Abstract
Puerose: To demonstrate our hypothesis that a correlation exists between oocyte maturity and a decrease in intraovarian blood flow resistance in assisted reproductive technology (ART) treatment cycles, oocyte maturity and total antioxidant status (TAOS) in follicular fluid was examined.
Methods: A total of 59 cycles involving 51 infertile patients undergoing ART treatment in our hospital were recruited in this study. The ART cycles were divided into two groups: deeply decreased (DD) group and not-deeply decreased (NDD) group, according to the pulsatility index (PI) values for perifollicular arterial blood flow before and after hCG administration. The PI values that showed a decrease in their PI after hCG administration of 10% or more were defined “deeply decreased” and showed a decrease of less than 10% were considered “not-deeply decreased.” The recovery rates of mature and immature oocytes and TAOS in follicular fluid were compared between the two groups.
Results: Mature and immature oocyte recovery rates in the DD group (62.5% and 17.2%) were significantly higher and lower, respectively, than those in the NDD group (41.7% and 38.3%, p < 0.05). The TAOS (1.55 ± 0.44 mmol/L) of the DD group was significantly lower than that in the NDD group (1.68 ± 0.47 mmol/L, p < 0.05).
Conclusions: The decrease in intraovarian arterial blood flow resistance measured after hCG administration may be a good indicator of retrieving mature oocyte.
Keywords: ART, Intraovarian blood flow resistance, Oocyte maturity, Total antioxidant status (TAOS)
Introduction
In all types of infertility treatment including assisted reproductive technology (ART), an accurate evaluation of oocyte maturity or embryo quality is a very important issue. It affects the rate of a good pregnancy outcome and permits the transfer a fewer number of good quality embryos into the uterus, thus preventing multiple pregnancies. We recently reported that it is possible to indirectly evaluate the maturity of oocytes or the quality of embryos by various methods such as measuring or monitoring intraovarian changes; evaluating the incidence of apoptotic granulosa cells [1], oxidative stress in granulosa cells [2], or alternations in the cell cycle of granulosa cells [3], and measuring the concentration of hyaluronan in follicular fluids [4]. Such intraovarian changes that take place during the periovulatory phase might be associated with intraovarian blood flow and arterial resistance.
Over the past 10 years, the development of vaginal color and pulsed Doppler ultrasound has made the noninvasive evaluation of blood flow changes in the ovary and the uterus a possibility [5–8]. As a result, this technique has recently been used to study the quality and developmental competence of oocytes by evaluating ovarian vascular changes in women undergoing IVF. It has been reported that perifollicular blood flow characteristics might be useful markers for evaluating the quality of oocytes and the developmental competence of the corresponding embryos [9, 10]. And it was also reported that measurement of peak systolic velocity of individual follicles could predict pregnancy outcome in ART treatment [11]. In these studies, follicles were evaluated by color Doppler imaging, and if intense coloration was observed, it was deemed to indicate a well-developed perifollicular vasculature. However, dynamic physiological changes in the follicles occur just towards ovulation. As a result, it would be important to evaluate blood flow changes from the LH surge to ovulation.
We recently measured the flow resistance of intraovarian arterial blood before and after follicular rupture in infertile women during natural cycles using transvaginal color flow Doppler imaging, and reported that a decrease in intraovarian blood vessel resistance was necessary in order to achieve a successful pregnancy [12]. This parameter is noninvasive and one of the available methods for evaluating the follicular environment during ovulation. Moreover, we also indicated that oligomenorrheal patients showed a disturbance in the ovulation process during the peri-ovulatory period with respect to intraovarian arterial blood flow resistance [13]. The induction of ovulation with a FSH low-dose step-up regimen improved the ovulation process of the oligomenorrheal patients with a deep decrease in intraovarian arterial blood flow resistance, and consequently, the pregnancy rate was also increased [13]. From these results, we conclude that mature oocytes with good quality develop and are ovulated when a substantial decrease in intraovarian arterial blood flow resistance occurs during the ovulation process. However, we were not able to demonstrate this hypothesis directly in the previous study because oocytes could not be observed in natural and ovulation induction cycles without follicle aspiration.
Oxidative stress is considered to be one of the main causes of molecular damage to cellular and tissue structures and it is known to be increased in patients with diabetes and advanced maternal age [14–16]. A reduction in the antioxidant state contributes to the development of oxidative stress. Although it is not always possible to directly measure oxidative stress in biological systems, several biomarkers have been identified that provide an indirect measurement of oxidative damage to biomolecules [17]. In this study, total antioxidant status (TAOS), defined as the ability of some fluids to quench free radical production, consisting of multicompartmental protection against molecular damage to the cell structure. It takes into account the complex interactions that occur between individual antioxidants in vivo.
TAOS is sensitive to changes in plasma antioxidant levels and the degree of oxidative stress. TAOS, a cumulative index of plasma antioxidant status and the levels of carbonyl groups, should be considered as an indicator of oxidative stress and its affects on the risk of cardiovascular disease in women with PCOS [18]. Ovulation has been linked to an inflammatory response associated with angiogenesis, a high permeability of blood vessels in the lutenizing follicles. TAOS can be a good measurement for estimating physiological and pathlogical oxidative status in follicles during follicle development and ovulation.
In the present study, we divided the ART cycles into two groups according to PI values before and after hCG administration: a deeply decreased (DD) group and a not-deeply decreased (NDD) group, and examined our hypothesis whether there was a correlation between the maturilty of oocytes and a decrease in intraovarian blood flow resistance exists, with regard to the recovery rate of mature oocytes and TAOS in follicular fluid.
Materials and methods
Patients and controlled ovarian hyperstimulation
From February 2003 through November 2004, a total of 59 cycles in 51 infertile patients undergoing ART treatment at the Division of Reproductive Medicine, Department of Perinatal Medicine and Maternal Care, National Center for Child Health and Development were selection for this study. Informed consent was obtained from all patients and the study was approved by the Institutional Review Board of the National Center for Child Health and Development.
The indications for ART treatment included tubal factor infertility (33.3%), unexplained infertility (33.3%), endometriosis (7.9%) and male factor infertility (25.5%). All male factor infertility cases were treated by intracytoplasmic sperm injection (ICSI).
All 59 cycles of the 51 patients were hyperstimulated according to our previous reports [19]. Briefly, the ovarian hyperstimulation protocol with GnRH-agonist was performed as follows: 600 μg of buserelin acetate (Suprecur nasal, Mochida, Tokyo) was administered daily intranasally from the midluteal phase of the pretreatment cycle to the day of human chorionic gonadtropin (hCG) injection. From the third day of the menstrual cycle 300 IU of human menopausal gonadotropin (hMG; Humegon; Organon, Tokyo, Japan) was administered on 2 consecutive days, and 225 IU of hMG was given daily until a dominant follicle reached 16 mm in diameter. HCG (10,000 IU; Gonatropin; Mochida, Tokyo, Japan) was administered 35 hours before oocyte retrieval.
IVF procedure and folicular fluid collection
The IVF procedure and follicular fluid (FF) collection has been previously described [4]. Oocytes were retrieved transvaginally using a needle guided technique, aided by ultrasonography. All follicles with a mean diameter of >15 mm were aspirated separately, using an 18-gauge needle connected to a tube and a 20-ml syringe for suction. The needle was removed after the aspiration of each follicle. The aspiration was interrupted and a new syringe was used if blood appeared in the tube connected to the syringe, thus avoiding contamination by blood. Culture media was not used for washing the follicle, and as a result, the FF was not mixed with culture media. After retrieving the oocyte and granulosa cell masses, each FF sample was centrifuged at 1,000×g for 10 min and stored at −20 °C until assayed for progesterone and TAOS.
Assay for TAOS in FF
The TAOS of FF was assessed using a commercially available kit (Randox Laboratories, Ltd., Crumlin, UK) [18]. The assay principle depends on the inhibition of ABTS (2, 2′-Azino-di[3-ethylbenzthiazoline sulfonate]) radical action by antioxidants present in the follicular fluid. ABTS radical is formed when ABTS is incubated with a peroxidase (metmyoblobin) and hydrogen peroxide (H2O2). The results are expressed as nmol/L. Intra-assay and inter-assay coefficient variation (CV) values were found to be less than 5%. For accuracy, Randox total antioxidant control (Cat. No. NX2331) was used.
Oocyte maturity
All retrieved oocytes did not undergo ICSI, and three quarters of retrieved oocytes were treated with conventional insemination and were not denuded just after oocyte retrieval [19]. Therefore, maturity of many retrieved oocytes could not be assessed directly. The oocytes maturity was assessed by the appearance of the corona radiata and cumulus granulosa cells, as described previously [20]. Oocyte-corona-cumulus complexes with a large and loose cumulus and distinct corona radiata were defined as mature, and complexes with a small and dense cumulus and opaque corona radiate were defined as immature. Overmature oocytes were surrounded by dark clumpy corona and few or no clumpy granulose cells, and complexes with clumpy granulosa cells and dark clumpy corona radiata were defined as dysmature.
Ultrasound and doppler examination
All patients were examined by transvaginal color flow Doppler imaging on the day of hCG administration and just before oocyte retrieval. Patients with three or less follicles in each ovary were included in this study to measure the perifollicular blood flow of the same blood vessel on the both measurement days, and the identification of the same blood vessel is the most important for the assessment the rate of change between before hCG administration and just before oocyte aspiration. Follicles could be easily identified individually by their size, location and rate of growth, during the daily vaginal ultrasound examination. Therefore, the examiner was able to detect the same peri-follicular artery on every measurement.
Color Doppler was used to visualize intraovarian blood flow, and pulsed Doppler signals were obtained using a 2 mm volume cursor. All examinations were performed with the patient in a lithotomy position, using a Mochida Sonovista-Color II (Mochida, Tokyo, Japan) with a 6.5-MHz transvaginal probe for imaging and a 6.5-MHz pulsed Doppler system for blood flow analysis. All scans were carried out by one operator (K.N.). Blood flow impedance was expressed as the pulsatility index (PI), calculated electronically from smooth curves fitted to waveforms over three cardiac cycles, as described in our previous report [12]. Wall filters (100 Hz) were used to eliminate low frequency signals arising from noise. The intra-assay coefficient variability was 4.3%. When the angle between the ultrasound beam and the longitudinal axis of the vessel becomes optimal for a Doppler measurement, the pulsatility index is a convenient parameter, in spite of the scanning angle because the angle parameter is not included in the formula for PI.
Statistics
Data are presented as real figures, rates and the mean ± S.E.M. (standard error of the mean). The significance of the differences was determined by the unpaired t-test and chi-square tests.
Results
Since the PI values from one side of the ovary were evaluated separately from the other side, the perifollicular arterial PI values of 88 ovaries and 59 cycles were included in this study. According to our previous study, the change in PI rate was calculated using the following formula; change in PI rate = {(PI just before follicle aspiration hCG−PI before hCG)/PI before hCG}×100. An ovary that showed a change in PI rate of −10% or less after hCG administration was classified as deeply decreased (DD), while an ovary that showed a more than −10% change was classified as not-deeply decreased (NDD). All 88 ovaries were divided according to this criterion (Table 1).
Table 1.
The oocyte recovery rate and mature oocyte recovery rate in both groups
| Deeply decreased (DD) group | Not-deeply decreased (NDD) group | P values | |
|---|---|---|---|
| Number of ovaries | 45 | 43 | |
| Number of ovaries that retrieved at least one oocyte | 40 | 37 | |
| Oocyte recovery rate (%; ovaries) | 88.9 | 86.0 | ns |
| Oocyte recovery rate (%; number of punctured follicles) | 71.9 | 70.5 | n.s. |
| Total number of retrieved oocytes | 64 | 60 | |
| Mature oocyte recovery rate (%) | 62.5 | 41.7 | P=0.02 |
| Immature oocyte recovery rate (%) | 17.2 | 38.3 | P<0.01 |
| Dysmature oocyte recovery rate (%) | 10.9 | 18.3 | n.s. |
| Overmature oocytes recovery rate (%) | 9.4 | 1.7 | n.s |
In the DD group, at least one oocyte was retrieved from 40 ovaries, with an oocyte recovery rate per ovaries of 88.9%, while in the NDD group one or more oocytes were retrieved from 37 ovaries, with a recovery rate of 86.0%. The rate of oocyte recovery per number of punctured follicles in the DD and NDD group was 71.9% (64/89) and 70.5% (60/85), respectively. No significant differences were found between these rates. The mature oocyte recovery rate in the DD group was 62.5%, significantly higher than that in the NDD group (41.7%; p = 0.02), and the immature oocyte recovery rate in the DD group was 17.2%, significantly lower than that in the NDD group (38.3%, p < 0.01). The overmature and dysmature oocyte recovery rates in the DD group were 9.4% and 10.9%, respectively, and the rates for the NDD group were similar (1.7% and 18.3%, respectively) (Table 1).
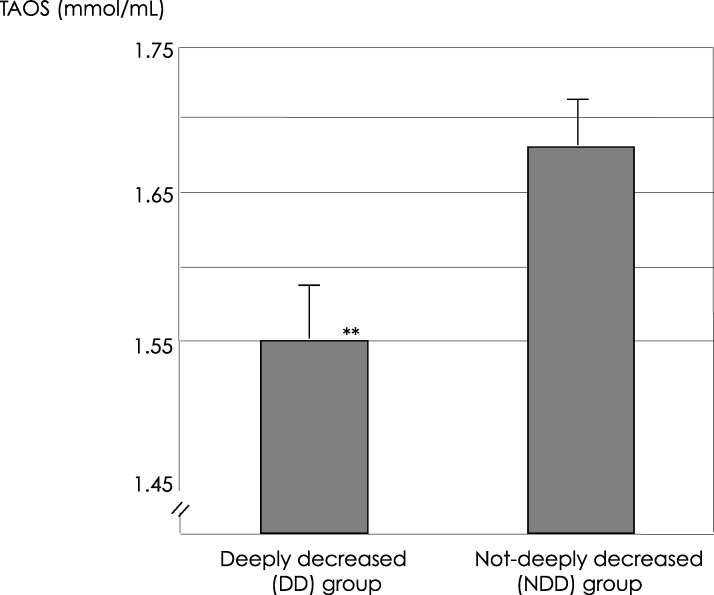
The average TAOS (mmol/L) in the FFs of the DD group was 1.55 ± 0.44 (mean ± S.E.M), also significantly lower than that in the NDD group (1.68 ± 0.47; p = 0.038; Fig. 1).
Fig. 1.
TAOS (mmol/L, mean±S.E.M) in the FF in the deeply decreased (DD) group was 1.55±0.44, also significantly lower than that in the not-deeply decreased (NDD) group (1.68±0.47, **p=0.038)
Discussion
In our previous studies, when the PI was used as a parameter for estimating the quality of the ovulation process, to our surprise, only half of the infertile women with regular menstrual cycles showed deeply decreased PI values after ovulation, while the other half showed increased or not-deeply decreased PI values after ovulation (Nakagawa et al., 2005). In this study, only half of the ovaries also showed deeply decreased PI values after hCG administration. Interestingly, several patients showed different PI changes after hCG administration between the both sides of ovary.
Recently, Coulam et al. demonstrated that measurement of peak systolic velocity (PSV) of individual follicles on the day of hCG administration could predict pregnancy outcome in ART treatment [11]. This report mentioned that the PSV ≥10 cm/s in at least on follicle more often became pregnant than those with <10 cm/s, and all pregnancies occurred in women with grade 3 (three-quarters of follicle was surrounded by colour) or 4 (entire follicle was surrounded by colour) [11]. In that study the measurement of follicular blood flow was performed only on the day of hCG administration, therefore, the changes of follicular and intrafollicular environment between hCG administration and oocyte retrieval were not be reflected. But in this study we measured arterial blood flow resistance at two points; on the day of hCG administration and just before oocyte retrieval, and the change of blood flow resistance might give us the important information of follicular final maturation. From this point of view, the previous report by Coulam et al. is different from this repot.
We also previously showed that a decrease in the intraovarian arterial PI value after ovulation indicated the establishment of a suitable follicular environment during ovulation [12]. From these results, we presumed that oocytes of good quality would be produced in the deeply decreased group. In the present study, the mature oocyte recovery rate in the DD group was 62.5%, significantly higher than that in the NDD group (41.6%; p = 0.02). Moreover, the average TAOS in the follicular fluids of the DD group were significantly lower than that in the NDD group.
Oxidative stress is an imbalance between the production of free radicals that contain unpaired electrons, which increase chemical reactivity, and antioxidant defenses buffering the oxidative damages. Since the balance is maintained by the presence of adequate amounts of antioxidants, measuring individual antioxidant levels as total antioxidant capacity, has been examined [21]. Although it is not always possible to directly measure this in biological systems, several biomarkers providing a measure of oxidative damage to biomolecules have been identified [17].
In this study TAOS, defined as the ability of FF to quench free radical production, was used as a biomarker to assess oxidative stress during ovulation. Because TAOS is thought to be sensitive to changes in plasma antioxidant levels and the degree of oxidative stress, it is a suitable biomarker for estimating the level of oxidative stress in follicular fluid. In ovulation, which is considered to be an inflammatory reaction, reactive oxygen species play a physiological role during ovulation that is similar in some respects to inflammation [22, 23]. Therefore, antioxidant capacity can reflect physiological and pathlogical oxidative status in follicles during follicle development and ovulation.
A low concentration of TAOS implies a reduction of antioxidant ability against oxidative stress. In this study, the average concentration of TAOS in follicular fluids of the DD group was significantly lower than in the NDD group. This, therefore, suggests that follicles in the DD group were exposed to more oxidative stress than those in the NDD group. The preovulatory follicle has a potent defense against antioxidants, which is depleted by intense peroxidation [21]. It is known that glutathione peroxidase functions to maintain a low concentration of hydroperoxides inside the follicle. However, after hCG exposure, the levels of antioxidants decrease and a high number of macrophases and neutrophils gather in the follicle wall, when ovulation is approaching. Moreover, prostaglandin levels, which might play an important role in follicular rupture, have been reported to be increased in follicular fluid [24]. These phenomena lead to an appropriate level of high oxidative conditions in the follicle, and a suitable environment for ovulation. Follicles in the NDD group in this study apparently lacked an appropriate level of oxidative stress, and the unphysiological preovulation process resulted in significantly lower recovery rates of mature oocytes.
In conclusion, intraovarian arterial blood flow resistance measured during ovulation may be a good indicator for assessing the ovulation process and consequently, oocyte maturity. Further studies will be necessary to develop procedures for decreasing PI values after hCG administration in infertility patients with both deeply and not-deeply decreased PI values.
References
- 1.Nakahara K, Saito H, Saito H, Ito M, Ohta N, Sakai N, Tezuka N, Hiroi M, Watanabe H. Incidence of apoptotic bodies in membrane granulosa of the patients participating in an in vitro fertilization program. Fertil Steril. 1997;67:302–3088. doi: 10.1016/S0015-0282(97)81915-2. [DOI] [PubMed] [Google Scholar]
- 2.Seino T, Saito H, Kaneko T, Takahashi T, Kawachiya S, Kurachi H. Eight-hydroxy-2′-deoxyguanosine in granulosa cells is correlated with the quality of oocytes and embryos in an in vitro fertilization-embryo transfer program. Fertil Steril. 2002;77:1184–1190. doi: 10.1016/S0015-0282(02)03103-5. [DOI] [PubMed] [Google Scholar]
- 3.Toya M, Saito H, Ohta N, Saito T, Kaneko T, Hiroi M. Moderate and severe endometriosis is associated with alterations in the cell cycle of granulosa cells in patients undergoing in vitro fertilization and embryo transfer. Fertil Steril. 2000;73:344–350. doi: 10.1016/S0015-0282(99)00507-5. [DOI] [PubMed] [Google Scholar]
- 4.Saito H, Kaneko T, Takahashi T, Kawachiya S, Saito T, Hiroi M. Hyaluronan in follicular fluids and fertilization of oocytes. Fertil Steril. 2000;74:1148–1152. doi: 10.1016/S0015-0282(00)01586-7. [DOI] [PubMed] [Google Scholar]
- 5.Campbell S, Bourne TH, Waterstone J, Reynolds KM, Crayford TJB, Jurkovic D, Okokon EV, Collins WP. Transvaginal color blood flow imaging of the periovulatory follicle. Fertile Steril. 1993;60:433–438. [PubMed] [Google Scholar]
- 6.Collins W, Jurkovic D, Bourne T, Kurjak A, Campbell S. Ovarian morphology, endocrine function and follicular blood flow during the peri-ovulatory period. Hum Reprod. 1991;6:319–324. doi: 10.1093/oxfordjournals.humrep.a137332. [DOI] [PubMed] [Google Scholar]
- 7.Sladkevicius P, Valentin L, Marsal K. Blood flow in the uterine and ovarian arteries during the normal menstrual cycle. Ultrasound Obstet Gynecol. 1993;3:199–208. doi: 10.1046/j.1469-0705.1993.03030199.x. [DOI] [PubMed] [Google Scholar]
- 8.Tan SL, Zaidi J, Campbell S, Doyle P, Collins W. Blood flow changes in the ovarian and uterine arteries during the normal menstrual cycle. Am J Obstet Gynecol. 1996;175:625–631. doi: 10.1053/ob.1996.v175.a73865. [DOI] [PubMed] [Google Scholar]
- 9.Van Blerkom J, Antczak M, Schrader R. The developmental potential of the human oocyte is related to the dissolved oxygen content of follicular fluid: association with vascular endothelial growth factor levels and perifollicular blood flow characteristics. Hum Reprod. 1997;12:1047–1055. doi: 10.1093/humrep/12.5.1047. [DOI] [PubMed] [Google Scholar]
- 10.Van Blerkom J. Epigenetic influences on oocyte developmental competence: perifollicular vascularity and intrafollicular oxygen. J Assist Reprod Genet. 1998;15:226–234. doi: 10.1023/A:1022523906655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coulam CB, Goodman C, Rinehart JS. Colour Doppler indices of follicular blood flow as predictors of pregnancy after in-vitro fertilization and embryo transfer. Hum Reprod. 1999;14:1979–1982. doi: 10.1093/humrep/14.8.1979. [DOI] [PubMed] [Google Scholar]
- 12.Nakagawa K, Ozawa N, Takamatsu K, Takahashi Y, Irahara M, Yoshimura Y, Saito H. A reduction in intraovarian arterial blood flow resistance after ovulation is necessary to achieve pregnancy in natural cycle. J Assist Reprod Genet. 2005;22:9–14. doi: 10.1007/s10815-005-0814-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakagawa K, Takahashi Y, Ito M, Horikawa T, Ohgi S, Irahara M, Saito H. Intraovarian arterial blod flow resistance in oligomenorrheal infertile women. J Assist Reprod Genet. 2006;23:105–110. doi: 10.1007/s10815-005-9007-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baynes JW. Role of oxidative stress in development of complications in diabetes. Diabetes. 1991;40:405–412. doi: 10.2337/diab.40.4.405. [DOI] [PubMed] [Google Scholar]
- 15.Giugliano D, Ceriello A, Paolisso G. Oxidative stress and diabetic vascular complications. Diabetes Care. 1996;19:257–267. doi: 10.2337/diacare.19.3.257. [DOI] [PubMed] [Google Scholar]
- 16.Tarin JJ, Vendrell Fj, ten J, Cano A. Antioxidant therapy counteracts the disturbring effects of diamide and maternal aging on meiotic division and chromosomal segregation in mouse oocytes. Mol Hum Reprod. 1998;4:281–282. doi: 10.1093/molehr/4.3.281. [DOI] [PubMed] [Google Scholar]
- 17.Betteridge DJ. What is the oxidative stress?a. Metabolism. 2000;49(suppl 1):3–8. doi: 10.1016/s0026-0495(00)80077-3. [DOI] [PubMed] [Google Scholar]
- 18.Fenkci V, Fenksi S, Yilmazer M, Serteser M. Decreased total antioxidant state and increased oxidative stress in women with polycystic ovary syndrome may contribute to the risk of cardiovascular disease. Fertil Steril. 2003;80:123–127. doi: 10.1016/S0015-0282(03)00571-5. [DOI] [PubMed] [Google Scholar]
- 19.Saito H, Takakazu S, Kaneko T, Sasagawa I, Kuramoto T, Hiroi M. Relatively poor oocyte quality is an indication for intracytoplasmic injection. Fertil Steril. 2000;73:465–469. doi: 10.1016/S0015-0282(99)00547-6. [DOI] [PubMed] [Google Scholar]
- 20.Saito H, Hiroi M. Corelation between the follicular gonadotropin inhibitor and the maturity of the ovum-corona cumulus complex. Fertil Steril. 1986;46:66–672. [PubMed] [Google Scholar]
- 21.Agarwal A, Gupta S, Sharma RK. Role of oxidative stress in female reproduction. Reprod Biol Endcrinol. 2005;3:28. doi: 10.1186/1477-7827-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Espey LL. Ovulation as an inflammatory reaction—a hypothesis. Biol Reprod. 1980;23:73–106. doi: 10.1095/biolreprod22.1.73. [DOI] [PubMed] [Google Scholar]
- 23.Fujii J, Iuchi Y, Okada F. Fundamental roles of reactive oxygen species and protective mechanism in the female reproductive system. Reprod Biol Endocrinol. 2005;3:43. doi: 10.1186/1477-7827-3-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoshimura Y, Espey LL, Hosoi Y, Adachi T, Atlas SJ, Ghodgaonkar RB, Dubin NH, Wallach EE. The effects of bradikinin on ovulation and prostaglandin production by the perfused rabbit ovary. Endocrinology. 1988;122:2540–2546. doi: 10.1210/endo-122-6-2540. [DOI] [PubMed] [Google Scholar]