acromegaly, but is likely to supersede somatostatin analogue therapy in the future. The safety of this drug in pregnancy and breast feeding has not yet been established and particular concerns remain given the risk of pegvisomant induced GH deficiency during fetal development.
Prior to this case report, there have been no reports on the effects of pegvisomant on a human pregnancy. Pegvisomant is currently regarded as a category B drug for use in pregnancy and as such should only be used if clearly necessary. Early embryonic development and teratology studies in pregnant rabbits have failed to demonstrate any evidence of teratogenic effects at subcutaneous doses of 1, 3, and 10 mg/kg/day. At the latter dose, a reproducible and slight increase in post-implantation loss was observed. This dose was however, 10-times the maximum human therapeutic dose based on body surface area and furthermore, animal studies are not always predictive of human responses to pharmaceutical agents. Nevertheless caution is still recommended when using pegvisomant in human pregnancies. The use of pegvisomant during breast-feeding should also be avoided as it is unclear whether this drug is excreted in breast milk.
The effects of pegvisomant on the developing fetus are unknown. Whilst studies of anencephalic fetuses and fetal decapitation experiments suggest GH plays no role in fetal development, studies in animal models with GHR mutations suggest that GH is important for the development of normal fetal height and weight [7]. Pegvisomant has a molecular weight of 42,000 Daltons, and although it is unlikely to cross the placenta by passive transfer alone [8], it is possible that it may do so by active mechanisms.
The success of modern medical and surgical treatments and the advent of assisted conception technology have increased the likelihood that women with acromegaly will seek to become pregnant. In such women the aim of therapy should be to conceive after normalization of prolactin and GH levels, avoidance of visual deterioration, diagnosis and management of gestational complications, and delivery of a normal infant. Although the incidence of subfertility is high in subjects with surgically treated GH secreting macroadenomas, avoiding surgery increases the risk of pituitary enlargement during pregnancy and thus visual deterioration. Patients with macroadenomas should have a formal assessment of their visual fields at four-six weekly intervals during pregnancy. When there is evidence of pregnancy associated tumor enlargement with visual loss, emergency transphenoidal resection should be considered.
It is likely that the use of pegvisomant in the treatment of acromegaly is likely to increase given its clinical effectiveness and metabolic advantages over other pharmaceutical agents. It is necessary therefore, that clinicians in the relevant specialties are familiar with this area and the potential issues that may arise. Pivotal to this understanding will be the documentation of case reports such as ours.
Acknowledgements
We thank Dr Isaac Manyonda for helpful discussion and advice in the preparation of this report.
Footnotes
This case report describes a successful pregnancy, managed by a functional multi-disciplinary team, following assisted conception in a subject with active acromegaly treated with pegvisomant.
References
- 1.Molitch ME. Pregnancy and the hyperprolactinemic woman. New England J Medi. 1985;312(21):1364–70. doi: 10.1056/NEJM198505233122106. [DOI] [PubMed] [Google Scholar]
- 2.Sauder SE, Frager M, Case GD, Kelch RP, Marshall JC. Abnormal patterns of pulsatile luteinizing hormone secretion in women with hyperprolactinemia and amenorrhea: responses to bromocriptine. J Clin Endocrinol Metab. 1984;59(5):941–8. doi: 10.1210/jcem-59-5-941. [DOI] [PubMed] [Google Scholar]
- 3.Wislawski J, Hartwig W, Kasperlik-Zaluska A, Ostrowski K. Treatment of acromegaly by the surgical approach through the sphenoid bone. Clinical results. Polish J Neurol Neurosurg. 1982;16(4):281–6. [PubMed] [Google Scholar]
- 4.Kupersmith MJ, Rosenberg C, Kleinberg D. Visual loss in pregnant women with pituitary adenomas. Ann Inter Med. 1994;121(7):473–7. doi: 10.7326/0003-4819-121-7-199410010-00001. [DOI] [PubMed] [Google Scholar]
- 5.Herman-Bonert V, Seliverstov M, Melmed S. Pregnancy in acromegaly: successful therapeutic outcome. J Clin Endocrinol Metab. 1998;83(3):727–31. doi: 10.1210/jc.83.3.727. [DOI] [PubMed] [Google Scholar]
- 6.Berelowitz M, HowGo E. Non-insulin diabetes mellitus secondary to other endocrine disorders. In: LeRoith D, Taylor SI, Olefsky JM eds. Diabetes mellitus. A fundamental and clinical text. New York: Lippincott-Raven 1996;496–502.
- 7.Parks JS. The ontogeny of growth hormone sensitivity. Hormone Res. 2001;55(Suppl 2):27–31. doi: 10.1159/000063470. [DOI] [PubMed] [Google Scholar]
- 8.Pacifici GM, Nottoli R. Placental transfer of drugs administered to the mother. Clin Pharmacokinet. 1995;28(3):235–69. doi: 10.2165/00003088-199528030-00005. [DOI] [PubMed] [Google Scholar]