Abstract
Purpose: To test whether environmental pollutants could affect fertility in humans.
Methods: 31 women and 16 men from Tanzania and 21 couples from Germany were included (n=89). Pesticides and polychlorinated biphenyls were measured in serum, follicular fluid or seminal plasma by gaschromatography and related to sperm quality and pregnancy rates.
Results: Higher concentrations of DDT+DDE and dieldrin in Tanzania and higher concentrations of PCBs in Germany and in men were detected. All compounds showed higher concentrations in serum and lowest concentrations in seminal plasma. A lower pregnancy rate in German women with high serum concentrations of DDT+DDE was observed. The toxins had no impact on sperm quality.
Conclusions: The distribution of toxins between agricultural and industrial countries is different. Seminal plasma seems to be inert against chemicals. In patients with high serum concentrations of DDT and DDE pregnancy rates were impaired.
Keywords: Africa, DDT, Fertility, PCB, Sperm quality
Introduction
In the last decades concern has risen to what extent chemicals might affect human health. A bulk of literature deals with the effects of environmental pollutants on infertility with inconsistent data. It is not well understood whether they could affect fertility in men and women [1, 2]. There are some indications that fertility in highly exposed people may be disturbed [3].
These contaminants are very heterogeneous and are characterized by various biological and chemical features. Polychlorinated biphenyls (PCBs) and organochlorine pesticides such as 1,1-bis-(4-chlorophenyl)-2,2,2-trichloroethane (DDT) and dieldrin are identified as endocrine disruptors. These substances are mimicking actions of hormones and could therefore potentially disturb the reproductive system. Exposure to endocrine disruptors can occur by contaminated water, air, food or soil [4]. Their accumulation in different compartments of the body, however, depends not only on the content of lipids. A previous study showed a high concentration of specific hydrocarbons in cervical mucus, which contains low amount of lipids [5].
PCBs have been used as industrial by-products in hydraulic fluids, capacitors, plasticizers and adhesives. Although they had been widely banned in 1977, they persist in the environment and in the food chain due to their lipophilic character and their stability. They are still present in many transformers and capacitors now in use. The main source of PCB is fish from waters PCB had been released to [6].
Another group of chlorinated hydrocarbons are used as biocides. DDT, dieldrin and hexachlorohexanes belong to this large group of chemicals. DDT has been used for a long time as an insecticide and was banned in most industrial countries in the early 1970s. It has, however, still been used in African countries, particularly for mosquito extinction, but also in general agriculture, due to high efficiency and relatively low cost. These compounds are still existent due to their resistance to biodegradation. The half-life of DTT and some of its metabolites can be more than 50 years [7].
Dieldrin is a human oral poison. It is rapidly absorbed through the skin, by ingestion and inhalation and may accumulate in the body during chronic exposure to low doses. Exposure to dieldrin occurs mostly by eating contaminated foods like seafood and fish [8]. The Environmental Protection Agency restricted its use in 1974 and banned it in 1987. Its manufacture was stopped in 1989. The lowest published lethal dose for dieldrin in humans is 28 mg/kg, for DDT 500 mg/kg (information about toxins were derived from the National Toxicology Program, USA [9]). The minimal risk levels (provided by the Agency for Toxic Substances and Disease Registry, USA) for dieldrin are 0.05 μg/kg/day, for DDT 5 μg/kg/day and for PCB 0.02 μg/kg/day [10].
The distribution of these contaminants is geographically different. In a previous study we obtained various body tissues and fluids from women undergoing caesarean section in Tanzania and Germany. Highest levels of insecticides like DDT and dieldrin were found in women from Tanzania, whereas the industrial by-products like PCB had higher levels in Germany. This different distribution pattern reflects the different economic situation of an agricultural versus an industrialized country [11]. Chlorinated hydrocarbons are widely distributed throughout the body with high concentrations in adipose tissues. It is not clear to which extent the concentration of these toxins in the serum reflects their concentration in other body fluids like seminal plasma or follicular fluid.
Here we compared the distribution of the most important environmental contaminants between infertile couples, referred to an IVF-ET program, from Tanzania and Germany. We investigated whether serum levels reflect levels of these chlorinated hydrocarbons in seminal plasma or follicular fluid. Furthermore we tested the hypothesis that PCBs and DDT could impair fertility.
Materials and methods
Patients and samples
Serum from 31 women and 16 men was collected in Tanzania. Seminal plasma and follicular fluid was obtained from 16 couples. In Germany 21 couples were included. Infertility was due to male subfertility. Patients underwent controlled ovarian hyperstimulation according to the long protocol with a GnRH agonist (Decapeptyl Gyn Depot®, Ferring Arzneimittel GmbH, Kiel, Germany) and gonadotropins (Menogon®, Ferring Arzneimittel GmbH, Kiel, Germany). Ovulation was induced by administration of 10,000 IU human chorionic gonadotropin (HCG) when the leading follicle size reached a diameter of about 18–20 mm. Transvaginal ovarian puncture was performed 36 h later. The same protocol was applied in patients from Tanzania and Germany.
Serum from both men and women was collected at the day of oocyte pick-up. Follicular fluid was harvested from non-blood contaminated punctates. At the same day a semen sample was collected and analysed. Seminal plasma was obtained after centrifugation. A total of 163 samples were obtained and frozen at −20°C.
Analysis of chlorinated hydrocarbons
Analysis of 14 different chemicals by gas chromatography was performed in 163 samples. Analyses were performed at the Federal Dairy Research Centre (Kiel, Germany) as described previously [11]. The samples were prepared according to a slightly modified procedure by Stivje and Cardinale [12]. Briefly, samples were put on a column filled with florisil and extrelut for cleansing. Organochlorids were eluted by petrolether and dichlormethane (4:1). Chemicals were measured by gas chromatography. The recovery rates were over 90%. Concentrations of 14 different chlorinated hydrocarbons were measured, i.e.: α-, β- and γ-1,2,3,4,5,6-hexachlorocyclohexane (a,b,g-HCH); 1,2,3,4,5,6-hexachl- orobenzene (HCB); 1,1-bis-(4-chlorophenyl)-2,2,2-trich- loroethane (p·p′-DDT); dieldrin; 1,1-bis-(4-chlorophenyl) 2,2-dichloroethene (p·p′-DDE); l,l-dichloro-2,2-bis-(4-chlorophenylethane), (p,p′-DDD); 2,4,4′-trichlorobiphenyl (PCB 28); 2,2′5,5′-tetrachlorobiphenyl (PCB 52); 2,2′, 4,5,5′-pentachlorobiphenyl (PCB 101); 2,2′3,4,4′5′-hexachlorobiphenyl (PCB 138); 2,2′,4,4′,5,5′-hexachlorobiphenyl (PCB 153); 2,2′3,4,4′5,5′-heptachlorobiphenyl (PCB 180). Congeners of the polychlorinated biphenyls (PCBs) have been numbered according to Ballschmiter and Zell [13].
PCB congeners 138 and 153 for industrial by-products and DDT and dieldrin for insecticides were included in the presented data, since they showed the most pronounced differences.
Fertility analysis
Since reliable parameters were only available for couples treated in Germany, we tested the influence of PCB and DDT on fertility in this subgroup of patients. For women we determined pregnancy rates and for men semen parameters like concentration, motility and morphology. Semen analysis was performed according to WHO criteria [14]. To estimate embryo quality, a modified cumulative embryo score was used as previously described [15]. This score adds the product of the number of blastomeres and embryo quality for all transferred embryos.
Data analysis
Data were expressed as mean±standard error of mean (SEM). Statistically significant differences between treatments were determined by one factor analysis of variance (ANOVA) followed by Newman–Keuls posttest. P values <0.05 were considered statistically significant.
Results
Distribution of toxins in females
Serum levels of PCB were significantly higher in women from Germany (0.35±0.049 μg/kg vs. 0.17±0.02 μg/kg, p < 0.01). No differences in PCB levels of follicular fluids could be observed between Germany and Tanzania (0.26±0.02 μg/kg vs. 0.22±0.06 μg/kg, n.s.). Serum levels mirror follicular fluid levels very closely. PCB concentrations in both compartments, follicular fluid and serum, and both countries, Germany and Tanzania, were in a similar range from 0.17 to 0.35 μg/kg (Fig. 1).
Fig. 1.
Distribution pattern of PCB (congener 153) in women from Tanzania and Germany. Serum from 31 women in Tanzania and 21 women in Germany and follicular fluid from 16 women in Tanzania and 21 women in Germany was collected. SWL (Serum females in Luebeck, Germany); FL (Follicular fluid in Luebeck, Germany); SWT (Serum females in Tanzania); FT (Follicular fluid in Tanzania); Asterisk indicates statistically significant differences p < 0.01
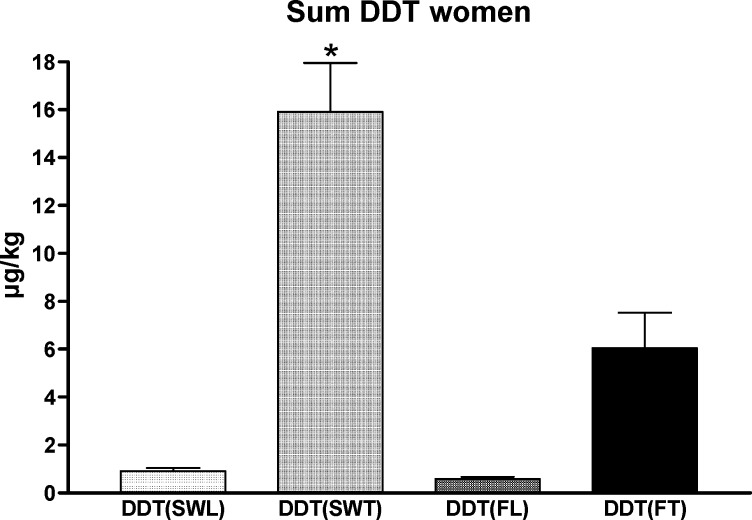
The insecticides DDT, its metabolite DDE and dieldrin showed a different distribution pattern (for DDE see Table 1). As expected, accumulation of DDT+DDE and dieldrin in women from Tanzania was significantly higher in serum and follicular fluid compared to German women (dieldrin serum: 0.50±0.07 μg/kg vs. 0.02±0.01 μg/kg; dieldrin follicular fluid: 0.17±0.02 μg/kg vs. 0.03±0.01 μg/kg; DDT+DDE serum: 15.92±2.04 μg/kg vs. 0.91±0.13 μg/kg; DDT+DDE follicular fluid: 6.06±1.47 μg/kg vs. 0.58±0.10 μg/kg). In contrast to PCB, levels of DDT+DDE in serum were roughly twofold higher compared to follicular fluid in Germany as well as in Tanzania. For example, DDT+DDE concentrations of women in serum were 15.92 μg/kg and 0.91 μg/kg compared to 6.06 μg/kg and 0.58 μg/kg in follicular fluid in Tanzania and Germany, respectively. Serum levels of insecticides did not show the same close correlation with follicular fluid levels we noted for PCB, but they still reflected the concentrations found in follicular fluid (Figs. 2 and 3).
Table 1.
DDE value of all subgroups
| Luebeck | Tanzania | |
|---|---|---|
| Males | ||
| Serum | 2.15±1.9 | 18.63±12.1 |
| Seminal plasma | 0.19±0.06 | 0.54 ± 0.37 |
| Females | ||
| Serum | 1.15±0.9 | 12.77±9.7 |
| Follicular fluid | 0.78±0.75 | 4.89±4.8 |
Note.Data of DDE in all subgroups presented as mean ± standard deviation.
Fig. 2.
Distribution pattern of DDT+DDE (as sum DDT) in women from Tanzania and Germany. Serum from 31 women in Tanzania and 21 women in Germany and follicular fluid from 16 women in Tanzania and 21 women in Germany was collected. SWL (Serum females in Luebeck, Germany); FL (Follicular fluid in Luebeck, Germany); SWT (Serum females in Tanzania); FT (Follicular fluid in Tanzania); Asterisks indicate statistically significant differences p < 0.05
Fig. 3.
Distribution pattern of dieldrin in women from Tanzania and Germany. Serum from 31 women in Tanzania and 21 women in Germany and follicular fluid from 16 women in Tanzania and 21 women in Germany was collected. SWL (Serum females in Luebeck, Germany); FL (Follicular fluid in Luebeck, Germany); SWT (Serum females in Tanzania); FT (Follicular fluid in Tanzania); Asterisks indicate statistically significant differences p < 0.01
Distribution of toxins in males
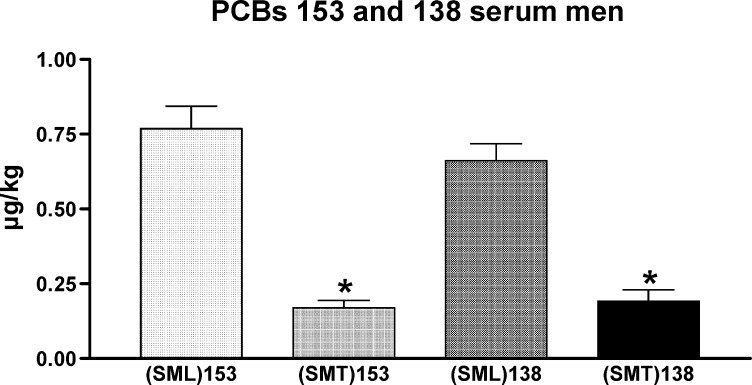
PCB (congeners 138 and 153) levels were significantly higher in the serum of men from Germany (0.66 μg/kg vs. 0.19 μg/kg and 0.79 μg/kg vs. 0.17 μg/kg, respectively; Fig. 4). No differences in PCB concentrations of seminal plasma could be demonstrated. Levels of both PCB congeners in seminal plasma from Tanzania and Germany were in the range of 0.2 μg/kg (Fig. 5). Concentrations of PCB in serum were three- to fourfold higher compared to seminal plasma in Germany. Therefore, serum concentrations of PCB cannot serve as surrogate parameter for seminal plasma concentrations.
Fig. 4.
Distribution pattern of PCB (congener 153 and congener 138) in serum of men from Tanzania and Germany. Serum was obtained from 16 men in Tanzania and 21 men in Germany. SML (Serum males in Luebeck, Germany); SMT (Serum males in Tanzania); Asterisks indicate statistically significant differences p < 0.001
Fig. 5.
Distribution pattern of PCB (congener 153 and congener 138) in seminal plasma of men from Tanzania and Germany. Seminal plasma was obtained from 16 men in Tanzania and 21 men in Germany. SL (Seminal plasma in Luebeck, Germany); ST (Seminal plasma in Tanzania)
DDT, its metabolite DDE and dieldrin levels were significantly higher in both, serum and seminal plasma of men from Tanzania than in German men (dieldrin serum 0.55±0.09 μg/kg vs. 0.08±0.01 μg/kg; dieldrin seminal plasma: 0.13±0.05 μg/kg vs. 0.03±0.01 μg/kg; DDT+DDE serum: 19.06±2.41 μg/kg vs. 1.92±0.35 μg/kg; DDT+DDE seminal plasma: 0.63±0.11 μg/kg vs. 0.19±0.01 μg/kg). Levels of these insecticides in serum were higher compared to seminal plasma (Figs. 6 and 7 and Table 1 for DDE).
Fig. 6.
Comparison of distribution pattern of DDT+DDE (as sum DDT) in serum and seminal plasma of men from Tanzania and Germany. Serum and seminal plasma was obtained from 16 men in Tanzania and 21 men in Germany. SL (Seminal plasma in Luebeck, Germany); ST (Seminal plasma in Tanzania); SML (Serum males in Luebeck, Germany); SMT (Serum males in Tanzania); Asterisks indicate statistically significant differences p < 0.001
Fig. 7.
Comparison of distribution pattern of dieldrin in serum and seminal plasma of men from Tanzania and Germany. Serum and seminal plasma was obtained from 16 men in Tanzania and 21 men in Germany. SL (Seminal plasma in Luebeck, Germany); ST (Seminal plasma in Tanzania); SML (Serum males in Luebeck, Germany); SMT (Serum males in Tanzania); Asterisks indicate statistically significant differences p < 0.01
Comparison of males and females
In general, levels of contaminants were higher in serum of men and lower in their seminal plasma compared to serum of women and their follicular fluid (Figs. 1, 4, 5).
PCB and DDT and fertility in Germany
Since fertilization and pregnancy records from Tanzania were not fully obtained, a critical evaluation revealed that these data are not reliable for investigating the impact of contaminants on fertility there. Those data were, however, recorded sufficiently and accurately in Germany.
To test the hypothesis that PCB and the insecticide DDT adversely affects fertility we correlated PCB and DDT concentrations in serum with sperm parameters and pregnancy rates in Germany. The semen parameters concentration, motility and morphology showed no correlation with serum PCB and DDT levels. PCB and DDT had no impact on semen parameters (data not shown). Furthermore, PCB has no impact on fertility in females, evaluated by oocyte number, fertilization and pregnancy rates (data not shown).
Two groups according to DDT levels in serum were generated in females from Germany. Patients with high and low levels of DDT were divided into two groups with a cut-off of 1 μg/kg. Comparing these two groups of high and low DDT concentrations, a relationship between high DDT levels above 1 μg/kg with low pregnancy rates could be demonstrated. Pregnancy rates in women with serum levels below 1 μg/kg were 53% compared to only 14% in women with serum concentrations of DDT above 1 μg/kg. The two patient groups did not differ significantly in infertility-related parameters like reason for infertility and age. Fertilization rates and oocyte quality are additionally shown in Table 2. We found an, however not significant, correlation with high DDT levels and low pregnancy rates.
Table 2.
Correlation of DDT+DDE with pregnancy rates
| High DDT | Low DDT | |
|---|---|---|
| Subjects (n) | 10 | 10 |
| Age | 33.2±4.8 | 31.1±3.9 |
| Oocyte no. | 10.8±5.8 | 12.1±5.7 |
| Fertilization rate (%) | 47.9±13.7 | 32.6±8.5 |
| CESm | 23.1±12.1 | 29.4±10.8 |
| Pregnancy rate (%) | 30 | 53 |
| 0.108 | ||
| 0.221 | ||
| 0.341 | ||
| 0.365 | ||
| 0.371 | ||
| 0.383 | ||
| 0.391 | ||
| 0.449 | ||
| 0.489 | ||
| 0.502 | ||
| 0.623 | ||
| 0.720 | ||
| 1.000 | ||
| 1.176 | ||
| 1.310 | ||
| 1.532 | ||
| 1.569 | ||
| 1.638 | ||
| 1.790 | ||
| 1.919 |
Notes. Fertilization rates, a modified embryo quality score, number of oocytes obtained and pregnancy rates in two groups are shown. The two groups are divided by serum DDT + DDE levels. In the data row below all DDT+DDE levels are shown. Pregnancies are indicated through Italic letters.
[DDT=DDT+DDE]; ±STD.
Italic: pregnancy.
Discussion
Although most of the contaminants tested here have been officially abandoned decades ago, they are still present in the food chain due to their lipophilic character and biological stability or in industrial products manufactured a long time ago. The populations of industrial and developing, more agricultural, countries are exposed to different patterns of pollutants which is reflected by the different distribution of these toxins in all body compartments tested. The burden of the biocides DDT and dieldrin is higher in Africa, the industrial by-products like PCB show higher levels in Germany. This is in line with our previous observation in maternal adipose tissue [11].
The literature concerning the effects of contaminants on male fertility delivers inconsistent and contradictory data. A recent study examined the effects of subchronic oral exposure to various organochlorines on reproductive tissues in mature male rats. Only minor, if any, effects on reproductive function were found [16]. Others, however, exposed juvenile guppies fish to DDE, which can act as an antiandrogen. Sperm count was reduced in adult males [17]. Similar findings were observed in adult male rats, exposed to 50 and 100 mg/kg DDT for 10 consecutive days. Sperm motility and testicular weight were reduced. PCB 77 and other PCBs applied to adult rats resulted in reduced sperm quality [18]. An adverse effect of DDT on male rat fertility was hypothesized [19]. Pflieger-Bruss and Schill reviewed the literature and noted that it might be unlikely that pesticides in vivo could affect sperm function, since in vivo concentrations are far lower than those used in in vitro experiments [20]. This was confirmed by a study having examined semen of 156 patients of an infertility unit in Germany. No correlation between PCB and semen parameters was observed [21]. Another study examined the sexual differentiation of boys and a possible impact of PCB. Prenatal exposure to PCB did not affect sexual differentiation in boys at the age of puberty [22]. Semen of a group of boys exposed to high levels of PCB through ingestion of contaminated cooking oil in Taiwan were examined 20 years after exposure. Reduced motility and altered morphology was described [23]. However, it must be kept in mind that the oil was also contaminated with dibenzofurans. Another report on reduced sperm motility in patients with high levels of PCB in seminal plasma adds evidence to negative effects of environmental chemicals on male fertility [24]. In contrast, Dalvie et al. (2004) found no strong evidence for an effect of DDT on semen quality, although DDT was negatively associated with semen count [25]. Others assessed sperm DNA integrity and detected that high serum levels of PCB may impair sperm chromatin integrity in a subgroup of patients, whereas DDE had no impact [26]. In accordance with that, Dallinga and co-workers (2002) suggested a negative correlation in the subgroup of patients with good semen quality between high PCB metabolites in serum and decreased sperm count. In the group with poor semen quality no such correlation was detected [27].
Taken together negative effects of insectizides or PCBs on male fertility were only detected in subgroups of patients for single agents and not for all chemicals tested. Furthermore, only specific semen parameters like sperm count, motility or DNA integrity have been affected. Seminal plasma seems to be very inert against environmental pollutants, since concentrations of all biocides investigated were lowest in seminal plasma. In contrast, serum concentrations of insecticides are highest in men. The saturation level of PCB in seminal plasma was 0.2 μg/kg, no matter how high the serum concentration was. This may deliver the explanation, why the tested toxins had no influence on sperm quality in our study.
First reports on the influence of insecticides on female reproductive function noted an eggshell thinning in birds exposed to organochlorine biocides [28]. Here, no impact of PCB levels on pregnancy rates could be demonstrated. This is in accord with previous studies. Anglers from Lake Ontario and women highly exposed to PCB through fish consumption in Michigan and Sweden have been examined in some other studies. Time to pregnancy as an indirect marker for fertility was not affected [29–31]. Another very recent study found only a weak and inconclusive correlation between exposure to PCBs and DDE and time to pregnancy. Women with highest serum levels of PCB experienced a longer waiting period for pregnancy. This did not apply for DDE [32]. Other authors tried to determine the effect of PCBs on fertilization in mice in vitro. PCB was added to IVF-medium. A negative effect of high doses of PCBs on fertilization, oocyte and embryo quality was observed [33]. Contrary to these findings Ensslen et al. could not establish an influence of PCB on fertilization of oocytes in women [34]. Again these divergent findings could be explained by the fact that the concentrations used for in vitro animal studies have been far higher than those detected in human tissues.
We observed a not statistically significant correlation between higher levels of DDT in German women and low pregnancy rates. The cut-off was set at 1 μg/kg. In seven women with DDT levels over 1 μg/kg only one pregnancy was achieved (14%). This is in contrast to a pregnancy rate of 53% in women with low serum DDT levels. Compared to seminal plasma, which we found to be almost inert against toxins, concentrations of contaminants in follicular fluid are far higher and reflect serum levels more closely. In concordance with our observations, abortion rates have been higher in women with high concentrations of DDT which might point to an effect of those toxins on early embryonic development [35]. Korrick et al. conducted a case control study to assess the association of DDT with spontaneous abortion [36]. Higher maternal serum levels of DDE, a metabolite of DDT, were associated with an increased risk for spontaneous abortion. Women attending a reproductive unit in Germany have been examined. Levels of hexachlorocyclohexane, pentachlorophenol and PCB were significantly higher in women with endometriosis or spontaneous abortion. Additionally, high concentrations of DDT have been associated with lower conception rates [37]. In keeping with that, Windham et al. (2005) advised an effect of DDE on ovarian function and consecutively on pregnancy rates. They found a shorter luteal phase length with higher DDT or DDE serum levels in asian women, but no impact of PCBs. Summarizing the data on female reproduction, no effect of PCBs, but a negative impact of DDT on ovarian function and pregnancy rates could be observed.
We conclude that DDT may adversely affect pregnancy rates in German women. Seminal plasma is quite insensitive against contaminants, since, despite of high serum levels, concentrations of all toxins in seminal plasma remained low. Additionally, sperm quality was not influenced by environmental contaminants. Serum levels of organochlorides in general are higher in men than in women. Concentrations of insecticides were higher in Tanzania, whereas concentrations of PCB were higher in Germany.
Footnotes
Prof. Dr. O. Bauer died at 12/3/2000 in an aeroplane crash. He gave the initial impetus to this project and set up all the necessary organisation. We dedicate this paper to his memory.
References
- 1.Hruska KS, Furth PA, Seifer DB, Sharara FI, Flaws JA. Environmental factors in infertility. Clin Obstet Gynecol. 2000;43:821–829. doi: 10.1097/00003081-200012000-00014. [DOI] [PubMed] [Google Scholar]
- 2.Sharara FI, Seifer DB, Flaws JA. Environmental toxicants and female reproduction. Fertil Steril. 1998;70:613–622. doi: 10.1016/S0015-0282(98)00253-2. [DOI] [PubMed] [Google Scholar]
- 3.Cohn BA, Cirillo PM, Wolff MS, et al. DDT and DDE exposure in mothers and time to pregnancy in daughters. Lancet. 2003;361:2205–2206. doi: 10.1016/S0140-6736(03)13776-2. [DOI] [PubMed] [Google Scholar]
- 4.Pocar P, Brevini TA, Fischer B, Gandolfi F. The impact of endocrine disruptors on oocyte competence. Reproduction. 2003;125:313–325. doi: 10.1530/rep.0.1250313. [DOI] [PubMed] [Google Scholar]
- 5.Wagner U, Schlebusch H, Van Der Ven H, Van Der Ven K, Diedrich K, Krebs D. Accumulation of pollutants in the genital tract of sterility patients. J Clin Chem Clin Biochem. 1990;28:683–688. [PubMed] [Google Scholar]
- 6.Chiu A, Beaubier J, Chiu J, Chan L, Gerstenberger S. Epidemiologic studies of PCB congener profiles in North American fish consuming populations. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2004;22:13–36. doi: 10.1081/GNC-120038004. [DOI] [PubMed] [Google Scholar]
- 7.Rivero-Rodriguez L, Borja-Aburto VH, Santos-Burgoa C, Waliszewskiy S, Rios C, Cruz V. Exposure assessment for workers applying DDT to control malaria in Veracruz, Mexico. Environ Health Perspect. 1997;105:98–101. doi: 10.1289/ehp.9710598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jorgenson JL. Aldrin and dieldrin: a review of research on their production, environmental deposition and fate, bioaccumulation, toxicology, and epidemiology in the United States. Environ Health Perspect. 2001;109(Suppl 1):113–139. doi: 10.1289/ehp.01109s1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Institute of Environmental Health Sciences. National Toxicology Program. 2002. http://www.niehs.nih.gov.
- 10.Agency for Toxic Substances and Disease Registry. 2002. http://www.atsdr.cdc.gov.
- 11.Van Der Ven K, Van Der Ven H, Thibold A, et al. Chlorinated hydrocarbon content of fetal and maternal body tissues and fluids in full term pregnant women: a comparison of Germany versus Tanzania. Hum Reprod. 1992;7(Suppl 1):95–100. doi: 10.1093/humrep/7.suppl_1.95. [DOI] [PubMed] [Google Scholar]
- 12.Stijve T, Cardinale E. Rapid determination of chlorinated pesticides, PCBs, and a number of phosphated insecticides in fatty foods. Mitt Lebensmittelunters Hyg. 1974;65:131–150. [Google Scholar]
- 13.Ballschmiter K, Zell M. Baseline studies of the global pollution. I. Occurrence of organohalogens in pristine European and antarctic aquatic environments. Int J Environ Anal Chem. 1980;8:15–35. doi: 10.1080/03067318008071876. [DOI] [PubMed] [Google Scholar]
- 14.WHO laboratory manual for the examination of human semen and sperm-cervical mucus. 4. Cambridge: Cambridge University Press; 1999. [Google Scholar]
- 15.Ludwig M, Schöpper B, Al Hasani S, Diedrich K. Clinical use of a pronuclear stage score following intracytoplasmic sperm injection: impact on pregnancy rates under the conditions of the German embryo protection law. Hum Reprod. 2000;15:325–329. doi: 10.1093/humrep/15.2.325. [DOI] [PubMed] [Google Scholar]
- 16.Wade MG, Foster WG, YoungLai EV, et al. Effects of subchronic exposure to a complex mixture of persistent contaminants in male rats: systemic, immune, and reproductive effects. Toxicol Sci. 2002;67:131–143. doi: 10.1093/toxsci/67.1.131. [DOI] [PubMed] [Google Scholar]
- 17.Bayley M, Junge M, Baatrup E. Exposure of juvenile guppies to three antiandrogens causes demasculinization and a reduced sperm count in adult males. Aquat Toxicol. 2002;56:227–239. doi: 10.1016/S0166-445X(01)00210-7. [DOI] [PubMed] [Google Scholar]
- 18.Faqi AS, Dalsenter PR, Mathar W, Heinrich-Hirsch B, Chahoud I. Reproductive toxicity and tissue concentrations of 3,3′,4,4′-tetrachlorobiphenyl (PCB 77) in male adult rats. Hum Exp Toxicol. 1998;17:151–156. doi: 10.1191/096032798678908459. [DOI] [PubMed] [Google Scholar]
- 19.Ben Rhouma K, Tebourbi O, Krichah R, Sakly M. Reproductive toxicity of DDT in adult male rats. Hum Exp Toxicol. 2001;20:393–397. doi: 10.1191/096032701682692946. [DOI] [PubMed] [Google Scholar]
- 20.Pflieger-Bruss S, Schill WB. Effects of chlorinated hydrocarbons on sperm function in vitro. Andrologia. 2000;32:311–315. doi: 10.1046/j.1439-0272.2000.00399.x. [DOI] [PubMed] [Google Scholar]
- 21.Ensslen SC, Riedel HH, Bluthgen H, Heeschen W. [Chlorinated hydrocarbons in seminal plasma and male fertility] Zentralbl Gynakol. 1990;112:817–821. [PubMed] [Google Scholar]
- 22.Mol NM, Sorensen N, Weihe P, et al. Spermaturia and serum hormone concentrations at the age of puberty in boys prenatally exposed to polychlorinated biphenyls. Eur J Endocrinol. 2002;146:357–363. doi: 10.1530/eje.0.1460357. [DOI] [PubMed] [Google Scholar]
- 23.Guo YL, Hsu PC, Hsu CC, Lambert GH. Semen quality after prenatal exposure to polychlorinated biphenyls and dibenzofurans. Lancet. 2000;356:1240–1241. doi: 10.1016/S0140-6736(00)02792-6. [DOI] [PubMed] [Google Scholar]
- 24.Bush B, Bennett AH, Snow JT. Polychlorobiphenyl congeners, p,p′-DDE, and sperm function in humans. Arch Environ Contam Toxicol. 1986;15:333–341. doi: 10.1007/BF01066399. [DOI] [PubMed] [Google Scholar]
- 25.Dalvie MA, Myers JE, Thompson ML, et al. The long-term effects of DDT exposure on semen, fertility, and sexual function of malaria vector-control workers in Limpopo Province, South Africa. Environ Res. 2004;96:1–8. doi: 10.1016/j.envres.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 26.Spano M, Toft G, Hagmar L, et al. Exposure to PCB and p, p′-DDE in European and Inuit populations: impact on human sperm chromatin integrity. Hum Reprod. 2005;20:3488–3499. doi: 10.1093/humrep/dei297. [DOI] [PubMed] [Google Scholar]
- 27.Dallinga JW, Moonen EJ, Dumoulin JC, Evers JL, Geraedts JP, Kleinjans JC. Decreased human semen quality and organochlorine compounds in blood. Hum Reprod. 2002;17:1973–1979. doi: 10.1093/humrep/17.8.1973. [DOI] [PubMed] [Google Scholar]
- 28.Ratcliffe DA. Decrease in eggshell weight in certain birds of prey. Nature. 1967;215:208–210. doi: 10.1038/215208a0. [DOI] [PubMed] [Google Scholar]
- 29.Axmon A, Rylander L, Stromberg U, Dyremark E, Hagmar L. Polychlorinated biphenyls in blood plasma among Swedish female fish consumers in relation to time to pregnancy. J Toxicol Environ Health A. 2001;64:485–498. doi: 10.1080/152873901753215948. [DOI] [PubMed] [Google Scholar]
- 30.Buck GM, Sever LE, Mendola P, Zielezny M, Vena JE. Consumption of contaminated sport fish from Lake Ontario and time-to-pregnancy. New York State Angler Cohort. Am J Epidemiol. 1997;146:949–954. doi: 10.1093/oxfordjournals.aje.a009221. [DOI] [PubMed] [Google Scholar]
- 31.Courval JM, DeHoog JV, Stein AD, et al. Sport-caught fish consumption and conception delay in licensed Michigan anglers. Environ Res. 1999;80:S183–S188. doi: 10.1006/enrs.1998.3909. [DOI] [PubMed] [Google Scholar]
- 32.Law DC, Klebanoff MA, Brock JW, Dunson DB, Longnecker MP. Maternal serum levels of polychlorinated biphenyls and 1,1-dichloro-2,2-bis(p-chlorophenyl)ethylene (DDE) and time to pregnancy. Am J Epidemiol. 2005;162:523–532. doi: 10.1093/aje/kwi240. [DOI] [PubMed] [Google Scholar]
- 33.Kholkute SD, Rodriguez J, Dukelow WR. Effects of polychlorinated biphenyls (PCBs) on in vitro fertilization in the mouse. Reprod Toxicol. 1994;8:69–73. doi: 10.1016/0890-6238(94)90069-8. [DOI] [PubMed] [Google Scholar]
- 34.Ensslen SC, Riedel HH, Bluthgen H, Heeschen W. [Demonstration of chlorinated hydrocarbons in follicular secretions] Zentralbl Gynakol. 1990;112:1223–1226. [PubMed] [Google Scholar]
- 35.Gerhard I, Daniel V, Link S, Monga B, Runnebaum B. Chlorinated hydrocarbons in women with repeated miscarriages. Environ Health Perspect. 1998;106:675–681. doi: 10.1289/ehp.98106675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Korrick SA, Chen C, Damokosh AI, et al. Association of DDT with spontaneous abortion: a case-control study. Ann Epidemiol. 2001;11:491–496. doi: 10.1016/S1047-2797(01)00239-3. [DOI] [PubMed] [Google Scholar]
- 37.Gerhard I, Monga B, Krahe J, Runnebaum B. Chlorinated hydrocarbons in infertile women. Environ Res. 1999;80:299–310. doi: 10.1006/enrs.1998.3890. [DOI] [PubMed] [Google Scholar]