Abstract
Objective
In the Fluid and Catheter Treatment Trial (FACTT)(NCT00281268), adults with acute lung injury (ALI) randomized to a conservative versus liberal fluid management protocol had increased days alive and free of mechanical ventilator support (ventilator-free days). Recruiting sufficient children with ALI into a pediatric trial is challenging. A Bayesian statistical approach relies on the adult trial for the a priori effect estimate, requiring fewer patients. Preparing for a Bayesian pediatric trial mirroring FACTT, we aimed to: a.)Identify an inverse association between fluid balance and VFDs; and b.)Determine if fluid balance over time is more similar to adults in the FACTT liberal or conservative arms.
Design
Multi-centered retrospective cohort study.
Setting
Five pediatric intensive care units.
Patients
Mechanically ventilated children (age ≥1 month to <18 years) with ALI admitted 2007–2010.
Interventions
None.
Measurements and Main Results
Fluid intake, output and net fluid balance were collected days 1–7 in 168 children with ALI (median age 3 years, median PaO2/FiO2 138) and weight-adjusted (ml/kg). Using multivariable linear regression to adjust for age, gender, race, admission day illness severity, PaO2/FiO2 and vasopressor use, increasing cumulative fluid balance (ml/kg) at day 3 was associated with fewer VFDs (p=0.02). Adjusted for weight, daily fluid balance on days 1–3 and cumulative fluid balance on days 1–7 were higher in these children compared to adults in the FACTT conservative arm (p<0.001, each day) and was similar to adults in the liberal arm.
Conclusions
Increasing fluid balance at day three in children with ALI at these centers is independently associated with fewer VFDs. Our findings and the similarity of fluid balance patterns in our cohort to adults in the FACTT liberal arm demonstrate the need to determine whether a conservative fluid management strategy improves clinical outcomes in children with ALI and support a Bayesian trial mirroring the FACTT trial.
Keywords: acute lung injury, acute respiratory distress syndrome, fluid balance, children, critical illness, fluid, mechanical ventilation
INTRODUCTION
Acute lung injury (ALI) and its more severe form, acute respiratory distress syndrome (ARDS), are devastating disorders of pulmonary inflammation causing diffuse alveolar damage, leading to pulmonary edema, hypoxemia and respiratory failure (1–5). The annual incidence of pediatric ALI in developed countries is estimated at 2 to 12/100,000 (6–12). ALI is associated with 2 to 10% of pediatric intensive care unit (PICU) admissions and is a factor in up to 30% of PICU deaths (12). Pediatric ALI mortality ranges from 22 to 65% (6–12) and approaches 80% in children with severe immune compromise. It has been estimated that each year 2,500 to 9,000 U.S. children will die from ALI (11).
Studies in children (16–24) and adults (25–31) show strong associations between positive fluid balance and worse outcomes, including death, in patients with respiratory failure and/or ALI. Flori and colleagues associated positive fluid balance in the first 3 days of illness with increased duration of mechanical ventilation and mortality in children with ALI (18). In mechanically ventilated children, Arikan et al. found percent fluid overload to be associated with worse oxygenation, increased duration of mechanical ventilation and increased PICU and hospital length of stay (19). Greater fluid overload in children at the initiation of renal replacement therapy is also associated with higher morbidity and mortality (21–23). The Fluid and Catheter Treatment Trial (FACTT, NCT00281268), a multi-centered randomized trial of adults with ALI, showed that a conservative fluid protocol compared to a liberal fluid protocol, increased days alive and free of mechanical ventilation (ventilator-free days or VFDs) without worsening non-pulmonary organ dysfunction (15).
The association of positive fluid balance with worse clinical outcomes has not strongly influenced pediatric practice; in 165 children with ALI across 12 North American and European countries, only 29% of patients were managed using a strategy to prevent or treat fluid overload (32). Because fluid overload may be a proxy for illness severity, a randomized trial in children with ALI is necessary to show that restrictive fluid management with diuresis leads to improved pediatric ALI outcomes (18). Recruiting sufficient pediatric ALI/ARDS patients into a traditionally designed trial of interventions aimed at mortality is very challenging (32–34), requiring an estimated recruitment across 60 large PICUs to enroll 800 pediatric ALI patients in four years (32). In 2009, Halpern et al. propose the use of a Bayesian statistical approach as a potential novel and alternative trial design method, given the infeasibility of enrolling a large number of children in clinical trials focused on critical illness conditions, such as ALI and ARDS A Bayesian statistical approach is an alternative trial design proposed for pediatric trials (33, 35) because it uses the a priori estimate of effect from a larger definitive trial, usually in adult patients, allowing the pediatric trial to gain power and have a smaller enrollment target (35).
We conducted this multicenter observational study in preparation for a future pediatric fluid management trial using a Bayesian statistical approach. Our primary objective was to identify an inverse association between cumulative fluid balance and VFDs, (the target outcome), in critically ill children with ALI at five proposed enrollment sites. Preliminary evidence supporting conservative fluid management in adults with ALI raised the issue that randomization of children to the liberal arm of the FACTT trial may be unethical if clinicians are adhering to a restrictive fluid management strategy in usual practice. Therefore, our secondary objective was to determine if the distribution of weight-adjusted daily net and cumulative fluid balance in children with ALI was similar to that of adults in the liberal arm of the FACTT trial, justifying its use as the comparative arm to the conservative fluid protocol. We hypothesized that increasing positive fluid balance at study day 3 was associated with fewer VFDs and the distribution of weight-adjusted fluid net and cumulative fluid balance was similar to that of adults in the FACTT liberal arm.
MATERIALS AND METHODS
Design, Setting and Patients
This multi-centered retrospective cohort study was conducted at the following five participating Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) pediatric intensive care units (PICUs) from January 2007 to March 2010: Children’s Hospital Boston; Benioff Children’s Hospital, University of California, San Francisco; Dell Children’s Medical Center of Central Texas; Children’s Hospital and Research Center Oakland; and Connecticut Children’s Medical Center. Institutional review board approval was obtained prior to data collection. Eligibility and exclusion criteria were adapted from the original FACTT protocol (15). Patients were identified retrospectively and enrolled. Patients from three of the centers were identified specific to this study. Patients at the other two centers were identified through the Pediatric Acute Lung Injury Cohort study database. Patients were identified for the study if they met all of the following inclusion criteria: 1.) age ≥ 1 month to < 18 years; 2.) receiving positive pressure ventilation through an endotracheal tube; 3.) meeting the American-European consensus criteria for ALI (36): a.) a ratio of partial pressure of arterial oxygen (PaO2) to fractional inspired oxygen (FiO2) of <300; b.) bilateral infiltrates on chest radiograph; c.) no evidence of left atrial hypertension; and d.) acute onset. We excluded patients with the following conditions: 1.) chronic conditions that could independently impair weaning from the ventilator including chronic ventilator dependence for chronic lung, neuromuscular disorders or other conditions; 2.) cyanotic heart disease; 3.) status post bone marrow, lung or renal transplantation; 4.) chronic renal failure 5.) burns >40% BSA and 6.) receiving continuous hemofiltration, hemodialysis or extracorporeal membrane oxygenation. The demographic profile of pediatric patients enrolled compared to those enrolled in the FACTT trial can be seen in the supplemental digital content, (Table, Supplementary Digital Content 1).
Data Collection
Study day 1 was defined as the first day eligible patients met ALI criteria. We collected data using an adapted case report form from the original FACTT trial. As done in the FACTT (15) trial, fluid intake, fluid output and fluid balance were collected daily closest to 8 AM on each study day. Daily fluid intake included blood products, total parenteral and enteral nutrition, intravenously administered fluids and medications. Daily fluid output included urine output, blood loss, gastric output and output from other body cavities. Insensible losses were not included in fluid output because they could not be adequately quantified (ventilated gases were humidified at all centers) and were not included in the original FACTT trial. Daily fluid balance was measured as the total 24hr (intake-output or I-O) per kilogram (kg) on each study day, using actual body weight. Cumulative fluid balance was measured as the daily running total 24hr (I-O)/kg from the start of the study, through study day 7. From the original FACTT dataset (15), we calculated weight-adjusted daily and cumulative fluid balance using the above calculation to make a weight-adjusted comparison with the pediatric cohort. Maintenance fluid intake was calculated using the Holliday-Segar method (37). Percent fluid overload was calculated using the formula described by Goldstein et al. (38). Vasopressor use was collected on admission and defined as intravenous dopamine (excluding dopamine ≤5 mcg/kg/min), norepinephrine, epinephrine or phenylephrine. Diuretic use was collected on admission and closest to 8 AM on study days 1 through 7. Duration of mechanical ventilation was measured as VFDs, which is measured as days free from mechanical ventilation until day 28 or death, at which time the VFDs are measured as zero (39). To assess illness severity, Pediatric Risk of Mortality (PRISM III) scoring was calculated for each patient during the first 24 hours following admission (40). Clinical and physiologic covariates were collected on study days 1 through 7, or until removal from mechanical ventilation or death, as done in the FACTT protocol (15).
Patients in the FACTT trial were managed with a low tidal volume, plateau pressure limited, mechanical ventilation strategy (15). The FACTT conservative protocol targeted fluid and diuretic therapies to achieve a goal CVP<4 mm Hg or PAOP<8 mm Hg while maintaining a mean arterial pressure ≥60 mm Hg, effective circulation and urine output ≥0.5cc/kg/hr. Those randomized to the FACTT liberal arm received fluid and diuretic therapies to achieve a goal CVP 10–14 or PAOP<14 while maintaining a mean arterial pressure ≥60 mm Hg, effective circulation and urine output ≥0.5cc/kg/hr. The protocol was only applied to patients not in shock. The FACTT protocol can be found at www.ardsnet.org. Fluid management and diuretic use in the pediatric cohort was not protocolized, and although these centers have implemented a modified version of the low tidal volume guidelines (41), guideline compliance was not monitored.
Data Analysis
Continuous demographic and clinical variables are reported as mean ± SD or median and inter-quartile range [IQR], depending on the normality. Categorical variables are represented as counts and percentages. Student’s t test was used to evaluate continuous variables. Multivariable linear regression was used to evaluate the association between VFDs and cumulative fluid balance at day three, adjusting for potential confounding factors. Clinical covariates were retained in the multivariable model if they were associated with VFDs at an alpha level of p < 0.2 or were clinically relevant, i.e. age, gender, and race. We chose to measure the association between cumulative fluid balance and VFDs at day 3 based on the Flori et al. study (18) as well as to capture the divergence between the fluid balance observed in the FACTT liberal and conservative fluid protocols (15) which occurred two days after enrollment and on average three days after patients were identified with ALI. Comparisons were considered significant if the two-sided p value was < 0.05. Analyses were performed using SAS (version 9.2, Cary, NC).
RESULTS
We identified 168 patients with ALI meeting eligibility criteria. Their baseline demographic and clinical characteristics are shown in Table 1. The median age of the pediatric cohort was 3 years (interquartile range [IQR] 0.8 to 11 years, range 0.1 to 17.9 years). ALI was triggered by direct pulmonary injury in 71% of the patients. Fifty-eight percent (58%) of the patients had at least one chronic condition on admission and 71% were admitted for a pulmonary reason. The median PaO2/FiO2 ratio on study entry was 138 [IQR 92, 178] and the median PRISM III score was 9 [IQR 3, 13].
Table 1.
Baseline characteristics of children with acute lung injury in these five pediatric intensive care units
| Baseline Characteristics | N=168 (%) |
|---|---|
| Age, median [IQR] | 3 [0.8, 11] |
| Gender, Male n (%) | 90 (54) |
| Race, White n (%) | 100 (59) |
| Direct lung injury, study entry, n (%) | 120 (71) |
| Reason for admission, n (%) | |
| Pulmonary | 120 (71) |
| Neurologic | 7 (4) |
| Infection | 14 (8) |
| Oncologic | 9 (6) |
| Immunologic | 13 (8) |
| Other | 13 (8) |
| Chronic condition, study entry, n (%) | 98 (58) |
| PaO2/FiO2 at study entry, median [IQR] | 138 [92, 178] |
| Vasopressor use at study entry, n (%) | 71 (42) |
| PRISM III, median [IQR] | 9 [3,13] |
IQR, Interquartile Range, PRISM, Pediatric Risk of Mortality
Mean daily net fluid balance and mean cumulative net fluid balance were positive study days 1 through 7, as shown in Figures 1 and 2. By study day 3, patients had received a mean total cumulative fluid intake of 269 ± 203 ml/kg and accumulated a mean cumulative positive fluid balance of 84 ± 93 ml/kg. Intravenous fluids comprised the greatest percentage of total cumulative fluid intake at day three, 86% ± 19%, followed by enteral nutrition, 7% ± 15%, packed red blood cells (pRBC), 3% ± 5%, 5% albumin, 2% ± 3%, fresh frozen plasma (FFP), 0.5% ± 2% and platelets 0.4% ± 2%. The mean total cumulative fluid output at day 3 measured 183 ± 139 ml/kg. Only 13% of the cohort (n=22) had achieved a negative fluid balance by day 3, and 58% (n=97) of the patients had received furosemide. The median cumulative furosemide dose (mg/kg) on study day 3 was 2 mg/kg [0.5, 4.3].
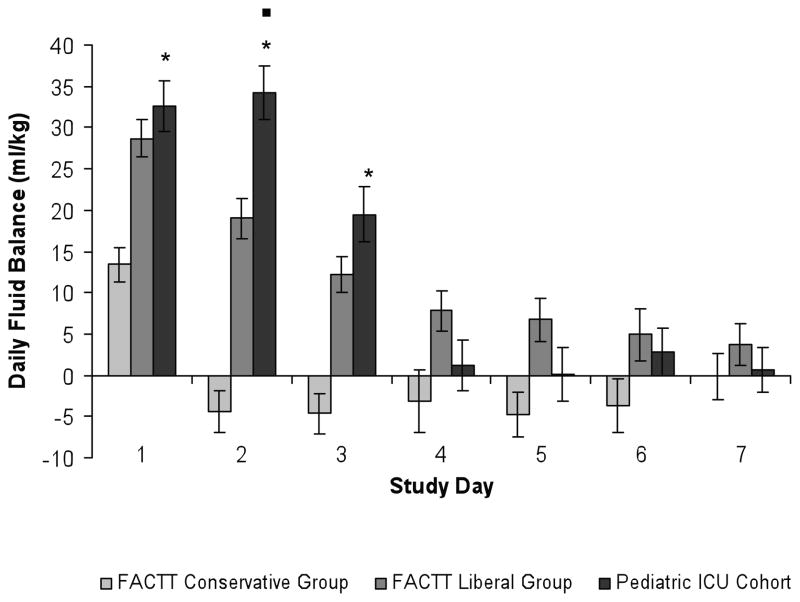
Figure 1.
Comparison of daily fluid balance (ml/kg/day) in the FACTT trial to the pediatric ICU cohort. Daily fluid balance (ml/kg/day) in the pediatric ICU was net positive on study days 1 through 7 and was significantly greater than that of the FACTT conservative group on study days 1 through 3, * = p < 0.001. Daily fluid balance in the pediatric ICU cohort was significantly greater than that of the FACTT liberal arm on study day 2, ■ = p <0.001. Error bars represent standard error (SE).
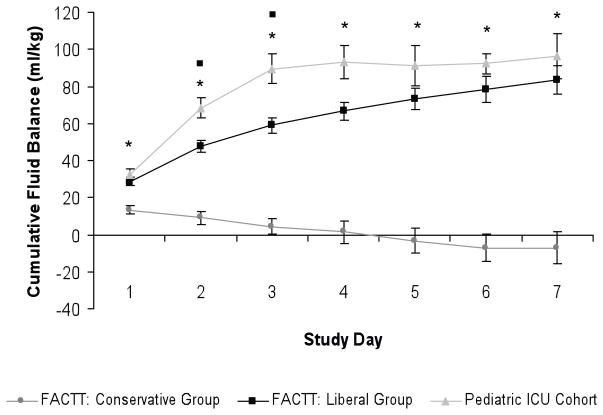
Figure 2.
Comparison of cumulative fluid balance (ml/kg/day) in the FACTT trial to the pediatric ICU cohort. Cumulative fluid balance (ml/kg/day) in the pediatric ICU was significantly greater than that of the FACTT conservative group on study days 1 through 7, * = p<0.001, and was significantly greater that that of the FACTT liberal arm on study days 2 and 3, ■ = p <0.02. Error bars represent standard error (SE).
The median number of VFDs in this cohort was 20 [IQR 10, 23] and did not differ significantly between the participating sites. Using linear regression, increasing cumulative fluid balance at day 3 was associated with fewer VFDs (p=0.02). Factors most strongly associated with VFDs in the univariate analysis included cumulative day 3 fluid balance, PRISM III score, vasopressor use, PaO2/FiO2 and age and are shown in Table 2. After adjusting for the above factors, as well as gender and race, increasing cumulative fluid balance on study day 3 remained significantly associated with fewer VFDs (p=0.01). The results of the multivariable analysis are shown in Table 3. Increasing cumulative fluid balance was also associated with fewer VFDs on study days 2 and 4, (p=0.01 and p=0.05, respectively), using the above multivariable model. In addition, increasing percent cumulative fluid overload was significantly associated with fewer VFDs on days 2 and 3 in our cohort, (p= 0.04, p=0.02, respectively), using the above multivariable model. The relationship between cumulative fluid balance (ml/kg) and VFDs did not vary by pediatric site. Cumulative furosemide dose (mg/kg) at day 3 was not significantly associated with VFDs.
Table 2.
Factors associated with ventilator-free days in the univariate analyses.
| Clinical Covariates | Regression Coefficient | p value |
|---|---|---|
| Cumulative Day 3 Fluid Balance (ml/kg) | −0.02 | 0.02 |
| PRISM III | −0.24 | 0.01 |
| Age (yrs) | −0.23 | 0.04 |
| Gender, Male | 0.06 | 0.97 |
| Race, White | −1.23 | 0.38 |
| PaO2/FiO2 at Study Entry | 0.01 | 0.02 |
| Vasopressor Use at Study Entry | −4.10 | 0.01 |
| Chronic Disease at Study Entry | 0.01 | 0.99 |
| Direct Lung Injury at Study Entry | 2.27 | 0.22 |
PRISM, Pediatric Risk of Mortality
Linear regression model was used to assess the association between clinical covariates and ventilator-free days. Clinical covariates were retained in the model if the univariate p value was < 0.2. Age, gender and race were kept in the multivariate model due to clinical relevance.
Table 3.
Results of the multivariable analysis of factors associated with ventilator-free days.
| Clinical Covariates | Regression Coefficient | p value |
|---|---|---|
| Cumulative Day 3 Fluid Balance (ml/kg) | −0.02 | 0.01 |
| PRISM III | −0.25 | 0.03 |
| Age (yrs) | −0.12 | 0.35 |
| Gender, Male | 0.16 | 0.91 |
| Race, White | −0.09 | 0.95 |
| PaO2/FiO2 at Study Entry | 0.02 | 0.08 |
| Vasopressor Use at Study Entry | −2.77 | 0.08 |
PRISM, Pediatric Risk of Mortality
Multivariable linear regression model was used to assess the association between clinical covariates and ventilator-free days. Clinical covariates were retained in the model if the univariate p value was < 0.2. Age, gender and race were kept in the multivariate model due to clinical relevance.
Infants may have inherently different fluid requirements than older children which may impact the association of fluid balance on VFDs in the younger population. To address this potential concern, we evaluated the univariate and multivariate association between fluid balance and VFDs in children ≥ 1 month to <1 years of age (n=50) using the above regression models. Cumulative fluid balance was associated with VFDs in the univariate and multivariate models both in children ≥ 1 year of age (p = <0.03 and p = 0.05, respectively) and in children ≥ 1 month to < 1 year of age, (p < 0.01 and p = 0.04, respectively). Finally, fluid intake, expressed as a percentage of maintenance fluid intake, did not differ significantly between children < 1 year compared to children ≥ 1 year.
The mortality rate in our pediatric cohort was 11.3%. Cumulative fluid balance was not significantly higher among non-survivors compared to survivors (119 ± 75 ml/kg vs. 80 ± 93 ml/kg, p=0.11, respectively) in the overall cohort. Cumulative fluid balance at day 3 was not significantly higher in patients receiving vasopressors on study entry compared to those not receiving vasopressors (109 ± 106 ml/kg vs. 80 ± 89 ml/kg, p=0.09, respectively).
In comparison to adult ALI patients in the FACTT trial (15), pediatric ALI patients were fluid net-positive on days 1 through 7, in a similar pattern observed in patients in the FACTT liberal arm, as shown in Figure 1. In contrast, patients in the FACTT conservative arm achieved a net-negative daily fluid balance (ml/kg) on study day 2, and continued to have a net-negative daily fluid balance (ml/kg) on study days 3 through 7. Daily fluid balance (ml/kg) in the pediatric cohort was significantly greater than that of the FACTT conservative arm on days 1, 2 and 3 (p < 0.001 for study days 1, 2, 3, Figure 1) and was even greater than the FACTT liberal arm on study day 2 (p<0.001). Cumulative fluid balance in the pediatric cohort remained positive through study day 7, was significantly greater that that of the FACTT conservative arm on study days 1 through 7, (p<0.001 on each study day, see Figure 2), and was significantly greater that that of the FACTT liberal arm on study days 2 and 3 (day 2: p=0.02, day 3: p=0.01, see Figure 2). Cumulative fluid balance (ml/kg) in the pediatric cohort was not significantly different from the FACTT liberal arm on the remainder of study days.
DISCUSSION
Our study identified a strong inverse association between cumulative fluid balance at day 3 and VFDs in children with ALI. Increasing fluid balance on study day 3 was significantly associated with fewer VFDs after adjusting for admission severity of illness, PaO2/FiO2 at study entry, vasopressor use, age, race and gender. In addition, fluid balance in the first week of illness in this cohort of children with ALI appears more similar to the consistently positive fluid balance pattern seen in adult patients in the liberal arm of the FACCT trial. Our findings demonstrate the need to prospectively evaluate the effect of fluid balance on pulmonary outcomes in children with ALI and support the use of a Bayesian statistical approach for the design of a pediatric trial of conservative fluid management trial in children with ALI.
The inverse relationship between increasing fluid balance and worsening pulmonary outcomes found in our study are similar to findings in previous pediatric clinical studies conducted prior to the FACTT trial (18–19, 42). We found that positive fluid balance at day 3 was associated with fewer days alive and free of mechanical ventilator support (VFDs), similar to Flori and colleagues (18) who found positive fluid balance at day 3 to be independently associated with duration of mechanical ventilation and survival, in children with ALI. Greater fluid overload at the initiation of continuous renal replacement therapy has been associated with higher mortality in critically ill children, even after adjusting for illness severity (34–36). Arikan et al. (19) found fluid overload to be associated with increased duration of mechanical ventilation. Similar to findings from Arikan et al.(19), by day 3, mean cumulative fluid overload in our cohort was 8.5% ± 10.5% and increasing percent fluid overload adjusted for admission severity of illness, vasopressor use, PaO2/FiO2, age, gender and race was significantly associated with VFDs on days 2 and 3 in our cohort, (p= 0.04, p=0.02, respectively). The similarity of our findings to these previous studies, as well as those found by Santschi et al. (32), suggest that the association between fluid balance and worse pulmonary outcomes has not influenced pediatric practice.
The fluid balance patterns and the association between increasing fluid balance and worsening pulmonary outcomes found in our study are also similar to findings in previous adult clinical studies (15, 25, 30). As reported by Schrier and colleagues (43), fluid balance patterns in ARDSNet studies (13–14) conducted prior to the FACTT Trial were similar to those in the FACTT liberal protocol group (15) suggesting that the liberal protocol was reflective of usual, non-protocolized, fluid management in adult patients with ALI (43). The similarity between fluid balance patterns in the pediatric cohort and that of adults in the liberal arm of the FACTT trial may suggest that pediatric fluid practices are similar to those of usual practice in adults prior to the FACTT trial.
One strength of this study is that we collected data across five large PICUs to evaluate fluid balance (ml/kg) in critically ill children with ALI across multiple institutions. Fluid balance (ml/kg) across these five PICUs was captured using the case report form, adapted for children, from the original FACTT (15) trial. However, because fluid management and diuretic use were not protocolized between the five pediatric centers and strict protocols were followed in the FACTT trial, making comparisons between the pediatric cohort and the fluid management arms of the FACTT trial must be limited. Additionally, although we converted the fluid parameters to ml/kg to allow comparison between adult and pediatric data, there are inherent differences in fluid requirements between infants and very young children compared to adults, limiting a weight-adjusted comparison. Infants are believed to have higher fluid requirements and insensible losses than adults (44). Insensible losses account for a percentage of losses but estimates are inaccurate and were not included in our study nor in the original FACTT trial. We attempted to evaluate the association between cumulative fluid balance and VFDs in infants separately from older children and similarly found cumulative fluid balance at day three to be significantly associated with VFDs in infants. Unfortunately, daily weights were not available but should be collected in the prospective trial.
We defined study day 1 as the first day patients met inclusion criteria for ALI whereas the FACTT trial defined study day 1 at the time of randomization. Study day 1 in the FACTT trial could be up to 48 hours after meeting inclusion criteria in some patients. Although this difference in timing could influence this comparison, we tried to adjust for it by using study day 3, whereas in the FACTT trial, patients were fluid negative on the conservative protocol on study day 2. Furthermore, we are dependent on data extracted from the clinical chart and documentation of fluids can be inaccurate (45–46).
Investigators have questioned the association between fluid balance and clinical outcomes, as it may be a surrogate for severity of illness, or shock. The children in our cohort may have received more fluid due to poor perfusion, hypotension or physician practice preferences and we do not have data on the degree of fluid overload prior to ICU admission. Fluid balance at day 3 may be a prognostic indicator of VFDs, reflecting the patient’s underlying illness, rather than be independently associated with VFDs. We attempted to evaluate the impact of fluid balance on VFDs, independent of shock and severity of illness, by controlling for vasopressor use on admission, severity of illness at admission, measured by PRISM III scores, admission PaO2/FiO2 and demographic factors including age, gender and race. However, the independent effect of fluid balance in children with ALI on VFDs can only be answered in a prospective trial. Use of protocolized mechanical ventilator management in the prospective trial will allow evaluation of the impact of fluid balance on duration of mechanical ventilation, oxygenation indices and length of stay. Once patients are on fluid management protocols, we will be able to evaluate the effect of these protocols on renal, hepatic, neurologic and cardiovascular function, oxygenation indices and coagulation profiles, which were not evaluated in this study.
The mortality in this cohort, which excluded patients after bone marrow transplant, was 11%. To power a traditionally designed clinical trial of the effect of fluid balance on mortality in children would require approximately 1500 patients per arm to see a 25% decrease (alpha ∝≤ 0.05, Beta = 0.8). Using VFDs as an outcome, to see a difference in 2.5 VFDs as seen in the FACTT trial (15), (alpha ∝≤ 0.05, Beta = 0.8), a pediatric trial would require 620 patients. Although composite outcomes like VFDs that combine mortality and duration of mechanical ventilation are not without controversy, VFDs was the reported outcome in the FACTT trial and statistical approaches have been developed for its use (39).
The use of a Bayesian statistical approach for the clinical trial of fluid management protocols in children with ALI is a rational study design choice (34–35). In clinical practice, lack of pediatric clinical trial data often leads pediatricians to extrapolate findings from adult studies to their patients. A Bayesian statistical approach is the natural step to making these extrapolations explicit (35), and although novel, is controversial. The extrapolation from adult to pediatric studies relies on the specification of “v”, the number representing how similar the effects of the treatment are in children to adults (35). This is obtained a priori, from medical expertise. The interpretation of the findings must be made in context with the degree of variation the clinician will accept between children and adults. Using the assumption that a conservative fluid strategy would decrease VFDs by 2.5 days, compared to a liberal fluid strategy, and using a coefficient of variance of 30%, v=0.74, we would need 200 patients in the pediatric trial to have an 80% chance of rejecting the null hypothesis at p=0.05. We estimate that 15 large PICUs could each enroll 3–5 children per year into a trial, allowing 200 patients to be enrolled over 4 years, a feasible goal.
CONCLUSION
In summary, our study has shown an independent association between positive fluid balance and fewer ventilator-free days in the same direction shown in the FACTT trial. Furthermore, we have shown a similar fluid balance trend in children to that of the liberal arm of the FACTT trial. Our findings support the need for a randomized controlled trial evaluating whether a conservative fluid strategy improve outcomes in children with ALI. The use of a Bayesian statistical approach to evaluate the effect of a conservative versus liberal fluid strategy in children with ALI, using the FACTT trial fluid management protocols modified for age and size, is a reasonable study choice. Using this novel methodology for a clinical trial will increase the feasibility of clinical trials in children in situations where enrollment of sufficient numbers of children is challenging and evidence in adult patients is available.
Supplementary Material
Acknowledgments
Financial Support: SV received research support from the NRSA Ruth T. Kirschstein Award, AHRQ T32 HS000063, as a fellow in the Harvard Pediatric Health Services Research Fellowship Program. AS received research support from NICHD HD047349 and NHLBI K23 HL085526. This work was conducted with support from the Harvard Catalyst | The Harvard Clinical and Translational Science Center (NIH Award #UL1 RR 025758 and financial contributions from Harvard University and its affiliated academic health care centers). The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic health care centers, the National Center for Research Resources, or the National Institutes of Health.
We thank Dr. David Schoenfeld for providing statistical advice. We thank Dr. Arthur Wheeler and Dr. Douglas Wilson for reviewing this manuscript and providing comments. This manuscript was prepared using FACTT Research Materials obtained from the NHLBI. We thank Andrea Harabin, PhD for facilitating access to the FACTT trial data and Dr. Michael Matthay for his support of this study. We acknowledge the ARDS Network FACTT trial investigators, as listed in Appendix 1, in the supplemental digital content (Supplemental Digital Content 2), for their collaboration. The following participating PALISI centers and site investigators contributed to this study: Children’s Hospital Boston: Dr. Adrienne Randolph, Dr. Stacey Valentine, Dionne Graham, PhD; Children’s Hospital and Research Center Oakland: Dr. Heidi Flori; University of California, San Francisco, Benioff Children’s Hospital: Dr. Anil Sapru, Maureen Convery; Connecticut Children’s Medical Center: Dr. Phillip Spinella, Laurie Karamessinis and Dell Children’s Medical Center of Central Texas: Dr. Renee Higgerson, Leeann Christie, RN.
Footnotes
The authors do not have any financial conflicts or ethical conflicts to disclose.
This research was performed at the following institutions: Children’s Hospital Boston, Boston, MA; Benioff Children’s Hospital, University of California, San Francisco, San Francisco, CA; Dell Children’s Medical Center of Central Texas, Austin, TX; Children’s Hospital and Research Center Oakland, Oakland, CA; Connecticut Children’s Medical Center, Hartford, CT
References
- 1.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 2.Luce JM. Acute lung injury and the acute respiratory distress syndrome. Crit Care Med. 1998;26:369–376. doi: 10.1097/00003246-199802000-00043. [DOI] [PubMed] [Google Scholar]
- 3.Ware LB. Pathophysiology of acute lung injury and the acute respiratory distress syndrome. Semin Respir Crit Care Med. 2006;27:337–349. doi: 10.1055/s-2006-948288. [DOI] [PubMed] [Google Scholar]
- 4.West JB. Invited Review: Pulmonary capillary stress failure. J Appl Physiol. 2000;89:2483–2489. doi: 10.1152/jappl.2000.89.6.2483. [DOI] [PubMed] [Google Scholar]
- 5.Calfee CS, Matthay MA. Nonventilatory treatments for acute lung injury and ARDS. Chest. 2007;131:913–920. doi: 10.1378/chest.06-1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Erickson S, Schibler A, Numa A, et al. Acute lung injury in pediatric intensive care in Australia and New Zealand: a prospective, multicenter, observational study. Pediatr Crit Care Med. 2007;8(4):317–323. doi: 10.1097/01.PCC.0000269408.64179.FF. [DOI] [PubMed] [Google Scholar]
- 7.Flori HR, Glidden DV, Rutherford GW, et al. Pediatric acute lung injury: Prospective evaluation of risk factors associated with mortality. Am J Respir Crit Care Med. 2005;171:995–1001. doi: 10.1164/rccm.200404-544OC. [DOI] [PubMed] [Google Scholar]
- 8.Goh AY, Chan PW, Lum LC, et al. Incidence of acute respiratory distress syndrome: A comparison of two definitions. Arch Dis Child. 1998;79:256–259. doi: 10.1136/adc.79.3.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Costil J, Cloup M, Leclerc F, et al. Acute respiratory distress syndrome (ARDS) in children: Multicenter Collaborative Study of the French Group of Pediatric Intensive Care. Pediatr Pulmonol Suppl. 1995;11:106–107. doi: 10.1002/ppul.1950191152. [DOI] [PubMed] [Google Scholar]
- 10.Dahlem P, van Aalderen WM, Bos AP. Pediatric acute lung injury. Paediatr Respir Rev. 2007;8:348–362. doi: 10.1016/j.prrv.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 11.Randolph A. Management of acute lung injury and acute respiratory distress syndrome in children. Crit Care Med. 2009;37:2488–2454. doi: 10.1097/CCM.0b013e3181aee5dd. [DOI] [PubMed] [Google Scholar]
- 12.Egan J. Acute lung injury in the child. Paediatr Respir Rev. 2010;11:171–175. doi: 10.1016/j.prrv.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 13.Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 14.Brower RG, Lanken PN, MacIntyre N, et al. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med. 2004;351:327–336. doi: 10.1056/NEJMoa032193. [DOI] [PubMed] [Google Scholar]
- 15.Wiedemann HP, Wheeler AP, Bernard GR, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354:2564–2575. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- 16.Trachsel D, McCrindle BW, Nakagawa S, et al. Oxygenation index predicts outcome in children with acute hypoxemic respiratory failure. Am J Respir Crit Care Med. 2005;172:206–211. doi: 10.1164/rccm.200405-625OC. [DOI] [PubMed] [Google Scholar]
- 17.Randolph AG, Forbes PW, Gedeit RG, et al. Cumulative fluid intake minus output is not associated with ventilator weaning duration or extubation outcomes in children. Pediatr Crit Care Med. 2005;6:642–647. doi: 10.1097/01.pcc.0000185484.14423.0d. [DOI] [PubMed] [Google Scholar]
- 18.Flori HR, Church G, Liu KD, et al. Positive fluid balance is associated with higher mortality and prolonged mechanical ventilation in pediatric patients with acute lung injury. Crit Care Res Pract. 2011;2011:854142. doi: 10.1155/2011/854142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arikan AA, Zappitelli M, Goldstein SL, et al. Fluid overload is associated with impaired oxygenation and morbidity in critically ill children. Pediatr Crit Care Med. 2011 doi: 10.1097/PCC.0b013e31822882a3. Epub July 14. [DOI] [PubMed] [Google Scholar]
- 20.Sutherland SM, Zappitelli M, Alexander SR, et al. Fluid overload and mortality in children receiving continuous renal replacement therapy: the prospective pediatric continuous renal replacement therapy registry. Am J Kidney Dis. 2010;55:316–325. doi: 10.1053/j.ajkd.2009.10.048. [DOI] [PubMed] [Google Scholar]
- 21.Goldstein SL, Somers MJ, Baum MA, et al. Pediatric patients with multi-organ dysfunction syndrome receiving continuous renal replacement therapy. Kidney Int. 2005;67:653–658. doi: 10.1111/j.1523-1755.2005.67121.x. [DOI] [PubMed] [Google Scholar]
- 22.Foland JA, Fortenberry JD, Warshaw BL, et al. Fluid overload before continuous hemofiltration and survival in critically ill children: a retrospective analysis. Crit Care Med. 2004;32:1771–1776. doi: 10.1097/01.ccm.0000132897.52737.49. [DOI] [PubMed] [Google Scholar]
- 23.Goldstein SL, Currier H, Graf C, et al. Outcome in children receiving continuous venovenous hemofiltration. Pediatrics. 2001;107:1309–1312. doi: 10.1542/peds.107.6.1309. [DOI] [PubMed] [Google Scholar]
- 24.Michael M, Kuehnle I, Goldstein SL. Fluid overload and acute renal failure in pediatric stem cell transplant patients. Pediatr Nephrol. 2004;19:91–95. doi: 10.1007/s00467-003-1313-z. [DOI] [PubMed] [Google Scholar]
- 25.Simmons RS, Berdine GG, Seidenfeld JJ, et al. Fluid balance and the adult respiratory distress syndrome. Am Rev Respir Dis. 1987;135:924–929. doi: 10.1164/arrd.1987.135.4.924. [DOI] [PubMed] [Google Scholar]
- 26.Humphrey H, Hall J, Sznajder I, et al. Improved survival in ARDS patients associated with a reduction in pulmonary capillary wedge pressure. Chest. 1990;97:1176–1180. doi: 10.1378/chest.97.5.1176. [DOI] [PubMed] [Google Scholar]
- 27.Schuller D, Mitchell JP, Calandrino FS, et al. Fluid balance during pulmonary edema. Is fluid gain a marker or a cause of poor outcome? Chest. 1991;100:1068–1075. doi: 10.1378/chest.100.4.1068. [DOI] [PubMed] [Google Scholar]
- 28.Alsous F, Khamiees M, DeGirolamo A, et al. Negative fluid balance predicts survival in patients with septic shock: a retrospective pilot study. Chest. 2000;117:1749–1754. doi: 10.1378/chest.117.6.1749. [DOI] [PubMed] [Google Scholar]
- 29.Sakr Y, Vincent JL, Reinhart K, et al. High tidal volume and positive fluid balance are associated with worse outcome in acute lung injury. Chest. 2005;128:3098–3108. doi: 10.1378/chest.128.5.3098. [DOI] [PubMed] [Google Scholar]
- 30.Rosenberg AL, Dechert RE, Park PK, et al. Review of a large clinical series: association of cumulative fluid balance on outcome in acute lung injury: a retrospective review of the ARDSnet tidal volume study cohort. J Intensive Care Med. 2009;24:35–46. doi: 10.1177/0885066608329850. [DOI] [PubMed] [Google Scholar]
- 31.Stewart RM, Park PK, Hunt JP, et al. Less is more: improved outcomes in surgical patients with conservative fluid administration and central venous catheter monitoring. J Am Coll Surg. 2009;208:725–735. doi: 10.1016/j.jamcollsurg.2009.01.026. [DOI] [PubMed] [Google Scholar]
- 32.Santschi M, Jouvet P, Leclerc F, et al. Acute lung injury in children: therapeutic practice and feasibility of international clinical trials. Pediatr Crit Care Med. 2010;11:681–689. doi: 10.1097/PCC.0b013e3181d904c0. [DOI] [PubMed] [Google Scholar]
- 33.Halpern SD, Randolph AG, Angus DC. No child left behind: Enrolling children and adults simultaneously in critical care randomized trials. Crit Care Med. 2009;37:2638–2641. doi: 10.1097/CCM.0b013e3181a59357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Randolph AG. The unique challenges of enrolling patients into multiple clinical trials. Crit Care Med. 2009;37:S107–111. doi: 10.1097/CCM.0b013e3181921c9d. [DOI] [PubMed] [Google Scholar]
- 35.Schoenfeld DA, Hui Z, Finkelstein DM. Bayesian design using adult data to augment pediatric trials. Clin Trials. 2009;6:297–304. doi: 10.1177/1740774509339238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149:818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 37.Holliday MA, Segar WE. The maintenance need for water in parenteral fluid therapy. Pediatrics. 1957;19:823–832. [PubMed] [Google Scholar]
- 38.Goldstein SL, Currier H, Graf JM, et al. Outcome in children receiving continuous venovenous hemofiltration. Pediatrics. 2001;107:1309–1312. doi: 10.1542/peds.107.6.1309. [DOI] [PubMed] [Google Scholar]
- 39.Schoenfeld DA, Bernard GR. Statistical evaluation of ventilator-free days as an efficacy measure in clinical trials of treatments for acute respiratory distress syndrome. Crit Care Med. 2002;30:1772–1777. doi: 10.1097/00003246-200208000-00016. [DOI] [PubMed] [Google Scholar]
- 40.Pollack MM, Patel KM, Ruttimann UE. PRISM III: An updated Pediatric Risk of Mortality score. Crit Care Med. 1996;24:743–752. doi: 10.1097/00003246-199605000-00004. [DOI] [PubMed] [Google Scholar]
- 41.Curley MA, Hibberd PL, Fineman LD, et al. Effect of prone positioning on clinical outcomes in children with acute lung injury: a randomized controlled trial. JAMA. 2005;294:229–237. doi: 10.1001/jama.294.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hu X, Qian S, Xu F, et al. Incidence, management and mortality of acute hypoxemic respiratory failure and acute respiratory distress syndrome from a prospective study of Chinese paediatric intensive care network. Acta Paediatr. 2010;99:715–721. doi: 10.1111/j.1651-2227.2010.01685.x. [DOI] [PubMed] [Google Scholar]
- 43.Schrier RW. Fluid administration in critically ill patients with acute kidney injury. Clin J Am Soc Nephrol. 2010;5:733–739. doi: 10.2215/CJN.00060110. [DOI] [PubMed] [Google Scholar]
- 44.Bush GH. Intravenous fluid requirements in paediatrics. Ann R Coll Surg Engl. 1971;49:92–101. [PMC free article] [PubMed] [Google Scholar]
- 45.Sheppard M. Monitoring fluid balance in acutely ill patients. Nurs Times. 2000;96:39–40. [PubMed] [Google Scholar]
- 46.Toto KH. Fluid balance assessment. The total perspective. Crit Care Nurs Clin North Am. 1998;10:383–400. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.