Abstract
Complement activation and the resulting inflammatory response is an important potential mechanism for multisystem organ injury in cardiac surgery. Novel therapeutic strategies using complement inhibitors may hold promise for improving outcomes for cardiac surgical patients by attenuating complement activation or its biologically active effector molecules. Recent clinical trials evaluating complement inhibitors have provided important data to further delineate the impact of complement activation and its inhibition on clinical outcomes. In this review we examine the role of complement activation and its inhibition as a therapeutic approach in cardiac surgery.
Introduction
The complement system is composed of more than 30 serum proteins that interact in a precise series of enzymatic cleavage and membrane binding events that lead to the generation of products with immunoprotective, immunoregulatory, and proinflammatory activities. Complement activation is associated with inflammatory injury in a heterogeneous group of clinical settings including cardiac surgery. Multiple pathways are responsible for complement activation in cardiac surgery including bioincompatibility of the cardiopulmonary bypass (CPB) circuit, reversal of heparinization, and activation via tissue ischemia and reperfusion. Recent advances in biotechnology and the development of complement inhibitors have allowed clinicians to examine the impact of inhibiting complement activation on clinical outcomes in cardiac surgery. This review will describe the complement system, mechanisms of activation, role of complement in inflammation and ischemia/reperfusion injury, and current therapeutic strategies for attenuating complement activation or its biologically active components in cardiac surgical patients.
The Complement System
Complement System Activation Pathways
The complement cascade comprises one of the main effector arms of both antibody-dependent and -independent mediated immunity. Complement was first identified as a heat-labile substance in serum that “complemented” antibodies in killing bacteria.1,2 The complement system serves as a bridge between innate and adaptive immunity in not only defending against bacterial infection, but also in disposing of both immune complexes and the products of inflammatory injury.1,2
The 30 plasma and cell surface proteins of the complement system together amount to more than 3 g per liter of plasma and make up nearly 15% of the globulin fraction.2 Activation occurs in a sequential manner with proteolytic cleavage of complement components yielding enzymatically active molecules that function as biological mediators and produce further downstream component activation. Understanding the sequence of complement activation is important because there are multiple therapeutic targets, which may attenuate its activation or antagonize biologically active metabolites.3
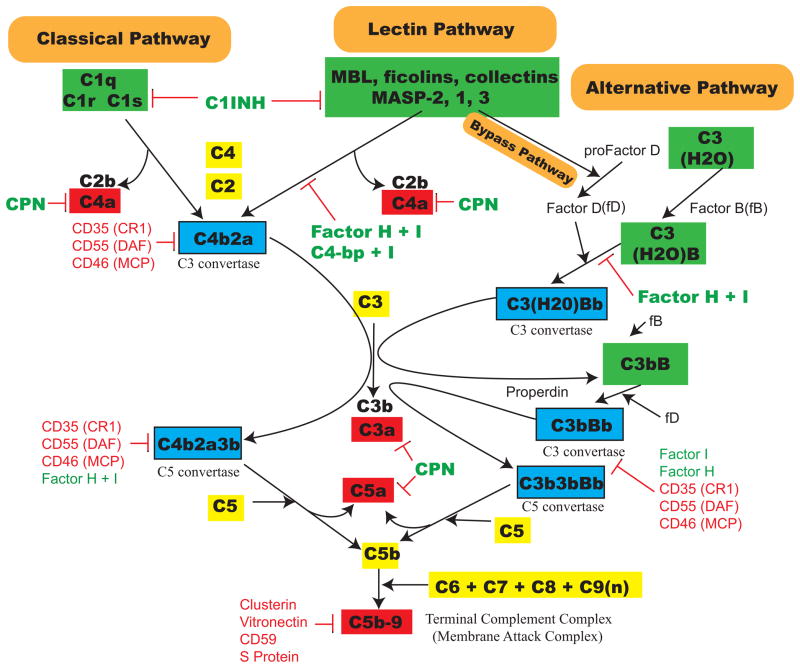
Three pathways of complement system activation have been described and include the classical, the alternative, and the mannose-binding lectin pathways (Figure 1 and Table 1). The classical pathway of complement activation is initiated when antibody-antigen complexes (immunoglobulin [IgG] or IgM) interact with the first complement component, C1. This protein in turn consists of a C1q component and two molecules each of the serine proteases, C1r and C1s. C1q binds to the Fc moiety of immune complexes to activate C1r and C1s. Subsequent cleavage of C2 and C4 leads to the generation of the classical pathway C3 convertase (i.e., C4b2a). In contrast to the classical pathway, the alternative pathway is activated by multiple mechanisms including bacterial products (endotoxin, exotoxin), yeast cell walls (zymosan), biomaterials (CPB and hemodialysis tubing), tissue type plasminogen activator, and the continuous interaction of C3 with water, which forms a C3b-like molecule known as C3H2O. The rate-limiting step of alternative pathway activation is the enzymatic cleavage of factor B by factor D to form the alternative pathway C3 convertase (i.e., C3bBb). The final pathway of complement activation, the lectin pathway, is primarily an antibody-independent pathway activated by binding of mannose-binding lectin (also known as mannan/mannose -binding lectin/protein; MBL), as well as binding of ficolins or collectin 11 (CL-K1), to carbohydrate structures present on the surface of bacteria, yeast, parasitic protozoa, and viruses.4,5 Associated with mannose-binding lectin are mannose-binding lectin associated serine proteases (MASP)-1/3 and MASP-2, serine proteases that cleave C2 and C4 to form the classical complement pathway C3 convertase. Mannose-binding lectin is structurally related to C1q, whereas MASP-1 and MASP-2 display remarkable homology to the classical pathway serine proteases, C1r and C1s.6 A bypass pathway involving complement activation by the mannose-binding lectin complex, in the absence of C2 and C4, has also been described and involves alternative pathway activation by MASP-1/3.7–10
Figure 1.
Three main complement pathways. An abbreviated diagram of the three complement pathways, along with regulators and endogenous inhibitors. Three major pathways are known as the classical, lectin and alternative pathways (orange boxes). A novel bypass pathway is also shown which activates the alternative pathway via MASP-1 or -3 within the mannose-binding lectin complex. Initiation molecules for each of the pathways are shown in green boxes. C3 and C5 convertases are shown in blue boxes. Biologically active complement components that have been studied extensively in the literature are shown in red boxes. Complement inhibitors/regulators (fluid phase and membrane bound) are shown attached to red inhibition blocking bars at the location of their interactions, which in some cases are at multiple locations. Abbreviations are further explained in Table 1.
Table 1.
Complement component abbreviations used in this review.
| C1INH – C1-inhibitor |
| C3a des Arg – inactivate C3a metabolite |
| C3bBb – alternative pathway C3 convertase |
| C3b3bBb – alternative pathway C5 convertase |
| C4-bp – C4-binding protein |
| C4b2a – classical/lectin pathway C3 convertase |
| C4b2a3b – classical/lectin pathway C5 convertase |
| C5b-9 – membrane attack complex; terminal complement complex |
| CPN – carboxypeptidase N |
| CR1 – complement receptor type 1; CD35 |
| DAF – decay accelerating factor; CD55 |
| MASP – mannose-binding lectin associated serine protease (1, 2 and 3) |
| MBL – mannose/mannan binding protein |
| MCP – membrane cofactor protein; CD46 |
All three complement activation pathways converge at C3, which is cleaved into C3a and C3b. C3a is a weak anaphylatoxin which is rapidly inactivated to C3a-des-arg by serum proteases. Attachment of C3b to either of the C3 convertases converts them into a C5 convertase, which can cleave C5 into C5a and C5b. C5a is a potent anaphylotoxin and induces alterations in smooth muscle, vascular tone and increases vascular permeability.11 Finally, C6, C7, C8, and multiple C9 units can be assembled to C5b and forms C5b-9, called the membrane attack complex or terminal complement complex, which is involved in host defense and cellular injury. C3a, iC3b, C5a, and C5b-9 are the most studied biologically active complement components and are considered the major complement mediators involved in inflammatory injury.3
Regulation of the Complement System
Complement activation is highly regulated by both plasma and integral membrane-bound proteins. Complement receptor type 1 (CR1; CD35) and membrane cofactor protein (MCP; CD46) are integral membrane proteins, while decay type accelerating factor (DAF; CD55) and CD59 are bound to the cell membrane phospholipid domain via a glycophosphatidylinositol anchor and can thus be easily cleaved by phospholipase C. CR1/CD35 plays a major role in opsonization of C3b-bound immune-complex leading to their clearance. Furthermore, MCP/CD46 acts as a cofactor for Factor I-mediated cleavage of C3b and C4b. DAF/CD55 prevents the assembly of both alternative and classical pathway C3 convertases12,13 and accelerates their decay by promoting dissociation of C2b from C4b2a or Bb from C3bBb.14 Thus, DAF/CD55, CR1/CD35, and MCP/CD46 inhibit complement activation at the level of C3. On the other hand, CD59 is a 20 kDa glycoprotein that interacts with both C8 and C9 during C5b-9 assembly to limit C9 insertion into the cell membrane.15,16 Additional protective mechanisms for nucleated cells against activated complement components include membrane attack complex internalization through endocytosis or ejection by exocytosis.17,18
Complement activation is further regulated by multiple humoral and membrane-based control mechanisms. The humoral or fluid-phase proteins involved in complement regulation include C1-inhibitor (C1INH), C4-binding protein (C4-bp), properdin, carboxypeptidase N (CPN), S-protein, clusterin and factors H and I.19–21 C1-inhibitor prevents excessive amplification of the classical pathway by covalently interacting with C1s and C1r, while still allowing free C1q to interact with its receptors.22 The classical complement pathway is also regulated by C4-binding protein, which binds the C3 convertase, C4b2a, and accelerates its decay by dissociating C2a.23 Factor H accelerates the decay of the alternative complement pathway C3 convertase. Finally, factor I is a serine protease, which regulates the classical and alternative complement pathway C3/C5 convertases by inactivating C4b and C3b by cleaving the C3b and C4b α-chains.24 Interestingly, properdin, which has historically been thought to stabilize the C3 convertase, promotes factor B binding to C3b and increase C3 convertase activity.25
Complement Modulation of Inflammation
Complement modulates vascular homeostasis and contributes to inflammatory injury through multiple mechanisms (Table 2). Complement activation generates proinflammatory mediators and amplifies injury via activated C5 and C3 leading to the release of peptides that serve as ligands for specific receptors on polymorphonuclear leukocytes, monocytes, macrophages, mast cells and other cells. Biologically active products of C3 and C5 cleavage include C3a/C3a des Arg, C5a/C5a des Arg, iC3b, and C5b-9.3,26 C5a is 1000-fold more potent than C3a as an inflammatory mediator and maintains biologic activity in the des Arg form, whereas C3a des Arg is without inflammatory activity. C5a also serves as the primary chemotactic factor for circulating neutrophils. C5a increases neutrophil adhesion to endothelium by mobilizing internal stores of neutrophil complement receptor 1, CD11b/CD18, CD62P and CD11c.27,28 Furthermore, C5a exacerbates tissue injury and inflammation by binding to neutrophils causing activation, aggregation and subsequent release of oxygen free radicals, proteolytic enzymes, and arachidonic acid metabolites.29 Finally, C5a facilitates the generation of chemokines, cytokines, and other proinflammatory mediators.30,31 C5 inhibition decreases neutrophil adhesion and transmigration, even though iC3b deposition still occurs in vivo.32–34 C5 inhibition also decreases expression of inflammatory molecules after ischemia/reperfusion injury.32,33,35 C3a interacts mainly with eosinophils, implicating C3a involvement in allergic inflammation.36,37 Even though iC3b, formed after C3b cleavage, is a specific ligand for neutrophil adhesion via CD11b/CD18 in vitro,38 iC3b formation in vivo does not support neutrophil adhesion in the presence of C5 inhibition.32,33,39 Thus, the specific role of iC3b as an in vivo ligand for neutrophil adhesion is not yet clearly defined.
Table 2.
Proinflammatory Components Resulting from Complement Activation
| Complement Component | Role in Inflammation |
|---|---|
| iC3b |
|
| C3a |
|
| C4a |
|
| C5a |
|
| C5b-9 (terminal membrane attack complex) |
|
Another important complement component, the membrane attack complex C5b-9, activates endothelial nuclear factor-kappa B (NF-κB) to increase leukocyte adhesion molecule transcription and expression.40 Moreover, this complex can lead to direct lysis of anucleated cells (e.g., bacteria and red blood cells). Endothelial leukocyte adhesion molecules influenced by complement activation include vascular cell adhesion molecule-1,41,42 intercellular adhesion molecule-1, and E-selectin.43 C5b-9 increases surface expression of P-selectin on platelets and endothelial cells from internal stores to increase neutrophil adhesion.15,44 C5b-9 also promotes leukocyte activation and chemotaxis by inducing endothelial interleukin-8 and monocyte chemoattractant protein-1 secretion.31 Furthermore, C5b-9 alters vascular tone by interfering with nitric oxide-mediated relaxation and decreasing endothelial cyclic guanosine monophosphate.42,45
Pathophysiology of Complement Activation and Its Implications for the Cardiac Surgical Patient
C1 Inhibitor Deficiency and Hereditary Angioedema
Hereditary angioedema is a syndrome where complement is activated by tissue injury during surgery or stress and characterized by excessive edema of the mucosa of the intestine and the larynx.46 The syndrome is primarily an autosomal dominant inherited disease that results in an inadequate level of C1 inhibitor that normally functions to inactivate serine esterases C1r and C1s, kallikrein of the kinin system, and activated factors XI and XII of the coagulation system. Plasmin degrades C1 inhibitor, and is an important trigger of angioedema. Excess bradykinin in the absence of C1 inhibitor is formed from the unregulated cleavage of high-molecular-weight kininogen by kallikrein.47 In type 1 hereditary angioedema, that accounts for 85% of cases C1 inhibitor levels are decreased, whereas in type 246 hereditary angioedema defective synthesis of C1 inhibitor results in normal C1 inhibitor levels but a dysfunctional protein.46 Another important, but rare, type of angioedema associated with C1 inhibitor deficiency is caused by the presence of autoantibodies against the protein. The latter syndrome usually occurs in elderly persons and is often associated with a lymphoproliferative disease (i.e., B cell lymphomas).48 Management of these patients for cardiac and other surgical procedures include addition of plasma-derived C1 inhibitor and the use of antifibrinolytics.49,50 In the future management of patients with hereditary angioedema may also include the use of recombinant human C1 inhibitor, which in addition to raising C1 inhibitor levels, binds and inactivates mannose-binding lectin and may give additional antiinflammatory actions above those observed using plasma-derived C1 inhibitor.51
Cardiac Surgery and Complement Activation
Complement activation is triggered by multiple mechanisms during cardiac surgery including the direct binding of C1q and/or mannose-binding lectin,52–55 or indirectly by binding to natural antibodies 5,56,57 or C-reactive protein (e.g., binding C1q).58 During CPB, direct adsorption of C3 onto the artificial surface of the extracorporeal surface may promote alternative pathway activation.59,60 However, compared with studies investigating the interaction of complement with biologic membranes, less is known regarding the factors initiating and controlling complement activation on artificial surfaces. Nonetheless, biomaterials have been developed to ameliorate excessive complement activation associated with extracorporeal circulation.61,62
Complement activation during cardiac surgery may also develop via non-CPB mediated mechanisms.63,64 For example, plasmin, produced as part of contact activation initiated by tissue injury, has been implicated in complement activation via direction activation of C3.65 Also, protamine reversal of heparinization activates the classical complement pathway and inhibits plasma carboxypeptidase N.66,67 Plasma carboxypeptidase N inhibition results in more inflammation from increased anaphylatoxin and kinin concentration. The magnitude of complement activation during cardiac surgery correlates with postoperative pulmonary shunting induced by classical complement pathway activation by protamine-heparin complexes.55 In addition, C4d-C-reactive protein, a marker for C-reactive protein-mediated complement activation, correlates with postoperative arrhythmias after coronary artery bypass graft (CABG).68 Thus, complement can be activated by multiple mechanisms during cardiac surgery, and may be associated with significant perioperative morbidity.
Complement Activation and Perioperative Myocardial Ischemia/Reperfusion Injury
The etiology of myocardial dysfunction after cardiac surgery is multifactorial.69 The myocardium may be particularly susceptible to ischemia during cardiac surgery due to underlying coronary artery disease, perioperative hemodynamic instability, inadequate protection during aortic cross-clamping and cardioplegic arrest, coronary artery embolization, and/or technical complications (i.e., incomplete revascularization, graft spasm or other nonphysiologic blood flow). Although prolonged myocardial ischemia alone jeopardizes cellular structural and biochemical integrity, oxygen deprivation that is limited to less than 20 minutes is usually associated with only transiently depressed myocardial contractility.70 Paradoxically, the restoration of blood flow after sustained myocardial ischemia results in a phenomenon known as myocardial ischemia/reperfusion injury, in which tissue injury is enhanced above that produced by ischemia alone.
Perioperative myocardial ischemia/reperfusion injury involves high-energy phosphate depletion, the generation of reactive oxygen species (ROS) upon the restoration of oxygen (O2) delivery, and alterations in intracellular calcium (Ca2+) homeostasis. In addition, the activation of several proinflammatory pathways including the coagulation, cytokine, and complement cascades can exacerbate tissue injury and functional impairment initiated by the original ischemic insult.71 Thus, myocardial ischemia/reperfusion injury involves a complex pathophysiological process, which significantly contributes to perioperative cardiac dysfunction and associated morbidity.
Activated complement components can contribute to myocardial ischemia/reperfusion injury via both indirect effects of anaphylatoxins and direct effects of C5b-9 that may modify leukocyte responses, alter vascular homeostasis, lead to cellular activation and ultimately induce tissue damage. Reperfusion exacerbates myocardial injury by activating complement, but complement activation may also be initiated during ischemia.72 During ischemia, disruption of myocyte membrane integrity may allow intracellular entry and complement activation, resulting in inflammation and tissue injury.19 Exposed basement membranes, subcellular organelles, mitochondrial particles, cardiolipin, certain sensitizing antibodies, or the coagulation/fibrinolytic system may directly activate complement.73–75
Complement activation plays an important early role in modifying myocardial injury not just during ischemia but also during the early phases of reperfusion of the ischemic myocardium. Activated complement components (e.g., anaphylatoxins, C5b-9) modify ROS production and Ca2+ flux that contribute to ischemia/reperfusion injury. For example, ROS generation is directly promoted via C5a-induced conversion of xanthine dehydrogenase and xanthine oxidase in endothelial cells.76 Ca2+ influx is also facilitated by complement activation via formation and membrane insertion of C5b-9.77,78
Activated complement components are particularly important facilitators of endothelial cell – leukocyte interactions that alter vascular homeostasis. The anaphylatoxins C3a, C5a, and C4a act directly on smooth muscle to promote vasodilatation.79–81 C5a independently promotes neutrophil chemotaxis, aggregation and the production of ROS, and arachidonic acid metabolites.82 Intracoronary C5a also promotes coronary vasoconstriction via thromboxane production from polymorphonuclear leukocytes/platelet interactions.83–87 C5a modifies neutrophil adherence to endothelium by eliciting the release of platelet activating factor, which subsequently activates neutrophils, up-regulatesβ2-integrins and induces L-selectin shedding.19,88 Furthermore, C5a may amplify the inflammatory response by inducing cytokine production.19 Additionally, iC3b functions as a specific ligand for sustaining leukocyte adhesion to the vascular endothelium via β2-integrins89 and facilitates the oxidative burst and release of proteolytic enzymes during neutrophil phagocytosis.90,91 C5b-9 modifies neutrophil-endothelial cell interactions by stimulating production of endothelial von Willebrand factor and rapid translocation of P-selectin from Weibel-Palade bodies to the endothelial surface.92 Endothelial NF-κB activation by C5b-9 increases leukocyte adhesion molecule transcription and expression.43,93 C5b-9 also alters vascular tone by inhibiting endothelium-dependent relaxation and decreasing endothelial cyclic guanosine monophosphate.45,93 Finally, C5b-9 promotes leukocyte activation and chemotaxis, and triggers neutrophils to release ROS, proteolytic enzymes and arachidonic acid metabolites (leukotriene B4; prostaglandin E2; thromboxane).31,94,95 Inhibition of many of these individual actions induced by complement protects the vasculature and tissues from inflammation, injury, and helps to maintain homeostasis. Thus, complement activation appears to be an “upstream” inducer of many proinflammatory and vascular dysfunction processes.
Complement activation associated with myocardial ischemia/reperfusion injury may also result from activation of the lectin complement pathway after binding of mannose-binding lectin to cell surface structures.5,56,96–99 Although mannose-binding lectin does not normally recognize the body’s own tissues, oxidative stress may alter cell surface membrane glycosylation leading to increased mannose-binding lectin deposition.72,98,100,101 Thus, complement activation through any of the three delineated pathways may significantly contribute to the pathogenesis of myocardial ischemia/reperfusion injury.
Genetics of Complement Activation and its Implications for Cardiovascular Disease and Adverse Perioperative Outcomes
Inflammation has evolved over time to become a crucial component of an organism’s immune response to pathogenic threats from microbial invasion or tissue injury. Anesthesia and surgery are also associated with a dramatically increased inflammatory response with concurrent suppression of cell-mediated immunity, which may play a role in short and long-term perioperative morbidity and mortality.102–107 Although perioperative systemic inflammation is universal, there is significant individual variation in the humoral response and associated perioperative morbidity. Consequently, sources of individual variation in the frequency and severity of postoperative adverse outcomes may be attributed not only to environmental, but also genetic influences.
Polymorphisms of several inflammatory genes, including those responsible for components of the complement cascade, are associated with differences in corresponding plasma protein levels and related cardiovascular disorders among nonsurgical populations. For example, mannose-binding lectin deficiency is associated with increased risk of various infections,108 increased incidence of sepsis and mortality in patients with systemic inflammatory response109 and severe atherosclerosis.110–112 Despite well-described associations between variations in genetic allotype, phenotypic expression and the role of inflammation in cardiovascular disease,113–115 there is little information available about patients undergoing cardiac surgical procedures. Nonetheless, several inflammatory gene polymorphisms have been identified which correlate with perioperative differences in plasma protein levels and adverse clinical outcomes.116
Allotypic variation of certain complement genes is associated with significant variability in the production of activated complement components and adverse clinical outcomes. The C4 gene is coded at two different loci, resulting in two major products designated C4A and C4B (that are distinguished from the activated complement product C4a).117 Activated C4A binds to immune complexes, whereas activated C4B has more affinity for erythrocyte membranes.55,118 Therefore, patients with the homozygous C4 null phenotype and lack the C4A isotype of C4 may have decreased clearance of activated C4-bound heparin-protamine complexes. For example, pediatric cardiac surgical patients, homozygous for the C4A null phenotype, have increased C3a and C4a levels, decreased lung compliance during CPB,119 and an increased risk for developing capillary leak syndrome.120 Consequently, prolonged circulation times for heparin-protamine complexes leads to further complement activation and anaphylatoxin generation. Heterozygosity of C4A null phenotype among adult patients undergoing cardiac surgery requiring CPB is also associated with increased complement activation induced by heparin-protamine complexes and greater pulmonary shunt fractions.55
Mannose-binding lectin deficiency is associated with arterial thrombosis in systemic lupus erythematosus among patients with variant alleles,121 and early venous bypass graft occlusion after CABG, suggesting a potential common genetic linkage between inflammation, hypercoagulability and adverse perioperative cardiovascular outcomes.122 However, median mannose-binding lectin levels between occlusion and non-occlusion groups were not significantly different, thus complicating data interpretation in this under-powered study.122 In contrast, mannose-binding lectin complexes have thrombin-like activity that mediates increased coagulation in vitro and in vivo in animal models.123–125 Therefore, clinical studies on mannose-binding lectin deficiency and coagulation interactions are clearly warranted.
Although historically complement activation as a component of perioperative systemic inflammation has been attributed to environmental factors, recent evidence has identified an important role of genetic influences on adverse clinical outcomes.126–130 In a prospective, longitudinal multi-institutional study of 978 patients undergoing primary CABG-only surgery with CPB, inclusion of the combined MBL2 LYQA secretor haplotype improved prediction models for perioperative myocardial injury based on traditional risk factors alone.128 In the near future, genotyping and phenotypic evaluation of inflammatory genes including those responsible for complement components may be included among current multivariate models of perioperative clinical risk assessment to help identify optimal interventional, antiinflammatory and immunomodulatory therapy, predict outcome and improve patient care.
Complement Inhibition as a Therapeutic Strategy in Cardiac Surgery
Multiple methods of inhibiting complement activation to reduce organ injury in cardiac surgical patients have been studied both experimentally and clinically (Table 3). Several recently published clinical trials involving large numbers of cardiac surgical patients have further supported the role of complement inhibition as a potential therapeutic strategy.131 The data from the clinical studies will be discussed.
Table 3.
Complement Inhibitors for Myocardial Ischemia/Reperfusion Injury
| Inhibitor | Target Protein (s) |
|---|---|
| C1 inhibitor | C1, MASP-1, MASP-2 |
| Rhucin (rec. human C1 inhibitor) | C1, MBL, MASP-1, MASP-2 |
| Soluble complement receptor type 1 | C3b, C4b, C3bBb, C3b2Bb C4b2a, C4b3b2a |
| Monoclonal Antibodies: | |
| Anti C5 | C5 |
| Anti C5a | C5a |
| Anti Properdin | Properdin |
| Anti MASP-2 | MASP-2 |
| Anti MBL | mannose-binding lectin |
| Anaphylatoxin Receptor Antagonists | |
| C5a Receptor Antagonist | C5aR |
| C3a Receptor Antagonist | C3aR |
| Synthetic Inhibitors: | |
| Compstatin | C3 |
| Nafamostat | C1s, factor D, C3bBb, C3b2Bb, C4b2a, C4b3b2a MASPs |
| Cobra Venom Factor | Complement depletion via alternative pathway activation |
| HC3-1496 | Complement depletion of C3, not C5 |
Plasma-Derived Complement Inhibitors
C1INH complexes with C1 (C1s and C1r) to inhibit C1 and components of the contact system proteases, including factors XIIa, XIa and kallikrein, as well as the lectin complement pathway.132–135 Cardioprotective effects of C1INH in myocardial ischemia/reperfusion injury is demonstrated in animal models.136–138 C1INH decreases infarct size and inflammation after myocardial ischemia/reperfusion in cats136 and pigs compared to controls.137,138 In a small clinical study, C1INH preserved hemodynamic performance and resulted in lower serum troponin levels compared with placebo-treated patients undergoing CABG.139 C1INH was used as “rescue therapy” for the treatment of myocardial ischemia/reperfusion injury in patients who became hemodynamically unstable after failed percutaneous transluminal angioplasty and required emergency surgical revascularization.140 Hemodynamic stabilization, weaning of aortic counterpulsation, and withdrawal of inotropic support were achieved within one day after the initiation of C1INH treatment. In addition to plasma-derived C1INH, a recombinant form of human C1INH (Ruconest™/Rhucin®; Pharming, Leiden, The Netherlands) has been developed and approved for hereditary angioedema in Europe (not yet approved in the USA). The recombinant form of human C1INH also binds and inhibits mannose-binding lectin suggesting that this form of C1INH may have additional advantages over plasma-derived C1INH.51
Pexelizumab
Pexelizumab (Alexion Pharmaceuticals, Inc., Cheshire, CT; Procter & Gamble, Cincinatti OH) is a recombinant single chain, monoclonal antibody that binds to C5 to inhibit C5a and C5b-9 formation. Early preclinical studies demonstrated that pexelizumab significantly inhibited C5 cleavage and both neutrophil and platelet activation in an in vitro CPB circuit.61 A subsequent Phase IIa trial evaluated 35 adult cardiac surgical patients receiving 0, 0.2, 0.5, 1, or 2 mg/kg of pexelizumab. Pexelizumab was well tolerated and was associated with a dose-dependent inhibition of C5 cleavage. Furthermore, dosing at 1 or 2 mg/kg decreased CD11b expression on polymorphonuclear leukocytes or monocytes, and significantly reduced cumulative CK-MB release and new visuospatial deficits.141 Finally, in a 914-patient, double-blind, placebo-controlled, 65-center study of patients undergoing CABG, pexelizumab had no significant effect on overall cognition. However, when domain specific effects were examined, a decline of at least 10% in the visuo-spatial domain was observed on postoperative day 4 in 56% of patients receiving placebo compared with 40% receiving pexelizumab by bolus and infusion (P = 0.003). Similarly, on postoperative day 30, a 10% decline was present in 21% of patients in the placebo group versus only 12% of the drug bolus plus infusion group (P=0.016).142
Based on these encouraging findings a double-blinded, placebo-controlled, prospective and randomized Phase IIb clinical study was subsequently performed in 914 cardiac surgical patients stratified into two groups: CABG-only requiring CPB and CABG with concomitant valve surgery on CPB.141 Patients were treated with placebo, pexelizumab 2.0 mg/kg bolus, or pexelizumab 2.0 mg/kg bolus followed by a 24-hr infusion at 0.05 mg/kg/hr. The patients were followed for 30 days to evaluate the safety and efficacy (CK-MB>60 ng/ml; Q-wave myocardial infarction (MI); new central neurological system deficit, left ventricular dysfunction; death; or a composite of death, MI, left ventricular dysfunction or new central neurological system deficit) of this intervention. Approximately 90% of the patients were in the CABG-only group (n = 796). Pexelizumab was well-tolerated and significantly inhibited complement activation (e.g., inhibition of hemolytic activity and decreased serum C5b-9 concentration) for 24 hours. There was no significant treatment effect of pexelizumab compared with placebo on the primary composite endpoint. Post hoc subgroup analysis though demonstrated that pexelizumab bolus plus infusion reduced the frequency of non-Q wave MI (when increasing the definition from a CK-MB > 60 ng/ml to 100 ng/mL) by 66% (2.7% vs 7.8%; p=0.01). Furthermore, the subgroup analysis found a reduction in the composite endpoint of death or MI (CK-MB>100 ng/ml independent of Q-wave) with pexelizumab bolus plus infusion (but not bolus dosing alone) compared with placebo on postoperative day 30 (3% vs 9%; p=0.004). Adverse events and infections were not significantly increased in pexelizumab versus placebo-treated patients. The composite incidence of death or MI (Q-wave or non-Q-wave) was 7.8% versus 13.2% of placebo patients at 30 days and was not risk adjusted. However, these preliminary data supported a potential therapeutic role for complement inhibition in the attenuation of non-Q wave MI and perhaps mortality after cardiac surgery and provided the rationale for subsequent larger clinical studies.141
In 2004, a larger phase III clinical trial called “Pexelizumab for Reduction in Infarction and Mortality in Coronary Artery Bypass Graft Surgery 1 (PRIMO-CABG)” evaluated the efficacy and safety of pexelizumab in reducing perioperative MI and mortality in CABG surgery.143 The study was a prospectively randomized, double-blind, placebo-controlled trial, involving 3099 adult patients undergoing CABG surgery with or without valve surgery. Patients were randomly assigned to receive IV pexelizumab (2.0 mg/kg bolus plus 0.05 mg/kg per hour for 24 hours; n = 1553) or placebo (n = 1546) before CPB initiation. The primary composite endpoint was the incidence of death or MI (CK-MB >100 ng/ml by postoperative day 4 independent of Q-wave; CK-MB>70 ng/ml by postoperative day 4 with Q-wave; new Q-wave by postoperative day 30 that was not present of postoperative day 4; MI with or without Q-wave evidence identified by the investigator and confirmed by a Clinical Events Committee by postoperative day 30) within 30 days of randomization in those undergoing CABG surgery only (n = 2746). Secondary analyses included the intent-to-treat analyses of the composite of death or MI at postoperative days 4 and 30 in all 3099 study patients. After 30 days, 134 (9.8%) of 1373 of patients receiving pexelizumab versus 161 (11.8%) of 1359 of patients receiving placebo died or experienced MI in the CABG-surgery only population (relative risk, 0.82; 95% confidence interval, 0.66–1.02; p=0.07). In the intent-to-treat analyses, 178 (11.5%) of 1547 patients receiving pexelizumab versus 215 (14.0%) of 1535 receiving placebo died or experienced MI (relative risk, 0.82; 95% confidence interval, 0.68–0.99; p=0.03) but were not risk adjusted and the trial was not powered to detect a reduction in mortality alone. In the CABG surgery-only group, pexelizumab was not associated with a significant reduction in the risk of the composite endpoint of death or MI in the 2746 patients evaluted with nonrisk-adjusted data. However, in risk-adjusted analysis, pexelizumab was associated with a statistically significant risk reduction in death or MI through postoperative day 30 among all 3099 patients undergoing CABG with or without valve surgery and provided the rationale for a larger clinical study of higher risk patients.
As a result of the risk adjusted data, an additional PRIMO-CABG II trial was undertaken as a prospectively randomized, double-blind, placebo-controlled trial, that enrolled 4254 patients undergoing CABG with or without valve surgery. The primary composite endpoint was the incidence of death or MI (defined as in PRIMO-CABG I) within 30 days of surgery. PRIMO-CABG II did not meet the primary composite endpoint of death or MI with 30 days of randomization (16.3% for placebo versus 15.2% for pexelizumab; p=0.20).144 However, a retrospective analysis of the combined results of PRIMO-CABG I and II (n=7353) was performed and the results were stratified by subsetting the patient populations according to their Society of Thoracic Surgery composite risk as opposed to their “PRIMO” risk that was simply defined as the number of risk factors. Based on this anlaysis, death at 30 days was significantly reduced for the greatest risk subset (n=2156, pexelizumab 5.7% versus placebo 8.1%, p=0.024). Furthermore, this mortality reduction persisted throughout the 180-day follow-up period (pexelizumab 11.1% versus placebo 14.4%, non risk adjusted, p=0.036). 144 The difference in PRIMO-CABG I and II trials may be a mortality benefit for high risk patients.144
Soluble Complement Receptor Type 1
Soluble complement receptor type 1 (TP10) is a recombinant soluble complement receptor type 1 (sCR1) antagonist that inhibits complement activation by accelerating the decay of C3 and C5 convertases and by acting as a cofactor in the proteolytic degradation of C3b and C4b by factor I.145 sCR1 attenuates tissue injury in animal models related to ischemia/reperfusion injury, experimental CPB and transplantation.146–148
Several prospective studies have evaluated TP10 in patients undergoing cardiac surgery. In infants < 1 year of age undergoing CPB (n=15) TP10 was administered in a phase I/II open-label prospective trial at a dose of 10 mg/kg before CPB, and 10 mg/100 mL prime volume added to the CPB circuit.149 All infants survived without TP10-related adverse events. C3a was lower 12 hours after CPB compared with baseline pre-CPB levels and remained lower 24 hours after CPB. TP10 concentration inversely correlated with the 12-hour post-CPB to pre-CPB ratio of C3a and with total fluid, net fluid and blood product administration/kg, through 24 hours after CPB. TP10 plasma concentration decreased to ≤60 μg/mL 12 hours after CPB. Based on these findings, an initial dose of 10 mg/kg over 0.5 hours followed by an infusion of 10 mg/kg over 23.5 hours should maintain TP10 concentrations 100–160 μg/mL for 24 hours after CPB. 149
A prospective, randomized, placebo-controlled, parallel group, phase II trial involving 594 high-risk, adult cardiac surgical patients requiring CPB evaluated the safety and efficacy of TP10.150 After anesthesia induction, patients were assigned to 1 of 5 groups consisting of TP10 administrated as a bolus (0, 1, 3, 5, or 10 mg/kg) CPB.150 The primary endpoint was the composite of death, MI, prolonged (≥ 24 hours) intraaortic balloon pump support, and prolonged tracheal intubation. TP10 significantly inhibited complement activity within 10–15 minutes of administration, an effect that persisted for 3 days postoperatively. Adverse events and infections were not significantly increased in TP10 versus placebo-treated patients. There was no difference in the primary endpoint between patients receiving TP10 in any dose versus placebo (31.4% TP10 vs 33.7% placebo; p=0.31). In subgroup analysis, however, the primary endpoint was reduced in male patients receiving TP10 by 30% (35.4% in placebo to 24.6% for TP10; p=0.026), and included a 36% reduction in death and MI (26.8% for placebo versus 17.1% for TP10; p=0.025). In contrast, no significant difference was observed in female patients receiving TP10 in the primary endpoint (28.8% in placebo to 44.7% for TP10; p=0.96) or in death and MI (17.8 for placebo versus 34% for TP10; p=0.98). In addition, among the TP10-treated patients, the incidence of death or MI was reduced in male patients undergoing CABG only by 43% comparaed with placebo (12.8% TP10 versus 22.6% placebo; p=0.043) and the requirement for prolonged intraaortic balloon pump support in male CABG and valve patients was reduced by 100% (p=0.019). No improvement was observed in female patients treated with TP10.
In summary, several prospectively randomized placebo-controlled clinical trials involving inhibitors of complement activation have been conducted in patients undergoing CABG surgery demonstrating some benefit in selected subgroups of patients for attenuating morbidity and mortality. In addition, despite theoretical concerns for an increased risk of infection associated with the antiinflammatory effects of complement inhibitors, the most recent trials involving both pexelizumab and TP10 have not revealed any significant differences in adverse events between treated and placebo groups. Pexelizumab and TP10 did not met their primary endpoints in their clinical trials and these biologics are not being further considered for cardiac surgery patients.
One explanation of the marginal positive findings of these clinical studies of complement inhibitors for improving outcomes of patients undergoing cardiac surgery is that they inhibit complement at a late stage of the cascade (e.g., C3/C5 convertase for TP10 and C5 for pexelizumab). As previously discussed, C1INH inhibits C1s and C1s, factors XIIa, XIa and MASP-1 and MASP-2 132–135 and was shown in a clinical study to preserved hemodynamic performance and lowered serum troponin levels compared to placebo treated patients undergoing CABG.139 Thus, C1INH inhibits not only classical and lectin complement cascades, but also the kinin-kallikrien and coagulation systems as well. Along these lines, thrombin is a multifunctional protease with proinflammatory, procoagulant, and proapoptotic effects.151 Thrombin has direct adverse effects on cardiomyocytes and endothelium and is a major contributor to myocardial ischemia/reperfusion during cardiac surgery.152 MASP-2 levels are decreased in cardiac surgery patients and MASP-2 inhibition or depletion is associated with cardioprotection suggesting that the mannose-binding lectin complex may play an important role in myocardial injury in patients undergoing cardiac surgery.126,153 Furthermore, MASP-1 and MASP-2 are involved in coagulation in vitro154 and recent evidence demonstrates that the mannose-binding lectin complex and in particular MASP-1, acts like thrombin in vivo.125 mannose-binding lectin deficiency in the APEX-AMI clinical trial was associated with significantly reduced mortality at 90 days after primary percutaneous coronary intervention.155 Additionally, a direct action of mannose-binding lectin on the microvasculature’s ability to vasodilate suggests that inhibition of this complement complex should be investigated further for its ability to provide cardioprotection in cardiac surgery patients.123,156 In summary, C1INH provides benefits in patients undergoing cardiac surgery. Since C1INH inhibits complement at multiple levels (e.g., C1r/s, MASPs), future clinical studies using C1INH, mannose-binding lectin inhibitors, or inhibitors against the early complement serine proteases (e.g., C1r/s and MASPs) would be warranted.
Conclusions
Complement was once considered to be a mysterious biochemical pathway involved in a few esoteric diseases. However, the role of complement in inflammatory injury and ischemia/reperfusion injury has recently been extensively studied. In addition, delineation of the complement cascade components, its endogenous regulatory mechanisms and interactions between different pathways has provided the foundation for developing novel, targeted pharmacological strategies aimed at reducing ischemia/reperfusion injury and associated morbidity. Several recently published clinical trials involving large numbers of cardiac surgical patients have supported the role of complement inhibition as a therapeutic strategy for improving outcomes of patients susceptible to ischemia/reperfusion injury. Further investigation will contribute to establishing the role of complement inhibition as a novel target for improving outcomes in cardiac surgical patients.
Acknowledgments
Funding: NIH grants: AI089781, HL056086 and HL099130
Footnotes
The authors declare no conflicts of interest.
Reprints will not be available from the authors.
DISCLOSURES:
Name: Gregory L. Stahl, PhD
Contribution: This author helped prepare the manuscript.
Name: Stanton K. Shernan, MD
Contribution: This author helped prepare the manuscript.
Name: Peter K. Smith, MD
Contribution: This author helped prepare the manuscript.
Name: Jerrold H. Levy, MD
Contribution: This author helped prepare the manuscript.
Recuse Note: Dr. Jerrold Levy is the Section Editor for Hemostasis and Transfusion Medicine for the Journal. This manuscript was handled by Dr. Charles W. Hogue, Jr, Associate Editor-in-Chief, and Dr. Levy was not involved in any way with the editorial process or decision.
Contributor Information
Gregory L. Stahl, Department of Anesthesiology, Perioperative and Pain Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts.
Stanton K. Shernan, Department of Anesthesiology, Perioperative and Pain Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts.
Peter K. Smith, Department of Surgery, Division of Cardiovascular and Thoracic Surgery, Duke University, Durham, North Carolina.
Jerrold H. Levy, Cardiothoracic Anesthesiology and Critical Care, Emory University School of Medicine, Atlanta, Georgia.
References
- 1.Walport MJ. Complement. Second of two parts. N Engl J Med. 2001;344:1140–4. doi: 10.1056/NEJM200104123441506. [DOI] [PubMed] [Google Scholar]
- 2.Walport MJ. Complement. First of two parts. N Engl J Med. 2001;344:1058–66. doi: 10.1056/NEJM200104053441406. [DOI] [PubMed] [Google Scholar]
- 3.Bhole D, Stahl GL. Therapeutic potential of targeting the complement cascade in critical care medicine. Crit Care Med. 2003;31:S97–104. doi: 10.1097/00003246-200301001-00014. [DOI] [PubMed] [Google Scholar]
- 4.Hansen S, Selman L, Palaniyar N, Ziegler K, Brandt J, Kliem A, Jonasson M, Skjoedt MO, Nielsen O, Hartshorn K, Jorgensen TJD, Skjodt K, Holmskov U. Collectin 11 (CL-11, CL-K1) Is a MASP-1/-3-Associated Plasma Collectin with Microbial-Binding Activity. J Immunol. 2010;185:6096–104. doi: 10.4049/jimmunol.1002185. [DOI] [PubMed] [Google Scholar]
- 5.McMullen ME, Hart ML, Walsh MC, Buras J, Takahashi K, Stahl GL. Mannose-binding lectin binds IgM to activate the lectin complement pathway in vitro and in vivo. Immunobiology. 2006;211:759–66. doi: 10.1016/j.imbio.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 6.Ji X, Azumi K, Sasaki M, Nonaka M. Ancient origin of the complement lectin pathway revealed by molecular cloning of mannan binding: Protein-associated serine protease from a urochordate, the Japanese ascidian, Halocynthia roretzi. Proc Natl Acad Sci USA. 1997;94:6340–5. doi: 10.1073/pnas.94.12.6340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iwaki D, Kanno K, Takahashi M, Endo Y, Matsushita M, Fujita T. The role of mannose-binding lectin-associated serine protease-3 in activation of the alternative complement pathway. J Immunol. 2011;187:3751–8. doi: 10.4049/jimmunol.1100280. [DOI] [PubMed] [Google Scholar]
- 8.Takahashi M, Ishida Y, Iwaki D, Kanno K, Suzuki T, Endo Y, Homma Y, Fujita T. Essential role of mannose-binding lectin-associated serine protease-1 in activation of the complement factor D. J Exp Med. 2010;207:29–3. doi: 10.1084/jem.20090633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Inal JM, Laich A, Miot S. Complement C2 bypass mechanism involving the C3-convertase C4bBb. J Immunol. 2007 Apr 1;178:53.16. [MeetingAbstracts] [Google Scholar]
- 10.Selander B, Martensson U, Weintraub A, Holmstrom E, Matsushita M, Thiel S, Jensenius JC, Truedsson L, Sjoholm AG. Mannan-binding lectin activates C3 and the alternative complement pathway without involvement of C2. J Clin Invest. 2006;116:1425–34. doi: 10.1172/JCI25982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kurizaki T, Abe M, Sanderson SD, Enke CA, Baranowska-Kortylewicz J. Role of polymorphonuclear leukocytes, nitric oxide synthase, and cyclooxygenase in vascular permeability changes induced by C5a agonist peptides. Mol Cancer Ther. 2004;3:85–91. [PubMed] [Google Scholar]
- 12.Nicholson-Weller A, Burge J, Fearon DT, Weller PF, Austen KF. Isolation of a human erythrocyte membrane glycoprotein with decay-accelerating activity for C3 convertase of the complement system. J Immunol. 1982;129:184–9. [PubMed] [Google Scholar]
- 13.Medof ME, Kinoshita T, Nussenzweig V. Inhibition of complement activation on the surface of cells after incorporation of decay-accelerating factor (DAF) into their membranes. J Exp Med. 1984;160:1558–78. doi: 10.1084/jem.160.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nicholson-Weller A. Decay accelerating factor (CD55) Curr Top Microbiol Immunol. 1992;178:7–30. doi: 10.1007/978-3-642-77014-2_2. [DOI] [PubMed] [Google Scholar]
- 15.Rollins SA, Zhao J, Ninomiya H, Sims PJ. Inhibition of homologous complement by CD59 is mediated by a species-selective recognition conferred through binding to C8 within C5b-8 or C9 within C5b-9. J Immunol. 1991;146:2345–51. [PubMed] [Google Scholar]
- 16.Meri S. Protectin (CD59). Complement lysis inhibitor and prototype domain in a new protein superfamily. Immunologist. 1994;2:149–55. [Google Scholar]
- 17.Morgan BP. Complement membrane attack on nucleated cells: resistance, recovery and non-lethal effects. Biochem J. 1989;264:1–14. doi: 10.1042/bj2640001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ohanian SH, Schlager SI. Humoral immune killing of nucleated cells: mechanisms of complement-mediated attack and target cell defense. Crit Rev Immunol. 1981;1:165–209. [PubMed] [Google Scholar]
- 19.Kilgore KS, Friedrichs GS, Homeister JW, Lucchesi BR. The complement system in myocardial ischaemia/reperfusion injury. Cardiovasc Res. 1994;28:437–44. doi: 10.1093/cvr/28.4.437. [DOI] [PubMed] [Google Scholar]
- 20.Morgan BP. Clinical complementology: recent progress and future trends. Eur J Clin Invest. 1994;24:219–28. doi: 10.1111/j.1365-2362.1994.tb01078.x. [DOI] [PubMed] [Google Scholar]
- 21.Morgan BP, Meri S. Membrane proteins that protect against complement lysis. Springer Semin Immunopathol. 1994;15:369–96. doi: 10.1007/BF01837366. [DOI] [PubMed] [Google Scholar]
- 22.Ziccardi RJ. The first component of human complement (C1): activation and control. Springer Semin Immunopathol. 1983;6:213–30. doi: 10.1007/BF00205874. [DOI] [PubMed] [Google Scholar]
- 23.Scharfstein J, Ferreira A, Gigli I, Nussenzweig V. Human C4-binding protein. I. Isolation and characterization. J Exp Med. 1978;148:207–22. doi: 10.1084/jem.148.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pangburn MK, Schreiber RD, Muller-Eberhard HJ. Human complement C3b inactivator: isolation, characterization, and demonstration of an absolute requirement for the serum protein beta1H for cleavage of C3b and C4b in solution. J Exp Med. 1977;146:257–70. doi: 10.1084/jem.146.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jelezarova E, Vogt A, Lutz HU. Interaction of C3b(2)--IgG complexes with complement proteins properdin, factor B and factor H: implications for amplification. Biochem J. 2000;349:217–23. doi: 10.1042/0264-6021:3490217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chenoweth DE, Cooper SW, Hugli TE, Stewart RW, Blackstone EH, Kirklin JW. Complement activation during cardiopulmonary bypass. Evidence for generation of C3a and C5a anaphylatoxins. N Engl J Med. 1981;304:497–503. doi: 10.1056/NEJM198102263040901. [DOI] [PubMed] [Google Scholar]
- 27.Mulligan MS, Schmid E, Till GO, Hugli TE, Friedl HP, Roth RA, Ward PA. C5a-dependent up-regulation in vivo of lung vascular P-selectin. J Immunol. 1997;158:1857–61. [PubMed] [Google Scholar]
- 28.Tonnesen MG, Anderson DC, Springer TA, Knedler A, Avdi N, Henson PM. Adherence of neutrophils to cultured human microvascular endothelial cells. Stimulation by chemotactic peptides and lipid mediators and dependence upon the Mac-1, LFA-1, p150,95 glycoprotein family. J Clin Invest. 1989;83:637–46. doi: 10.1172/JCI113928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sacks T, Moldow CF, Craddock PR, Bowers TK, Jacob HS. Oxygen radicals mediate endothelial cell damage by complement- stimulated granulocytes: an in vitro model of immune vascular damage. J Clin Invest. 1978;61:1161–7. doi: 10.1172/JCI109031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saadi S, Holzknecht RA, Patte CP, Platt JL. Endothelial cell activation by pore-forming structures: pivotal role for interleukin-1alpha. Circulation. 2000;101:1867–73. doi: 10.1161/01.cir.101.15.1867. [DOI] [PubMed] [Google Scholar]
- 31.Kilgore KS, Flory CM, Miller BF, Evans VM, Warren JS. The membrane attack complex of complement induces interleukin-8 and monocyte chemoattractant protein-1 secretion from human umbilical vein endothelial cells. Am J Pathol. 1996;149:953–61. [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou W, Farrar CA, Abe K, Pratt JR, Marsh JE, Wang Y, Stahl GL, Sacks SH. Predominant role for C5b-9 in renal ischemia/reperfusion injury. J Clin Invest. 2000;105:1363–71. doi: 10.1172/JCI8621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vakeva AP, Agah A, Rollins SA, Matis LA, Li L, Stahl GL. Myocardial infarction and apoptosis after myocardial ischemia and reperfusion. Role of the terminal complement components and inhibition by anti-C5 therapy. Circulation. 1998;97:2259–67. doi: 10.1161/01.cir.97.22.2259. [DOI] [PubMed] [Google Scholar]
- 34.Zhao H, Montalto MC, Pfeiffer KJ, Hao L, Stahl GL. Murine model of gastrointestinal ischemia associated with complement-dependent injury. J Appl Physiol. 2002;93:338–45. doi: 10.1152/japplphysiol.00159.2002. [DOI] [PubMed] [Google Scholar]
- 35.Wada K, Montalto MC, Stahl GL. Inhibition of complement C5 reduces local and remote organ injury after intestinal ischemia/reperfusion in the rat. Gastroenterology. 2001;120:126–33. doi: 10.1053/gast.2001.20873. [DOI] [PubMed] [Google Scholar]
- 36.Jagels MA, Daffern PJ, Hugli TE. C3a and C5a enhance granulocyte adhesion to endothelial and epithelial cell monolayers: epithelial and endothelial priming is required for C3a-induced eosinophil adhesion. Immunopharmacology. 2000;46:209–22. doi: 10.1016/s0162-3109(99)00178-2. [DOI] [PubMed] [Google Scholar]
- 37.DiScipio RG, Daffern PJ, Jagels MA, Broide DH, Sriramarao P. A comparison of C3a and C5a-mediated stable adhesion of rolling eosinophils in postcapillary venules and transendothelial migration in vitro and in vivo. J Immunol. 1999;162:1127–36. [PubMed] [Google Scholar]
- 38.Marks RM, Todd RFI, Ward PA. Rapid induction of neutrophil-endothelial adhesion by endothelial complement fixation. Nature. 1989;339:314–7. doi: 10.1038/339314a0. [DOI] [PubMed] [Google Scholar]
- 39.Zhao H, Montalto MC, Pfeiffer KJ, Hao L, Stahl GL. Murine model of gastrointestinal ischemia associated with complement dependent injury. J Appl Physiol. 2002;93:338–45. doi: 10.1152/japplphysiol.00159.2002. [DOI] [PubMed] [Google Scholar]
- 40.Kilgore KS, Schmid E, Shanley TP, Flory CM, Maheswari V, Tramontini NL, Cohen H, Ward PA, Friedl HP, Warren JS. Sublytic concentrations of the membrane attack complex of complement induce endothelial interleukin-8 and monocyte chemoattractant protein-1 through nuclear factor-kappaB activation. Am J Pathol. 1997;150:2019–31. [PMC free article] [PubMed] [Google Scholar]
- 41.Tedesco F, Pausa M, Nardon E, Introna M, Mantovani A, Dobrina A. The cytolytically inactive terminal complement complex activates endothelial cells to express adhesion molecules and tissue factor procoagulant activity. J Exp Med. 1997;185:1619–27. doi: 10.1084/jem.185.9.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Collard CD, Lekowski R, Jordan JE, Agah A, Stahl GL. Complement activation following oxidative stress. Mol Immunol. 1999;36:941–8. doi: 10.1016/s0161-5890(99)00116-9. [DOI] [PubMed] [Google Scholar]
- 43.Kilgore KS, Shen JP, Miller BF, Ward PA, Warren JS. Enhancement by the complement membrane attack complex of tumor necrosis factor-a-induced endothelial cell expression of E-selectin and ICAM-1. J Immunol. 1995;155:1434–41. [PubMed] [Google Scholar]
- 44.Rollins SA, Johnson KK, Li L, Birks C, Matis LA, Rother RP. Role of porcine P-selectin in complement-dependent adhesion of human leukocytes to porcine endothelial cells. Transplantation. 2000;69:1659–67. doi: 10.1097/00007890-200004270-00023. [DOI] [PubMed] [Google Scholar]
- 45.Stahl GL, Reenstra WR, Frendl G. Complement mediated loss of endothelium-dependent relaxation of porcine coronary arteries. Role of the terminal membrane attack complex. Circ Res. 1995;76:575–83. doi: 10.1161/01.res.76.4.575. [DOI] [PubMed] [Google Scholar]
- 46.Zuraw BL, Christiansen SC. Pathophysiology of hereditary angioedema. Am J Rhinol Allergy. 2011;25:373–8. doi: 10.2500/ajra.2011.25.3661. [DOI] [PubMed] [Google Scholar]
- 47.Nussberger J, Cugno M, Amstutz C, Cicardi M, Pellacani A, Agostoni A. Plasma bradykinin in angio-oedema. Lancet. 1998;351:1693–7. doi: 10.1016/S0140-6736(97)09137-X. [DOI] [PubMed] [Google Scholar]
- 48.Gelfand JA, Boss GR, Conley CL, Reinhart R, Frank MM. Acquired C1 esterase inhibitor deficiency and angioedema: a review. Medicine (Baltimore) 1979;58:321–8. doi: 10.1097/00005792-197907000-00004. [DOI] [PubMed] [Google Scholar]
- 49.Wall RT, Frank M, Hahn M. A review of 25 patients with hereditary angioedema requiring surgery. Anesthesiology. 1989;71:309–11. doi: 10.1097/00000542-198908000-00025. [DOI] [PubMed] [Google Scholar]
- 50.Chaney JD, Adair TM, Lell WA, McGiffin DC, Nielsen VG. Hemostatic analysis of a patient with hereditary angioedema undergoing coronary artery bypass grafting. Anesth Analg. 2001;93:1480–2. doi: 10.1097/00000539-200112000-00025. [DOI] [PubMed] [Google Scholar]
- 51.Gesuete R, Storini C, Fantin A, Stravalaci M, Zanier ER, Orsini F, Vietsch H, Mannesse ML, Ziere B, Gobbi M, De Simoni MG. Recombinant C1 inhibitor in brain ischemic injury. Ann Neurol. 2009;66:332–42. doi: 10.1002/ana.21740. [DOI] [PubMed] [Google Scholar]
- 52.Mulligan MS, Yeh CG, Rudolph AR, Ward PA. Protective effects of soluble CR1 in complement- and neutrophil- mediated tissue injury. J Immunol. 1992;148:1479–85. [PubMed] [Google Scholar]
- 53.Wurzner R, Schulze M, Happe L, Franzke A, Bieber FA, Oppermann M, Gotze O. Inhibition of terminal complement complex formation and cell lysis by monoclonal antibodies. Complement Inflamm. 1991;8:328–40. doi: 10.1159/000463204. [DOI] [PubMed] [Google Scholar]
- 54.Evans MJ, Rollins SA, Wolff DW, Rother RP, Norin AJ, Therrien DM, Grijalva GA, Mueller JP, Nye SH, Squinto SP, Wilkins JA. In vitro and in vivo inhibition of complement activity by a single-chain Fv fragment recognizing human C5. Mol Immunol. 1995;32:1183–95. doi: 10.1016/0161-5890(95)00099-2. [DOI] [PubMed] [Google Scholar]
- 55.Shastri KA, Logue GL, Stern MP, Rehman S, Raza S. Complement activation by heparin-protamine complexes during cardiopulmonary bypass: effect of C4A null allele. J Thorac Cardiovasc Surg. 1997;114:482–8. doi: 10.1016/S0022-5223(97)70197-1. [DOI] [PubMed] [Google Scholar]
- 56.Busche MN, Pavlov V, Takahashi K, Stahl GL. Myocardial ischemia and reperfusion injury is dependent on both IgM and mannose-binding lectin. Am J Physiol Heart Circ Physiol. 2009;297:H1853–H1859. doi: 10.1152/ajpheart.00049.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thomas TC, Rollins SA, Rother RP, Giannoni MA, Hartman SL, Elliott EA, Nye SH, Matis LA, Squinto SP, Evans MJ. Inhibition of complement activity by humanized anti-C5 antibody and single-chain Fv. Mol Immunol. 1996;33:1389–401. doi: 10.1016/s0161-5890(96)00078-8. [DOI] [PubMed] [Google Scholar]
- 58.McGrath FD, Brouwer MC, Arlaud GJ, Daha MR, Hack CE, Roos A. Evidence that complement protein C1q interacts with C-reactive protein through its globular head region. J Immunol. 2006;176:2950–7. doi: 10.4049/jimmunol.176.5.2950. [DOI] [PubMed] [Google Scholar]
- 59.Janatova J. Activation and control of complement, inflammation, and infection associated with the use of biomedical polymers. ASAIO J. 2000;46:S53–S62. doi: 10.1097/00002480-200011000-00038. [DOI] [PubMed] [Google Scholar]
- 60.Kazatchkine MD, Carreno MP. Activation of the complement system at the interface between blood and artificial surfaces. Biomaterials. 1988;9:30–5. doi: 10.1016/0142-9612(88)90066-x. [DOI] [PubMed] [Google Scholar]
- 61.Rinder CS, Rinder HM, Smith BR, Fitch JCK, Smith MJ, Tracey JB, Matis LA, Squinto SP, Rollins SA. Blockade of C5a and C5b-9 generation inhibits leukocyte and platelet activation during extracorporeal circulation. J Clin Invest. 1995;96:1564–72. doi: 10.1172/JCI118195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lappegard KT, Fung M, Bergseth G, Riesenfeld J, Mollnes TE. Artificial surface-induced cytokine synthesis: effect of heparin coating and complement inhibition. Ann Thorac Surg. 2004;78:38–44. doi: 10.1016/j.athoracsur.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 63.Kirklin JK, Westaby S, Blackstone EH, Kirklin JW, Chenoweth DE, Pacifico AD. Complement and the damaging effects of cardiopulmonary bypass. J Thorac Cardiovasc Surg. 1983;86:845–57. [PubMed] [Google Scholar]
- 64.Gu YJ, Mariani MA, Boonstra PW, Grandjean JG, van OW. Complement activation in coronary artery bypass grafting patients without cardiopulmonary bypass: the role of tissue injury by surgical incision. Chest. 1999;116:892–8. doi: 10.1378/chest.116.4.892. [DOI] [PubMed] [Google Scholar]
- 65.Amara U, Flierl MA, Rittirsch D, Klos A, Chen H, Acker B, Bruckner UB, Nilsson B, Gebhard F, Lambris JD, Huber-Lang M. Molecular intercommunication between the complement and coagulation systems. J Immunol. 2010;185:5628–36. doi: 10.4049/jimmunol.0903678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tan FT, Jackman H, Skidgel RA, Zsigmond EK. Protamine inhibits plasma carboxypeptidase N, the inactivator of anaphylatoxins and kinins. Anesthesiology. 1989;70:267–75. doi: 10.1097/00000542-198902000-00015. [DOI] [PubMed] [Google Scholar]
- 67.Rent R, Ertel N, Eisenstein R, Gewurz H. Complement activation by interaction of polyanions and polycations. I. Heparin-protamine induced consumption of complement. J Immunol. 1975;114:120–4. [PubMed] [Google Scholar]
- 68.Bruins P, te Velthuis H, Yazdanbakhsh AP, Jansen PGM, van Hardevelt FWJ, de Beaumont EMFH, Wildevuur CRH, Eijsman L, Trouwborst A, Hack CE. Activation of the complement system during and after cardiopulmonary bypass surgery. Postsurgery activation involves C-reactive protein and is associated with postoperative arrhythmia. Circulation. 1997;96:3542–8. doi: 10.1161/01.cir.96.10.3542. [DOI] [PubMed] [Google Scholar]
- 69.Shernan SK. Perioperative myocardial ischemia reperfusion injury. Anesthesiol Clin North America. 2003;21:465–85. doi: 10.1016/s0889-8537(03)00038-5. [DOI] [PubMed] [Google Scholar]
- 70.Park JL, Lucchesi BR. Mechanisms of myocardial reperfusion injury. Ann Thorac Surg. 1999;68:1905–12. doi: 10.1016/s0003-4975(99)01073-5. [DOI] [PubMed] [Google Scholar]
- 71.Royston D. Systemic inflammatory responses to surgery with cardiopulmonary bypass. Perfusion. 1996;11:177–89. doi: 10.1177/026765919601100302. [DOI] [PubMed] [Google Scholar]
- 72.Stahl GL. Repefusion-injury during surgery: Role of the endothelium, oxidative stress and complement activation. New Surgery. 2001;1:62–6. [Google Scholar]
- 73.Rossen RD, Michael LH, Kagiyama A, Savage HE, Hanson G, Reisberg MA, Moake JN, Kim SH, Self D, Weakley S, Giannini E, Entman ML. Mechanism of complement activation after coronary artery occlusion: Evidence that myocardial ischemia in dogs causes release of constituents of myocardial subcellular origin that complex with human C1q in vivo. Circ Res. 1988;62:572–84. doi: 10.1161/01.res.62.3.572. [DOI] [PubMed] [Google Scholar]
- 74.Rossen RD, Michael LH, Hawkins HK, Youker K, Dreyer WJ, Baughn RE, Entman ML. Cardiolipin-protein complexes and initiation of complement activation after coronary artery occlusion. Circ Res. 1994;75:546–55. doi: 10.1161/01.res.75.3.546. [DOI] [PubMed] [Google Scholar]
- 75.DiScipio RG. The activation of the alternative pathway C3 convertase by human plasma kallikrein. Immunology. 1982;45:587–95. [PMC free article] [PubMed] [Google Scholar]
- 76.Friedl HP, Till GO, Ryan US, Ward PA. Mediator-induced activation of xanthine oxidase in endothelial cells. FASEB J. 1989;3:2512–8. doi: 10.1096/fasebj.3.13.2806779. [DOI] [PubMed] [Google Scholar]
- 77.Kim SH, Carney DF, Hammer CH, Shin ML. Nucleated cell killing by complement: effects of C5b-9 channel size and extracellular Ca2+ on the lytic process. J Immunol. 1987;138:1530–6. [PubMed] [Google Scholar]
- 78.Berger H-J, Taratuska A, Smith TW, Halperin JA. Activated complement directly modifies the performance of isolated heart muscle cells from guinea pig and rat. Am J Physiol Heart Circ Physiol. 1993;265:H267–H272. doi: 10.1152/ajpheart.1993.265.1.H267. [DOI] [PubMed] [Google Scholar]
- 79.Abe M, Shibata K, Akatsu H, Shimizu N, Sakata N, Katsuragi T, Okada H. Contribution of anaphylatoxin C5a to late airway responses after repeated exposure of antigen to allergic rats. J Immunol. 2001;167:4651–60. doi: 10.4049/jimmunol.167.8.4651. [DOI] [PubMed] [Google Scholar]
- 80.Humbles AA, Lu B, Nilsson CA, Lilly C, Israel E, Fujiwara Y, Gerard NP, Gerard C. A role for the C3a anaphylatoxin receptor in the effector phase of asthma. Nature. 2000;406:998–1001. doi: 10.1038/35023175. [DOI] [PubMed] [Google Scholar]
- 81.Bautsch W, Hoymann HG, Zhang Q, Meier-Wiedenbach I, Raschke U, Ames RS, Sohns B, Flemme N, Meyerz V, Grove M, Klos A, Kohl J. Cutting edge: guinea pigs with a natural C3a-receptor defect exhibit decreased bronchoconstriction in allergic airway disease: evidence for an involvement of the C3a anaphylatoxin in the pathogenesis of asthma. J Immunol. 2000;165:5401–5. doi: 10.4049/jimmunol.165.10.5401. [DOI] [PubMed] [Google Scholar]
- 82.Chenoweth DE. Complement mediators of inflammation. In: Ross GD, editor. Immunobiology of the complement system: An introduction for research and clinical medicine. Orlando: Academic Press; 1986. pp. 88–120. [Google Scholar]
- 83.Martin SE, Chenoweth DE, Engler RL, Roth DM, Longhurst JC. C5a decreases regional coronary blood flow and myocardial function in pigs: Implications for a granulocyte mechanism. Circ Res. 1988;63:483–91. doi: 10.1161/01.res.63.2.483. [DOI] [PubMed] [Google Scholar]
- 84.Stahl GL, Amsterdam EA, Symons JD, Longhurst JC. Role of thromboxane A2 in the cardiovascular response to intracoronary C5a. Circ Res. 1990;66:1103–11. doi: 10.1161/01.res.66.4.1103. [DOI] [PubMed] [Google Scholar]
- 85.Stahl GL, Fletcher MP, Amsterdam EA, Longhurst JC. Role of granulocytes and C5a in myocardial response to zymosan- activated serum. Am J Physiol Heart Circ Physiol. 1991;261:H29–H37. doi: 10.1152/ajpheart.1991.261.1.H29. [DOI] [PubMed] [Google Scholar]
- 86.Fletcher MP, Stahl GL, Longhurst JC. C5a-induced myocardial ischemia: role for CD18-dependent PMN localization and PMN-platelet interactions. Am J Physiol. 1993;265:H1750–H1761. doi: 10.1152/ajpheart.1993.265.5.H1750. [DOI] [PubMed] [Google Scholar]
- 87.Stahl GL, Morse DS, Martin SL. Eicosanoid production from porcine neutrophils and platelets: Differential production with various agonists. Am J Physiol. 1997;272:C1821–C1828. doi: 10.1152/ajpcell.1997.272.6.C1821. [DOI] [PubMed] [Google Scholar]
- 88.Braquet P, Paubert-Braquet M, Bourgain RH, Bussolino F, Hosford D. PAF/cytokine auto-generated feedback networks in microvascular immune injury: consequences in shock, ischemia and graft rejection. J Lipid Mediators. 1989;1:75–112. [PubMed] [Google Scholar]
- 89.Ueda T, Rieu P, Brayer J, Arnaout MA. Identification of the complement iC3b binding site in the b2 integrin CR3 (CD11b/CD18) Proc Natl Acad Sci U S A. 1994;91:10680–4. doi: 10.1073/pnas.91.22.10680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Takizawa F, Tsuji S, Nagasawa S. Enhancement of macrophage phagocytosis upon iC3b deposition on apoptotic cells. FEBS Letters. 1996;397:269–72. doi: 10.1016/s0014-5793(96)01197-0. [DOI] [PubMed] [Google Scholar]
- 91.Ohkuro M, Ogura-Masaki M, Kobayashi K, Sakai M, Takahashi K, Nagasawa S. Effect of iC3b binding to immune complexes upon the phagocytic response of human neutrophils: Synergistic functions between FcgammaR and CR3. FEBS Lett. 1995;373:189–92. doi: 10.1016/0014-5793(95)01036-e. [DOI] [PubMed] [Google Scholar]
- 92.Hattori R, Hamilton KK, McEver RP, Sims PJ. Complement proteins C5b-9 induce secretion of high molecular weight multimers of endothelial von Willebrand factor and translocation of granule membrane protein GMP-140 to the cell surface. J Biol Chem. 1989;264:9053–60. [PubMed] [Google Scholar]
- 93.Collard CD, Agah A, Reenstra WR, Buras J, Stahl GL. Endothelial nuclear factor-kB translocation and vascular cell adhesion molecule-1 induction by complement: Inhibition with anti-human C5 therapy or cGMP analogues. Arterioscler Thromb Vasc Biol. 1999;19:2623–9. doi: 10.1161/01.atv.19.11.2623. [DOI] [PubMed] [Google Scholar]
- 94.Cybulsky AV, Monge JC, Papillon J, McTavish AJ. Complement C5b-9 activates cytosolic phospholipase A2 in glomerular epithelial cells. Am J Physiol Renal, Fluid Electrolyte Physiol. 1995;269:F739–F749. doi: 10.1152/ajprenal.1995.269.5.F739. [DOI] [PubMed] [Google Scholar]
- 95.Morgan BP, Dankert JR, Esser AF. Recovery of human neutrophils from complement attack: Removal of the membrane attack complex by endocytosis and exocytosis. J Immunol. 1987;138:246–53. [PubMed] [Google Scholar]
- 96.Busche MN, Walsh MC, McMullen ME, Guikema BJ, Stahl GL. Mannose-binding lectin plays a critical role in myocardial ischaemia and reperfusion injury in a mouse model of diabetes. Diabetologia. 2008;51:1544–51. doi: 10.1007/s00125-008-1044-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Walsh MC, Bourcier T, Takahashi K, Shi L, Busche MN, Rother RP, Solomon SD, Ezekowitz RA, Stahl GL. Mannose-binding lectin is a regulator of inflammation that accompanies myocardial ischemia and reperfusion injury. J Immunol. 2005;175:541–6. doi: 10.4049/jimmunol.175.1.541. [DOI] [PubMed] [Google Scholar]
- 98.Collard CD, Montalto MC, Reenstra WR, Buras JA, Stahl GL. Endothelial oxidative stress activates the lectin complement pathway: role of cytokeratin 1. Am J Pathol. 2001;159:1045–54. doi: 10.1016/S0002-9440(10)61779-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jordan JE, Montalto MC, Stahl GL. Inhibition of mannose-binding lectin reduces postischemic myocardial reperfusion injury. Circulation. 2001;104:1413–8. doi: 10.1161/hc3601.095578. [DOI] [PubMed] [Google Scholar]
- 100.Montalto MC, Collard CD, Buras JA, Reenstra WR, McClaine R, Gies DR, Rother RP, Stahl GL. A keratin peptide inhibits mannose-binding lectin. J Immunol. 2001;166:4148–53. doi: 10.4049/jimmunol.166.6.4148. [DOI] [PubMed] [Google Scholar]
- 101.Collard CD, Vakeva A, Morrissey MA, Agah A, Rollins SA, Reenstra WR, Buras JA, Meri S, Stahl GL. Complement activation following oxidative stress: Role of the lectin complement pathway. Am J Pathol. 2000;156:1549–56. doi: 10.1016/S0002-9440(10)65026-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.McBride WT, Armstrong MA, McBride SJ. Immunomodulation: an important concept in modern anaesthesia. Anaesthesia. 1996;51:465–73. doi: 10.1111/j.1365-2044.1996.tb07793.x. [DOI] [PubMed] [Google Scholar]
- 103.Salo M. Effects of anaesthesia and surgery on the immune response. Acta Anaesthesiol Scand. 1992;36:201–20. doi: 10.1111/j.1399-6576.1992.tb03452.x. [DOI] [PubMed] [Google Scholar]
- 104.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 105.Vallejo R, Hord ED, Barna SA, Santiago-Palma J, Ahmed S. Perioperative immunosuppression in cancer patients. J Environ Pathol Toxicol Oncol. 2003;22:139–46. doi: 10.1615/jenvpathtoxoncol.v22.i2.70. [DOI] [PubMed] [Google Scholar]
- 106.Tsuchiya Y, Sawada S, Yoshioka I, Ohashi Y, Matsuo M, Harimaya Y, Tsukada K, Saiki I. Increased surgical stress promotes tumor metastasis. Surgery. 2003;133:547–55. doi: 10.1067/msy.2003.141. [DOI] [PubMed] [Google Scholar]
- 107.Moudgil GC, Singal DP. Halothane and isoflurane enhance melanoma tumour metastasis in mice. Can J Anaesth. 1997;44:90–4. doi: 10.1007/BF03014331. [DOI] [PubMed] [Google Scholar]
- 108.Garred P, Madsen HO, Hofmann B, Svejgaard A. Increased frequency of homozygosity of abnormal mannan-binding-protein alleles in patients with suspected immunodeficiency. Lancet. 1995;346:941–3. doi: 10.1016/s0140-6736(95)91559-1. [DOI] [PubMed] [Google Scholar]
- 109.Garred P, JJS, Quist L, Taaning E, Madsen HO. Association of mannose-binding lectin polymorphisms with sepsis and fatal outcome, in patients with systemic inflammatory response syndrome. J Infect Dis. 2003;188:1394–403. doi: 10.1086/379044. [DOI] [PubMed] [Google Scholar]
- 110.Best LG, Davidson M, North KE, MacCluer JW, Zhang Y, Lee ET, Howard BV, DeCroo S, Ferrell RE. Prospective analysis of mannose-binding lectin genotypes and coronary artery disease in American Indians: the Strong Heart Study. Circulation. 2004;109:471–5. doi: 10.1161/01.CIR.0000109757.95461.10. [DOI] [PubMed] [Google Scholar]
- 111.Madsen HO, Videm V, Svejgaard A, Svennevig JL, Garred P. Association of mannose-binding lectin deficiency with severe atherosclerosis. Lancet. 1998;352:959–60. doi: 10.1016/S0140-6736(05)61513-9. [DOI] [PubMed] [Google Scholar]
- 112.Rugonfalvi-Kiss S, Endresz V, Madsen HO, Burian K, Duba J, Prohaszka Z, Karadi I, Romics L, Gonczol E, Fust G, Garred P. Association of Chlamydia pneumoniae with coronary artery disease and its progression is dependent on the modifying effect of mannose-binding lectin. Circulation. 2002;106:1071–6. doi: 10.1161/01.cir.0000027137.96791.6a. [DOI] [PubMed] [Google Scholar]
- 113.Marenberg ME, Risch N, Berkman LF, Floderus B, de FU. Genetic susceptibility to death from coronary heart disease in a study of twins. N Engl J Med. 1994;330:1041–6. doi: 10.1056/NEJM199404143301503. [DOI] [PubMed] [Google Scholar]
- 114.Mehrabian M, Lusis AJ. Genetic markers for studies of atherosclerosis and related risk factors. In: Lusis AJ, Rotter JI, Sparkes RS, editors. Molecular genetics of coronary artery disease: candidate genes and processes in atherosclerosis. Karger; 1992. pp. 363–418. [Google Scholar]
- 115.Murata M, Kawano K, Matsubara Y, Ishikawa K, Watanabe K, Ikeda Y. Genetic polymorphisms and risk of coronary artery disease. Semin Thromb Hemost. 1998;24:245–50. doi: 10.1055/s-2007-995849. [DOI] [PubMed] [Google Scholar]
- 116.Fox AA, Shernan SK, Body SC. Predictive genomics of adverse events after cardiac surgery. Semin Cardiothorac Vasc Anesth. 2004;8:297–315. doi: 10.1177/108925320400800404. [DOI] [PubMed] [Google Scholar]
- 117.Awdeh ZL, Alper CA. Inherited structural polymorphism of the fourth component of human complement. Proc Natl Acad Sci U S A. 1980;77:3576–80. doi: 10.1073/pnas.77.6.3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Law SK, Dodds AW, Porter RR. A comparison of the properties of two classes, C4A and C4B, of the human complement component C4. EMBO J. 1984;3:1819–23. doi: 10.1002/j.1460-2075.1984.tb02052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhang S, Li Q, Yao S. Effect of complement 4 genetic polymorphism on complement activation during cardiopulmonary bypass in open heart surgery among children. Zhonghua Yi Xue Za Zhi. 2001;81:918–20. [PubMed] [Google Scholar]
- 120.Zhang S, Wang S, Yao S. Evidence for development of capillary leak syndrome associated with cardiopulmonary bypass in pediatric patients with the homozygous C4A null phenotype. Anesthesiology. 2004;100:1387–93. doi: 10.1097/00000542-200406000-00009. [DOI] [PubMed] [Google Scholar]
- 121.Ohlenschlaeger T, Garred P, Madsen HO, Jacobsen S. Mannose-binding lectin variant alleles and the risk of arterial thrombosis in systemic lupus erythematosus. N Engl J Med. 2004;351:260–7. doi: 10.1056/NEJMoa033122. [DOI] [PubMed] [Google Scholar]
- 122.Limnell V, Aittoniemi J, Vaarala O, Lehtimaki T, Laine S, Virtanen V, Palosuo T, Miettinen A. Association of mannan-binding lectin deficiency with venous bypass graft occlusions in patients with coronary heart disease. Cardiology. 2002;98:123–6. doi: 10.1159/000066313. [DOI] [PubMed] [Google Scholar]
- 123.Takahashi K, Chang WC, Takahashi M, Pavlov V, Ishida Y, La Bonte LR, Shi L, Fujita T, Stahl GL, Van Cott EM. Mannose-binding lectin and its associated proteases (MASPs) mediate coagulation and its deficiency is a risk factor in developing complications from infection, including disseminated intravascular coagulation. Immunobiology. 2011;216:96–102. doi: 10.1016/j.imbio.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Krarup A, Wallis R, Presanis JS, Gal P, Sim RB. Simultaneous activation of complement and coagulation by MBL-associated serine protease 2. PLoS ONE. 2007;2:e623. doi: 10.1371/journal.pone.0000623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.La Bonte LR, Pavlov VI, Tan YS, Takahashi K, Takahashi M, Banda NK, Zou C, Fujita T, Stahl GL. Mannose-Binding Lectin-Associated Serine Protease-1 Is a Significant Contributor to Coagulation in a Murine Model of Occlusive Thrombosis. J Immunol. 2011;188:885–91. doi: 10.4049/jimmunol.1102916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Schwaeble WJ, Lynch NJ, Clark JE, Marber M, Samani NJ, Ali YM, Dudler T, Parent B, Lhotta K, Wallis R, Farrar CA, Sacks S, Lee H, Zhang M, Iwaki D, Takahashi M, Fujita T, Tedford CE, Stover CM. Targeting of mannan-binding lectin-associated serine protease-2 confers protection from myocardial and gastrointestinal ischemia/reperfusion injury. Proc Natl Acad Sci U S A. 2011;108:7523–8. doi: 10.1073/pnas.1101748108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Walsh MC, Shaffer LA, Guikema BJ, Body SC, Shernan SK, Fox AA, Collard CD, Fung M, Taylor RP, Stahl GL. Fluorochrome-linked immunoassay for functional analysis of the mannose binding lectin complement pathway to the level of C3 cleavage. J Immunol Meth. 2007;323:147–59. doi: 10.1016/j.jim.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Collard CD, Shernan SK, Fox AA, Bernig T, Chanock SJ, Vaughn WK, Takahashi K, Ezekowitz AB, Jarolim P, Body SC. The MBL2 “LYQA Secretor” Haplotype is an Independent Predictor of Postoperative Myocardial Infarction in Caucasians Undergoing Coronary Artery Bypass Graft Surgery. Circulation. 2007;116:106–12. doi: 10.1161/CIRCULATIONAHA.106.679530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Fox A, Shernan S, Collard C, DeSantis S, Liu KY, Jarolim P, Body S. Preoperative B-type Natriuretic Peptide Independently Predicts Ventricular Dysfunction after Primary Coronary Artery Bypass Graft Surgery. J Thorac Cardiovasc Surg. 2007 doi: 10.1016/j.jtcvs.2007.12.036. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Reiner AP, Carlson CS, Rieder MJ, Siscovick DS, Liu K, Chandler WL, Green D, Schwartz SM, Nickerson DA. Coagulation factor VII gene haplotypes, obesity-related traits, and cardiovascular risk in young women. J Thromb Haemost. 2007;5:42–9. doi: 10.1111/j.1538-7836.2006.02279.x. [DOI] [PubMed] [Google Scholar]
- 131.Shernan SK, Collard CD. Role of the complement system in ischaemic heart disease: potential for pharmacological intervention. BioDrugs. 2001;15:595–607. doi: 10.2165/00063030-200115090-00004. [DOI] [PubMed] [Google Scholar]
- 132.Cedzynski M, Madalinski K, Gregorek H, Swierzko AS, Nowicka E, Obtulowicz K, Dzierzanowska-Fangrat K, Wojda U, Rabczenko D, Kawakami M. Possible disease-modifying factors: the mannan-binding lectin pathway and infections in hereditary angioedema of children and adults. Arch Immunol Ther Exp (Warsz ) 2008;56:69–75. doi: 10.1007/s00005-008-0004-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Rossi V, Cseh S, Bally I, Thielens NM, Jensenius JC, Arlaud GJ. Substrate specificities of recombinant mannan-binding lectin-associated serine proteases-1 and-2. J Biol Chem. 2001;276:40880–7. doi: 10.1074/jbc.M105934200. [DOI] [PubMed] [Google Scholar]
- 134.Rossi V, Teillet F, Thielens NM, Bally I, Arlaud GJ. Functional characterization of complement proteases C1s/mannan-binding lectin-associated serine protease-2 (MASP-2) chimeras reveals the higher C4 recognition efficacy of the MASP-2 complement control protein modules. J Biol Chem. 2005;280:41811–8. doi: 10.1074/jbc.M503813200. [DOI] [PubMed] [Google Scholar]
- 135.Davis AE., III Biological effects of C1 inhibitor. Drug News Perspect. 2004;17:439–46. doi: 10.1358/dnp.2004.17.7.863703. [DOI] [PubMed] [Google Scholar]
- 136.Buerke M, Murohara T, Lefer AM. Cardioprotective effects of a C1 esterase inhibitor in myocardial ischemia and reperfusion. Circulation. 1995;91:393–402. doi: 10.1161/01.cir.91.2.393. [DOI] [PubMed] [Google Scholar]
- 137.Horstick G, Heimann A, Gotze O, Hafner G, Berg O, Boehmer P, Becker P, Darius H, Rupprecht H-J, Loos M, Bhakdi S, Meyer J, Kempski O. Intracoronary application of C1 esterase inhibitor improves cardiac function and reduces myocardial necrosis in an experimental model of ischemia and reperfusion. Circulation. 1997;95:701–8. doi: 10.1161/01.cir.95.3.701. [DOI] [PubMed] [Google Scholar]
- 138.Horstick G, Berg O, Heimann A, Gotze O, Loos M, Hafner G, Bierbach B, Petersen S, Bhakdi S, Darius H, Horstick M, Meyer J, Kempski O. Application of C1-esterase inhibitor during reperfusion of ischemic myocardium: dose-related beneficial versus detrimental effects. Circulation. 2001;104:3125–31. doi: 10.1161/hc5001.100835. [DOI] [PubMed] [Google Scholar]
- 139.Fattouch K, Bianco G, Speziale G, Sampognaro R, Lavalle C, Guccione F, Dioguardi P, Ruvolo G. Beneficial effects of C1 esterase inhibitor in ST-elevation myocardial infarction in patients who underwent surgical reperfusion: a randomised double-blind study. Eur J Cardiothorac Surg. 2007;32:326–32. doi: 10.1016/j.ejcts.2007.04.038. [DOI] [PubMed] [Google Scholar]
- 140.Bauernschmitt R, Bohrer H, Hagl S. Rescue therapy with C1-esterase inhibitor concentrate after emergency coronary surgery for failed PTCA. Intensive Care Med. 1998;24:635–8. doi: 10.1007/s001340050629. [DOI] [PubMed] [Google Scholar]
- 141.Shernan SK, Fitch JC, Nussmeier NA, Chen JC, Rollins SA, Mojcik CF, Malloy KJ, Todaro TG, Filloon T, Boyce SW, Gangahar DM, Goldberg M, Saidman LJ, Mangano DT. Impact of pexelizumab, an anti-C5 complement antibody, on total mortality and adverse cardiovascular outcomes in cardiac surgical patients undergoing cardiopulmonary bypass. Ann Thorac Surg. 2004;77:942–9. doi: 10.1016/j.athoracsur.2003.08.054. [DOI] [PubMed] [Google Scholar]
- 142.Mathew JP, Shernan SK, White WD, Fitch JC, Chen JC, Bell L, Newman MF. Preliminary report of the effects of complement suppression with pexelizumab on neurocognitive decline after coronary artery bypass graft surgery. Stroke. 2004;35:2335–9. doi: 10.1161/01.STR.0000141938.00524.83. [DOI] [PubMed] [Google Scholar]
- 143.Verrier ED, Shernan SK, Taylor KM, Van de WF, Newman MF, Chen JC, Carrier M, Haverich A, Malloy KJ, Adams PX, Todaro TG, Mojcik CF, Rollins SA, Levy JH. Terminal complement blockade with pexelizumab during coronary artery bypass graft surgery requiring cardiopulmonary bypass: a randomized trial. JAMA. 2004;291:2319–27. doi: 10.1001/jama.291.19.2319. [DOI] [PubMed] [Google Scholar]
- 144.Smith PK, Shernan SK, Chen JC, Carrier M, Verrier ED, Adams PX, Todaro TG, Muhlbaier LH, Levy JH. Effects of C5 complement inhibitor pexelizumab on outcome in high-risk coronary artery bypass grafting: combined results from the PRIMO-CABG I and II trials. J Thorac Cardiovasc Surg. 2011;142:89–98. doi: 10.1016/j.jtcvs.2010.08.035. [DOI] [PubMed] [Google Scholar]
- 145.Weisman HF, Bartow T, Leppo MK, Marsh HCJ, Jr, Carson GR, Concino MF, Boyle MP, Roux KH, Weisfeldt ML, Fearon DT. Soluble human complement receptor type 1: in vivo inhibitor of complement suppressing post-ischemic myocardial inflammation and necrosis. Science. 1990;249:146–51. doi: 10.1126/science.2371562. [DOI] [PubMed] [Google Scholar]
- 146.Rioux P. TP-10 (AVANT Immunotherapeutics) Current Opinions on Investigation Drugs. 2001;2:364–71. [PubMed] [Google Scholar]
- 147.Lazar HL, Bao Y, Gaudiani J, Rivers S, Marsh H. Total complement inhibition: an effective strategy to limit ischemic injury during coronary revascularization on cardiopulmonary bypass. Circulation. 1999;100:1438–42. doi: 10.1161/01.cir.100.13.1438. [DOI] [PubMed] [Google Scholar]
- 148.Chai PJ, Nassar R, Oakeley AE, Craig DM, Quick G, Jr, Jaggers J, Sanders SP, Ungerleider RM, Anderson PA. Soluble complement receptor-1 protects heart, lung, and cardiac myofilament function from cardiopulmonary bypass damage. Circulation. 2000;101:541–6. doi: 10.1161/01.cir.101.5.541. [DOI] [PubMed] [Google Scholar]
- 149.Li JS, Sanders SP, Perry AE, Stinnett SS, Jaggers J, Bokesch P, Reynolds L, Nassar R, Anderson PA. Pharmacokinetics and safety of TP10, soluble complement receptor 1, in infants undergoing cardiopulmonary bypass. Am Heart J. 2004;147:173–80. doi: 10.1016/j.ahj.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 150.Lazar HL, Bokesch PM, van LF, Fitzgerald C, Emmett C, Marsh HC, Jr, Ryan U. Soluble human complement receptor 1 limits ischemic damage in cardiac surgery patients at high risk requiring cardiopulmonary bypass. Circulation. 2004;110:II274–II279. doi: 10.1161/01.CIR.0000138315.99788.eb. [DOI] [PubMed] [Google Scholar]
- 151.Van de Wouwer M, Collen D, Conway EM. Thrombomodulin-protein C-EPCR system: integrated to regulate coagulation and inflammation. Arterioscler Thromb Vasc Biol. 2004;24:1374–83. doi: 10.1161/01.ATV.0000134298.25489.92. [DOI] [PubMed] [Google Scholar]
- 152.Raivio P, Lassila R, Petaja J. Thrombin in myocardial ischemia-reperfusion during cardiac surgery. Ann Thorac Surg. 2009;88:318–25. doi: 10.1016/j.athoracsur.2008.12.097. [DOI] [PubMed] [Google Scholar]
- 153.Zhang M, Hou YJ, Cavusoglu E, Lee DC, Steffensen R, Yang L, Bashari D, Villamil J, Moussa M, Fernaine G, Jensenius JC, Marmur JD, Ko W, Shevde K. MASP-2 activation is involved in ischemia-related necrotic myocardial injury in humans. Int J Cardiol. 2011 doi: 10.1016/j.ijcard.2011.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Krarup A, Gulla KC, Gal P, Hajela K, Sim RB. The action of MBL-associated serine protease 1 (MASP1) on factor XIII and fibrinogen. Biochim Biophys Acta. 2008;1784:1294–300. doi: 10.1016/j.bbapap.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 155.Trendelenburg M, Theroux P, Stebbins A, Granger C, Armstrong P, Pfisterer M. Influence of functional deficiency of complement mannose-binding lectin on outcome of patients with acute ST-elevation myocardial infarction undergoing primary percutaneous coronary intervention. Eur Heart J. 2010 doi: 10.1093/eurheartj/ehp597. [DOI] [PubMed] [Google Scholar]
- 156.Pavlov VI, La Bonte LR, Baldwin WM, Markiewski M, Lambris J, Stahl GL. Absence of mannose-binding lectin prevents hyperglycemic cardiovascular complications. Am J Pathol. 2012;180:104–12. doi: 10.1016/j.ajpath.2011.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]